Abstract
This study stimulated functional microbial adaptation to the extreme environment of the Luliang oilfield reservoir and assessed the impact of indigenous microbial enhanced oil recovery treatment on the microbial community and oil recovery. By high-resolution pyro-sequencing, Acinetobacter, Pseudomonas, Sphingomonas, Thauera, Arcobacter and Hyphomonas were regarded as suitable candidates and a customised nutrient mixture was selected to activate the target microbes. The interfacial tension of crude oil and water decreased from 29.76 mN m−1 to 0.65 mN m−1 and the emulsion stability index increased from 5% to 92% after customised nutrient culture. The oil biodegradation experiments after the cultivation exhibited positive effects in degrading aromatics, resins and asphaltenes fractions and improving the mobility. Six target genera were activated markedly after 96 h of incubation. The oil recovery was enhanced from 51.2% to 60.7% using fermentation broth injection. Thus, injecting a customised nutrient mixture with injection brine into the stratum to stimulate functional microbes has the potential to enhance oil recovery in the Luliang oilfield.
Introduction
Stimulation of indigenous microbial enhanced oil recovery (IMEOR) has been widely used in oil exploitation to successfully increase oil production [Citation1,Citation2], especially in oil fields where residual oil is no longer recovered efficiently by ordinary water flooding [Citation3]. IMEOR often involves the injection of nutrients with a small amount of inoculum [Citation4] with the objective of stimulating microbes to produce biosurfactants or emulsifiers in the reservoir to emulsify the crude oil, modify the properties of crude oil and enhance oil recovery [Citation5]. Therefore, hydrocarbon degrading bacteria, as functional bacteria in IMEOR, have been the target of in situ nutrient stimulation and have been studied to extract additional oil [Citation6]. A previous study using 16S rRNA gene pyrosequencing showed that the injection of nutrients in the Huabei oilfield in China resulted in a microbial community dominated by hydrocarbon-utilising genera, which was determined by the type of available nutrient [Citation7]. This is thought to be closely related to the incremental oil production [Citation8].
In recent stimulation studies, field candidates of IMEOR were evaluated based on the remaining oil in place, average porosity and average permeability [Citation9]. The optimum nutrients and incubation conditions for the given oil fields were optimised by laboratory experiments [Citation9–12]. A novel study used an exogenous Bacillus subtilis to strengthen the IMEOR process [Citation13]. IMEOR relies of the combination of various mechanisms, such as oil emulsification, oil bio-degradation, gas production, acid production and viscosity reduction [Citation11,Citation14,Citation15]. In a large-scale IMEOR pilot field, 210,000 tonnes of crude oil were produced cumulatively by injection of nutrients with water [Citation16].
However, many stimulation experiments in the laboratory, could not even confirm whether the activated bacteria could survive in the oil reservoir, let alone achieve displacement efficiency in the oilfield. Therefore, our target bacteria, which existed in both the injection and formation brine, and had a much higher abundance in the formation brine, were regarded as suitable candidates for MEOR. For this purpose, stimulation experiments aimed at the target bacteria were performed and the enhanced oil recovery potential was assessed in this study.
Materials and methods
Reservoir characteristics and sample collection
In this study, three brine samples from the Luliang block in the Xinjiang Oilfield were collected. The depth of the petroleum reservoir was 1200 m, with a temperature of 34 °C and pressure of 14.99 MPa. Brine sample Y1 was injection brine from injected well Y1; Y3 and Y5 were formation brines collected from produced wells Y3 and Y5, respectively. The samples were sieved thoroughly to remove oil and sediment and achieve homogeneity. A portion of each sample was collected in a 200 mL centrifuge tube, placed in an ice-box and transferred to the laboratory. The tubes were stored at −80 °C until DNA extraction.
Fermentation broth preparation
The nutrient combination (molasses 1%, yeast 0.06% and ethanol 0.2%) was selected based on stimulation of common functional genera between injection brine and formation brines, according to other reports and preliminary experiments. Cultivation was carried out in 250 mL Erlenmeyer flasks containing 100 mL of injection brine supplemented with selected nutrients and 2% crude oil. Flasks were incubated at 35 °C for 96 h with agitation at 120 rpm.
DNA extraction
Brine samples and fermentation broth were centrifuged at 12,000 rpm for 15 min using a Hettich universal 320R centrifuge (Hettich, Tuttlingen, Germany) to collect bacterial cells. DNA was extracted from the bacteria using an AxyPrep Bacterial Genomic DNA Miniprep kit (Axygen Biosciences, Union City, CA) following the manufacturer’s instructions. The extracted DNA was diluted in Tris-EDTA (TE) buffer (10 mmol L−1 Tris–HCl, 1 mmol L−1 ethylenediaminetetraacetic acid, pH 8.0) and stored at −20 °C until use.
Pyro-sequencing
The V1-V3 hypervariable regions of the bacterial 16S rRNA gene were amplified using the extracted DNA as a template and primers 27F (5′-AGA GTT TGA TCT GGC TCA G-3′) and 533R (5′-ATT ACC GCG GCT GCT GG-3′). Polymerase chain reactions (PCR) were performed in a 25-μL mixtures containing 0.5 μL of each primer, 1 μL of template DNA and PCR SuperMix (Invitrogen, Shanghai, China). The following thermal program was used for amplification by a Bio-Rad C1000 Touch thermal cycler (Bio-Rad, Hercules, CA): 94 °C for 5 min; followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; followed by extension at 72 °C for 10 min [Citation17]. An equal amount of the PCR product from each sample was combined in a single tube to be run on a Roche FLX 454 pyro-sequencing machine at Majorbio BioPharm Technology Co., Ltd, Shanghai, China.
Construction of 16S rDNA gene libraries
Amplification of 16S rDNA of bacteria in the fermentation broth was performed using the extracted DNA as a template and primers 8F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1541R (5′-AAG GAG GTG ATC CAG CC-3′) [Citation18]. The following thermal program was used for amplification: 95 °C for 5 min; followed by 30 cycles of 94 °C for 45 s, 52 °C for 45 s and 72 °C for 90 s; followed by extension at 72 °C for 10 min. The obtained 16S rDNA fragments were inserted into Peasy®-T3 cloning vector and transformed into competent cells. The transformed cells were grown to form colonies and 210 clones were randomly selected for verification using primers T7 (5′-TAA TAC GAC TCA CTA TAG GG-3′) and SP6 (5′ -ATT TAG GTG ACA CTA TAG AA-3′) to remove false positive clones. The clones were sequenced at Majorbio BioPharm Technology Co., Ltd, Shanghai, China. The determined sequences were compared with similar sequences from reference organisms in the GenBank database of the National Center of Biotechnology Information (NCBI) using a BLAST search.
Analysis of crude oil
After the cultivation, the culture was extracted with equal volumes of hexane, dichloromethane and chloroform for the subsequent measurements. The extracts were dried at room temperature by evaporation in a safety cabinet and were used as the residual crude oil [Citation19]. Then, the residual crude oil was separated into saturates, aromatics, resins and asphaltenes (SARA) fractions using column chromatography with several different developing solvents, and then weighted according to the standard (SY/T 5119-2008) in China. The viscosity measurements of the crude oil were carried out using a Brookfield DV-II programmable viscometer (Brookfield, Middleboro, MA).
Interfacial tension (IFT), biomass and emulsifying properties
To investigate the crude oil-emulsifying performance of the activation system, the biomass, the oil-water IFT, the emulsion stability index and the diameters of the emulsified crude oil droplets were studied in the stimulation test. The fermentation broth was centrifuged at 8000 rpm for 10 min using a Hettich universal 320R centrifuge; the collected cells were dried in an oven at 80 °C, and the dry weight of the biomass was determined. The oil–water IFT was measured using a TX-500C IFT meter. The emulsion state of the crude oil in the fermentation broth was observed using an optical microscope. The images of crude oil captured from the microscope were processed using Micro-image Analysis & Process software to obtain the drop sizes. To determine the average diameters of crude oil droplets, 500 were taken droplets at each experimental time. The emulsion stability index was defined as the inverse of the change in transmission and backscattering in unit time and is positively correlated with the stability of the emulsion.
Core flooding experiments for oil recovery
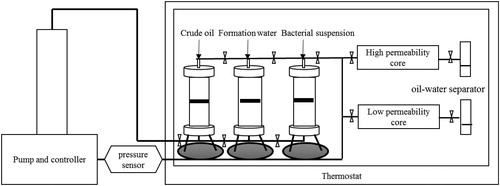
The effect of the nutrient activator was evaluated by artificial core flooding experiments. A schematic diagram of the experimental setup for the core flooding experiments is shown in . The oil from the Luliang production well was used as the oleic phase, whereas the injection brine came from well Y1. Artificial cores made from oil sand of Luliang were 29.7 cm in length and 3.8 cm in diameter. Porosity and permeability were also achieved based on the same block. First, the artificial oil-bearing core was injected with injection brine until no more oil was recovered from it. Subsequently, the columns were injected with 0.4 pore volumes (PV) of formation water, 0.4 PV of a nutrient package prepared by the formation water, and 0.4 PV fermentation broth, respectively. After incubation at 35 °C for 7 days, ultimate oil recovery and enhanced oil recovery were calculated with successive water flooding.
Results and discussion
Composition of the microbial communities
The microbial community structure of the injection brine and formation brines from oil wells Y3 and Y5 in the Luliang oilfield were analysed using 454 pyro-sequencing. In total, we obtained 10,738 high quality sequences from the three samples, and 3056–3861 sequences were obtained per sample (an average of 3579). Of these sequences, 84% could be classified. The dominant classes (relative abundance >3%) across the three samples were Alphaproteobacteria, Betaproteobacteria, Epsilonproteobacteria, Deltaproteobacteria and Deferribacteres, and these classes accounted for 72% of the bacterial sequences.
Analysis of common functional bacteria
The reservoir environment was unsuitable for the growth of bacteria that existed in the injection brine, but not in the formation brine. However, the microbes that were present in the formation brine but not in the injection brine were non-dominant in the formation brine and represented non-functional bacteria in this experiment. The common bacteria that existed in both the injected brine and formation brine, and had a higher abundance in the formation brine compared with injection water, were more suitable for growth in the reservoir.
As shown in , a total of 15 genera were common in the three samples. Some functional genera showed increased abundance. For instance, only 0.04% of Sphingomonas was detected in injection brine Y1, but 28.58% was detected in oil well Y5 and 0.92% in Y3, representing an increase of more than 728 times in Y5. The relative abundance of Pseudomonas was 0.22% in oil well Y3 and 0.23% in Y5, which was nearly five times higher than that in injection brine Y1. The relative abundance of Acinetobacter was 0.18% in oil well Y3 and 0.14% in Y5, which was near three times higher than its abundance in the injection brine Y1. The relative abundance of Thauera increased by 12 times in Y3 compared with that in Y1 and also increased in Y5. All of these bacteria are capable of degrading hydrocarbons in either anaerobic or aerobic conditions, and have been commonly found in other oilfields [Citation7]. Acinetobacter and Pseudomonas, which are known to produce metabolites (i.e. biosurfactants and extracellular polysaccharides) to emulsify crude oil, are hydrogen-oxidizing bacteria and are a focus for MEOR [Citation20–22]. Sphingomonas is reported to be able to degrade aromatic compounds. Thauera, a denitrifying facultative anaerobe, can anaerobically degrade aromatic compounds. The relative abundance of Arcobacter was 0.2% in Y5, which was 55 times higher than its abundance in Y1. The relative abundance of Hyphomonas increased by 9-fold in Y3 compared with that in Y1 and was also increased in Y5. Arcobacter and Hyphomonas, which can reduce nitrate and participate in hydrocarbon degradation, inhibit the growth of sulphate-reducing bacteria (SRB). Their presence helps slow down the damage from facility corrosion. The results showed that the six functional genera, which were provided by the injection brine, were able to adapt to the extreme environment of the oil reservoir and showed rapid growth. To improve oil recovery, the nutrient composition for MEOR stimulation was adjusted according to the needs of these six functional genera, especially for Acinetobacter, Pseudomonas, Sphingomonas and Thauera, which could degrade crude oil and were adapted to the oil environment in Luliang oilfield.
Table 1. Common genera and their abundance proportions in the three samples.
Bacterial community after stimulation
Using a nutrient activator containing 1.5% molasses and 0.06% yeast, the functional genera Acinetobacter and Pseudomonas of Daqing oilfield in China were stimulated [Citation23,Citation24]; however, Thauera and Sphingomonas were activated at relatively low abundance. An Acinetobacter strain was found to be enriched and produced an emulsifying agent when grown on ethanol medium in a fed batch reactor [Citation25]. Based on these studies and pre-experiments, the nutrient activator with molasses, yeast and ethanol was selected to stimulate the common functional genera in the injection brine.
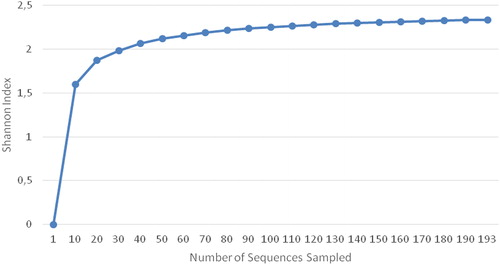
We constructed a gene library to detect bacterial species in the enrichment culture incubated for 96 h, and then sequenced 191 clones (approximately 1500 bp) from the library. The Shannon–Wiener curve () for the library was close to saturation, which indicated that the sequencing results had good coverage of the species in the culture.
We determined 27 genera from 191 clones in the enrichment culture. As shown in , the dominant genera (relative abundance >3.53%) in the enrichment culture were Acinetobacter, Pseudomonas, Arcobacter, Sphingomonas and Hyphomonas and these genera accounted for more than 71.4% of the bacterial sequences. The abundance of genera Acinetobacter and Pseudomonas increased rapidly, and they became the most dominant genera, at 740-fold and 515-fold higher abundance than that in the produced water Y1, respectively. The relative abundance of Arcobacter and Sphingomonas increased by 47 and 200 times compared with that in Y1, respectively. The genus Thauera was also detected, but was not abundant. However, the abundance of Thauera was also higher than that in injection brine Y1 and formation brines Y3 and Y5. This indicated that the target genera were greatly activated after 96 h of incubation in the selected nutrients, and the two most dominant genera were hydrogen-oxidizing bacteria, Acinetobacter and Pseudomonas.
Table 2. Genera in the enrichment culture and their abundance proportions.
In fact, MEOR is dependent on the influence of a large number of bacteria and the stimulated bacteria are the most important elements. If the bacteria, which were stimulated and exhibited enhanced oil recovery in laboratory experiments, did not survive in the oil reservoir, it would be impossible to achieve the goal of enhanced oil recovery. In this study, our functional target bacteria could be highly abundant as a result of natural selection in the stratum, without nutritional stimulation. A natural rule-based process was performed that added nutrients to enhance the abundance of the functional target bacteria. Therefore, the stimulation experiments based on this principle were more likely to be repeated in the oilfield, thus showing the advantages of this experiment.
Biodegradation and viscosity alteration of the crude oil
The proportion of heavy components in the crude oil, such as asphaltenes and resins, strongly affects the crude oil viscosity and the degree of difficulty in oil reservoir development [Citation26]. As shown in , Luliang Oil contained 61.28% saturated hydrocarbons. After the cultivation, the relative percentage of saturates increased by 6.8%, whereas the percentages of aromatic hydrocarbons and resins decreased by 6.1% and 20.7%, respectively. Meanwhile, the asphaltenes of the degraded crude oil were found to decrease slightly compared with the original oil. The increase in the saturates content mainly resulted from the consumption of the other three fractions. The degradation of crude oil had a clear impact; the viscosity of the crude oil decreased significantly from 45.2 mPa s to 29.7 mPa s. Comparing these results with other reports (in the ‘Bacterial community after stimulation’), it showed that the stimulated bacterial community had the ability to degrade the heavy fractions and change the chemical and physical properties of Luliang crude oil after stimulation by the selected nutrients.
Table 3. Oil components before and after cultivation.
Emulsifying properties after stimulation
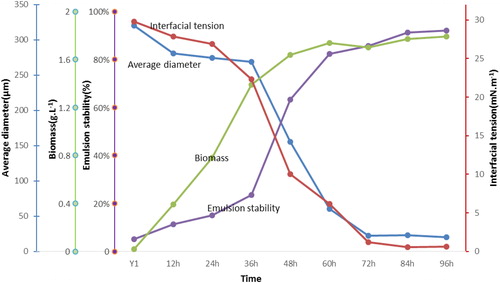
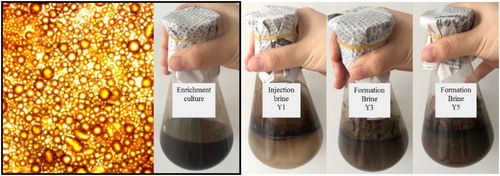
Biomass, oil–water IFT, the emulsion stability index and the average diameters of emulsified crude oil droplets are shown in . A maximum biomass of 1.77 g L−1 was obtained at 84 h. The IFT decreased from 29.76 mN m−1 to 1.21 mN m−1 (72 h) and a stable IFT below 1 was observed from 84 h. The average diameters of the crude oil droplets decreased to 22.15 μm at 72 h. Greater crude oil emulsification began to appear from 48 h, which lagged behind cell growth. The best emulsification was observed at 72 h, while the emulsion stability index increased from 5.1% to 92% during the entire period of nutritional stimulation. In contrast, with the formation brines, IFTs of 21.6 mN m−1 and 20.87 mN m−1 and emulsion stability indices of 13.4% and 12.9% were observed in formation brine Y3 and Y5, respectively. shows the emulsions of the activation system, injection brine Y1 and formation brines Y3 and Y5. The emulsification performance was more pronounced in the activation system than in the injection and formation brines. Emulsification in the activation system was mainly attributed to biosurfactants produced by the bacteria rather than cell growth. This result demonstrated that the complex bacteria were stimulated significantly by nutritional stimulation and could use crude oil, metabolise and produce biosurfactants, which led to a reduced IFT and an excellent emulsification performance for crude oil. Reducing interfacial tensions between brines and oil, and forming micro-emulsions, are important mechanisms involved in MEOR [Citation27,Citation28]. In this study, nutrients accelerated the growth of functional bacteria that were adapted to the stratum in the injection brine and promoted crude oil emulsification in 4 days compared with formation brines.
Enhanced oil recovery in the core flooding experiment
The ultimate goal of MEOR is to enhance the oil recovery or the oil production rate. As shown in , the oil displacement efficiency increased by 9.5% in the core-flooding tests with the injected fermentation broth and the water drive recovery was 51.2%, which exhibited a great potential to promote the oil influx into the well. The total water drive recovery after injection of the nutrient activator was 58.1%, with 7.6% enhanced oil recovery. In artificial core flooding tests, enhanced oil recovery was demonstrated and such enhancements resulted from the collaborated metabolic activities of the complex enriched bacterial system, especially the six stimulated target genera. The results implied that the nutrients selected for the target genera of Luliang oilfield in this study had great potential to increase the oil recovery.
Table 4. Results of the core-flooding tests.
The results suggested that the method using selected nutrients to stimulate functional bacteria had more benefits, such as raising the quality of crude oil, reducing the oil viscosity and improving the flow characteristics by degrading the heavy fractions and producing biosurfactant to emulsify crude oil. Affected by all these factors, the enhanced oil recovery was similar to that achieved by injecting (bio)surfactants directly [Citation29,Citation30]. However, compared with the injection of (bio)surfactants, this method has low energy consumption and is non-hazardous and cost-effective. Therefore, it could be more advantageous than the direct injection of (bio)surfactants.
Conclusions
The results from this study showed that six dominant functional genera: Acinetobacter, Pseudomonas, Arcobacter, Sphingomonas, Hyphomonas and Thauera, were provided by the injection brine, and were able to adapt to the extreme environments of the oil reservoir in Luliang, which provided the basic conditions for IMEOR. Under the given reservoir conditions, biostimulation was carried out and a maximum biomass of 1.77 g L−1 was observed in the injection brine supplemented with selected nutrients. With the injection of nutrients, the six functional genera were greatly stimulated and could produce metabolites, such as biosurfactants, to modify the oil emulsifying properties. It resulted in the decrease of interfacial tension to 0.65 mN m−1. Meanwhile, a stable emulsion was formed by dispersed crude oil, with the average droplet diameter decreasing to 20.37 μm and the emulsion stability increasing to 92%. After the cultivation, the percentages of aromatics, resins and asphaltenes fractions decreased by 6.1%, 20.7% and 1.8%, respectively. As a result of this, the mobility was improved through the viscosity reduction. Core-flooding experiments showed an enhanced oil recovery of 9.5% with injection of the fermentation broth and 7.6% with the nutrient activator. Therefore, the nutrient combination selected for the six functional genera was confirmed as effective and could be used in stimulation-based IMEOR in the Luliang oilfield.
Additional information
Funding
References
- Lazar I, Petrisor IG, Yen TF. Microbial enhanced oil recovery (MEOR). Petrol Sci Technol. 2007;25(11):1353–1366.
- Youssef N, Elshahed MS, Mcinerney MJ. Microbial processes in oil fields: culprits, problems, and opportunities. Adv Appl Microbiol. 2009;66(66):141–251.
- Huang L, Yu L, Luo Z, et al. A microbial-enhanced oil recovery trial in huabei oilfield in China. Petrol Sci Technol. 2014;32(5):584–592.
- Gao P, Li G, Dai X, et al. Nutrients and oxygen alter reservoir biochemical characters and enhance oil recovery during biostimulation. World J Microbiol Biotechnol. 2013;29(11):2045–2054.
- Cui QF, Sun SS, Luo YJ, et al. Comparison of in-situ and ex-situ microbial enhanced oil recovery by strain Pseudomonas aeruginosa WJ-1 in laboratory sand-pack columns. Petrol Sci Technol. 2017;35(21):2044–2050.
- Nazina TN, Grigor’Yan AA, Shestakova NM, et al. [Microbiological investigations of high-temperature horizons of the Kongdian petroleum reservoir in connection with field trial of a biotechnology for enhancement of oil recovery]. Mikrobiologiia. 2007;76(3):329–339. Russian.
- You J, Wu G, Ren F, et al. Microbial community dynamics in Baolige oilfield during MEOR treatment, revealed by Illumina MiSeq sequencing. Appl Microbiol Biotechnol. 2015;100(3):1469–1478.
- Li G, Gao P, Wu Y, et al. Microbial abundance and community composition influence production performance in a low-temperature petroleum reservoir. Environ Sci Technol. 2014;48(9):5336–5344.
- Zhan Y, Wang Q, Chen C, et al. Potential of wheat bran to promote indigenous microbial enhanced oil recovery. J Ind Microbiol Biot. 2017;44(6):1–11.
- Gao P, Li G, Le J, et al. Succession of microbial communities and changes of incremental oil in a post-polymer flooded reservoir with nutrient stimulation. Appl Microbiol Biotechnol. 2018;102(4):2007–2017.
- Astuti DI, Ariadji T, Aditiawati P, et al., editors. A comprehensive preparation study for microbial nutrient injection of microbial enhanced oil recovery: Reservoir screening and laboratory analysis – Case study Bentayan Field. Proceeding of SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition; 2017 Oct 17–19; Jakarta, Indonesia. Richardson (TX): Society of Petroleum Engineers; 2016 (Document ID: SPE-186249-MS).
- Alkan H, Klueglein N, Mahler E, et al., editors. An Integrated German MEOR Project, Update: Risk Management and Huff’n Puff Design. Proceeding of SPE Improved Oil Recovery Conference; 2016 Apr 11–13; Tulsa, OK. Richardson (TX): Society of Petroleum Engineers; 2016 (Document ID: SPE-179683-MS).
- Gao P, Li G, Li Y, et al. An exogenous surfactant-producing Bacillus subtilis facilitates indigenous microbial enhanced oil recovery. Front Microbiol. 2016;7(505):186. [14 p.] DOI: 10.3389/fmicb.2016.00186
- Chen C-M, Wang J-L, Kim JB, et al. Laboratory studies of rice bran as a carbon source to stimulate indigenous microorganisms in oil reservoirs. Pet Sci. 2016;13(3):572–583.
- Tian H, Gao P, Dai X, et al. [Optimization of a new viscously slow-release nutrient and oil displacement potential evaluation]. Chinese J Bioprocess Eng. 2016;14(3):33–38 (in Chinese).
- Ke CY, Lu GM, Li YB, et al. A pilot study on large-scale microbial enhanced oil recovery (MEOR) in Baolige Oilfield. Int Biodeter Biodegr. 2018;127:247–253.
- Liu J, Sui Y, Yu Z, et al. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol Biochem. 2014;70:113–122.
- Yamane K, Hattori Y, Ohtagaki H, et al. Microbial diversity with dominance of 16S rRNA gene sequences with high GC contents at 74 and 98 °C subsurface crude oil deposits in Japan. FEMS Microbiol Ecol. 2011;76(2):220–235.
- Xia W, Shen W, Yu L, et al. Conversion of petroleum to methane by the indigenous methanogenic consortia for oil recovery in heavy oil reservoir. Appl Energ. 2016;171:646–655.
- Safdel M, Anbaz MA, Daryasafar A, et al. Microbial enhanced oil recovery, a critical review on worldwide implemented field trials in different countries. Renew Sust Energ Rev. 2017;74:159–172.
- Nazina TN, Sokolova DS, Babich TL, et al. Microorganisms of low-temperature heavy oil reservoirs (Russia) and their possible application for enhanced oil recovery. Microbiol. 2017;86(6):773–785.
- Hanafy AEME, Anwar Y, Mohamed SA, et al. Isolation and identification of bacterial consortia responsible for degrading oil spills from the coastal area of Yanbu, Saudi Arabia. Biotechnol Biotechnol Equip. 2016;86(1):1763–1775.
- Ji K. [Biochemical Evaluation of Indigenous Microbial Flooding Process in Reservoir after Polymer Flooding] [Master’s thesis]. Tianjin (China): Nankai University; 2012 (in Chinese).
- Zhao L. [Molecular Ecology Analysis of Reservoir Microorganisms] [Master’s thesis]. Tianjin (China): Nankai University; 2009 (in Chinese).
- Navon-Venezia S, Zosim Z, Gottlieb A, et al. Alasan, a new bioemulsifier from Acinetobacter radioresistens. Appl Environ Microbiol. 1995;61(9):3240–3244.
- Farag S, Soliman NA, Abdel-Fattah YR. Statistical optimization of crude oil bio-degradation by a local marine bacterium isolate Pseudomonas sp. sp48. J Genet Eng Biotechnol. 2018 [cited 2018 Mar 31]. [12 p.] DOI: 10.1016/j.jgeb.2018.01.001
- Mnif S, Chamkha M, Labat M, et al. Simultaneous hydrocarbon biodegradation and biosurfactant production by oilfield-selected bacteria. J Appl Microbiol. 2011;111(3):525–536.
- Voordouw G. Production-related petroleum microbiology: progress and prospects. Curr Opin Biotechnol. 2011;22(3):401–405.
- Al-Ghailani T, Al-Wahaibi YM, Joshi SJ, et al., editors. Alkaline-biosurfactant-biopolymer process and its potential for enhancing oil recovery in omani oil field. Proceeding of SPE EOR Conference at Oil and Gas West Asia; 2018 Mar 26–28; Muscat, Oman. Richardson (TX): Society of Petroleum Engineers; 2018 (Document ID: SPE-190380-MS).
- Dhanarajan G, Rangarajan V, Bandi C, et al. Biosurfactant-biopolymer driven microbial enhanced oil recovery (MEOR) and its optimization by an ANN-GA hybrid technique. J Biotechnol. 2017;256:46–56.