Abstract
Mutations conferring tolerance to diverse stresses (i.e. multistress tolerance) on budding yeast Saccharomyces cerevisiae are useful for industrial yeast strains such as baker’s and wine yeast. However, little is known about the mutations conferring multistress tolerance. Previously, we developed a simple method for isolating multistress-tolerant semidominant mutants of S. cerevisiae by one-step selection under lethal hydrogen peroxide stress, which we named the LCH method. In this study, we applied a combination of genetics and next-generation sequencing (NGS) technology to identify the causal mutation for multistress tolerance of the mutant isolated using the LCH method. The haploid mutant strain was crossed with the wild-type strain and the resulting diploids were sporulated. The 20 haploid progeny strains showing multistress tolerance were mixed and subjected to DNA extraction for NGS. CDC25-P1306L, a novel mutant allele of CDC25 encoding a Ras guanine nucleotide exchange factor, was detected 86 times; however, a wild-type CDC25 allele was not detected in the NGS data from the mixture of the multistress-tolerant progeny strains, suggesting that all of the progeny strains showing multistress tolerance have a CDC25-P1306L allele instead of a wild-type CDC25 allele. Substitution of CDC25 in the wild-type strain with CDC25-P1306L rendered the strain tolerant to ethanol, heat shock, freeze-thaw, chronological aging and high concentrations of glucose. These results indicate that CDC25-P1306L is a multistress-tolerant mutation and is promising for breeding multistress-tolerant S. cerevisiae strains for food production.
Introduction
When the budding yeast Saccharomyces cerevisiae is applied to fermentation processes in the production of foods such as bread, Japanese sake and wine, it is exposed to various stresses, such as high temperature [Citation1,Citation2], freeze-thaw [Citation1,Citation3], high concentrations of sugar [Citation4,Citation5], chronological aging [Citation6,Citation7] and ethanol [Citation8,Citation9]. Therefore, breeding of stress tolerant strains of S. cerevisiae for these diverse stresses (i.e. multistress-tolerant strains) is important for improving the quality of the foods, improving the efficiency or reducing the costs of food production and developing novel types of breads and alcoholic beverages that are difficult to produce because of these stresses.
For breeding multistress-tolerant strains for food production, isolation of multistress-tolerant mutant strains from diploid industrial S. cerevisiae strains is one of the most useful procedures. The main reason is that gene-recombination technologies, unfavourable for food production because of public unacceptability [Citation10,Citation11], are not required for isolation of the mutant strains. However, the mutant strains tend to lose their useful properties, because heterozygous mutations in the cells of diploid industrial strains are gradually lost by a phenomenon designated as ‘loss of heterozygosity’ in the course of subculture of the strains [Citation12,Citation13]. On the other hand, loss of heterozygosity does not occur in haploid strains and diploid strains harbouring homozygous mutations, because of an absence of the other allele. Therefore, identification of the mutated genes is useful for breeding genetically stable diploid industrial strains harbouring homozygous mutations. Previously, we have developed a simple method for isolating multistress-tolerant semidominant mutants of S. cerevisiae by one-step selection under lethal hydrogen peroxide (H2O2) stress condition, which we named the lethal concentration of H2O2 (LCH) method [Citation14]. By using the LCH method on cells of a haploid laboratory S. cerevisiae strain mutagenised with ethyl methanesulfonate (EMS), we isolated a multistress-tolerant mutant strain of S. cerevisiae, in which a single gene mutation designated MLT2-1 is responsible for the multistress tolerance [Citation14]. However, MLT2-1 has not yet been identified. Identification of the mutation conferring multistress tolerance is important for understanding the mechanism of multistress tolerance, and would make it possible to use this mutation for breeding multistress-tolerant and genetically stable diploid industrial strains of S. cerevisiae for food production harbouring homozygous mutations.
In this study, we attempted to identify the MLT2-1 mutation gene. The candidates for the causal mutation for multistress tolerance were successfully identified by a combination of genetics and next-generation sequencing (NGS) technology. Among the candidate mutations, we identified CDC25-P1306L, a novel mutant allele of CDC25, as the MLT2-1 mutation gene by substituting CDC25 in the parental wild-type strain to CDC25-P1306L. Since the strain harbouring CDC25-P1306L, instead of CDC25, was tolerant to ethanol, heat shock, freeze-thaw, chronological aging and high concentrations of glucose, CDC25-P1306L is promising for breeding industrial strains for production of foods, such as bread, Japanese sake and wine.
Materials and methods
Microorganisms and media
The S. cerevisiae strains used in this study are listed in . Escherichia coli strain JM109 [Citation15] was used as a host for the propagation and manipulation of plasmid DNA. Yeast strains were grown in YPDA medium (1% yeast extract, 2% polypeptone, 2% glucose and 0.04% adenine) or synthetic dextrose (SD) medium (0.67% yeast nitrogen base without amino acids and 2% glucose) supplemented with 0.002% L-histidine·HCl·H2O, 0.04% adenine, 0.002% uracil (if required), 0.002% L-tryptophan (if required) and 0.01% L-leucine (if required) [Citation16] unless otherwise specified. Solid media were prepared using 2% agar. A diploid yeast strain was sporulated on minimal sporulation medium (1% potassium acetate and 2% agar) supplemented with 0.0005% L-histidine·HCl·H2O, 0.01% adenine and 0.0005% uracil [Citation16]. 5-Fluoroorotic acid (5-FOA) medium for selection of uracil auxotrophic strains was prepared as described by Akada et al. [Citation17]. E. coli cells harbouring plasmid DNA were cultivated in Luria–Bertani (LB) medium (1% tryptone, 0.5% yeast extract and 0.5% NaCl) containing 50 µg/mL ampicillin.
Table 1. S. cerevisiae strains used in this study.
Genetic, biochemical and physiological methods
Mating of haploid yeast strains was performed on YPDA solid medium and the resulting diploid strain was selected on SD solid medium supplemented with 0.002% L-histidine·HCl·H2O, 0.04% adenine and 0.002% uracil. Asci formed on the minimal sporulation medium by the diploid strain were treated with a filter-sterilised solution of 0.02% Zymolyase 100T (Seikagaku Corporation, Tokyo, Japan) for 10 min or more at room temperature. The tetrads in asci were dissected on YPDA solid medium using a micromanipulator Singer MSM Manual (Singer Instruments, Somerset, UK) in accordance with the manufacturer’s instructions. S. cerevisiae cells were transformed using a Frozen-EZ Yeast Transformation II Kit (Zymo Research, Irvine, CA, USA). Preparation of yeast chromosomal DNA was performed as described by Hereford et al. [Citation18] with modifications. Briefly, the cells were incubated in solution A (0.2 mol/L Tris and 5% 2-mercaptoethanol) for 30 min at room temperature. Then, the cells were spun down and incubated in solution B (1.0 mol/L sorbitol, 40 mmol/L potassium phosphate buffer (pH 6.8) and 0.0125% Zymolyase 100T) for 1 h at 30 °C. Then, the spheroplasts were spun down and lysed by incubation for 30 min or more at 60 °C in solution C (50 mmol/L Tris-HCl (pH 8.5), 0.2 mol/L NaCl, 0.1 mol/L EDTA disodium salt and 5% SDS). Then, nucleic acids were purified by phenol:chloroform:isoamyl alcohol extraction [Citation15]. The thread-like chromosomal DNA was recovered by ethanol precipitation [Citation15], dried and dissolved in Tris EDTA (TE) buffer (pH 8.0) [Citation15]. The chromosomal DNA was incubated for 1 h at 37 °C with 0.1 mg/mL of ribonuclease A. Then, the chromosomal DNA was purified, recovered and dissolved in TE buffer (pH 8.0) as described above. Genomic polymerase chain reaction (PCR) was performed using PrimeSTAR HS DNA polymerase (Takara Bio Inc., Shiga, Japan) in accordance with the manufacturer’s instructions. Restriction enzymes were purchased from New England Biolabs Japan Inc. (Tokyo, Japan) and used in accordance with the manufacturer’s instructions. DNA ligation was performed using T4 DNA ligase (Takara Bio Inc.) [Citation15]. Plasmid DNAs were prepared from E. coli cells using LaboPass Plasmid Mini Purification Kit (Hokkaido System Science, Hokkaido, Japan). Spot assays for analyzing stress tolerance were performed as described previously [Citation14], except that the assay for chronological aging stress tolerance was performed after incubation of the cells in sterile distilled water at 30 °C for 7 days and that the assay for freeze-thaw stress tolerance was performed after the samples were subjected to freeze-thaw treatment (frozen at −80 °C for 24 h and thawed at 30 °C for 10 min) five times in 5 days. The plates for spot assays were incubated at 30 °C for 2 days, except for the plates containing 50% (w/v) glucose and 15% (v/v) ethanol, which were incubated for 5 days and 7 days before photography, respectively.
Preparation of mixed genomic DNA and NGS sequencing
The multistress-tolerant strain JSEOA8 was crossed with the wild-type strain YN101 and the resulting diploid strain was sporulated and subjected to tetrad analyses as described previously [Citation14]. Then the 20 segregants which exhibited stress tolerance to H2O2, ethanol, heat shock and high concentrations of glucose were collected as multistress-tolerant progeny strains. The cells of these strains were cultivated in 10 mL of YPDA medium independently, mixed, subjected to preparation of yeast chromosomal DNA as described above and designated as MLT2-1 Mix. The DNA samples of MLT2-1 Mix, the parental wild-type strain YN57 and the wild-type strain YN101 with opposite mating type were sequenced using an Illumina HiSeq 2000 sequencer (paired-end, 100 bp) by Macrogen Japan Corp. (Tokyo, Japan).
Identification of candidates for the multistress-tolerant mutation
The fastq.gz files of MLT2-1 Mix containing total bases of 2.1 Giga generated by NGS and the fastq.gz files of YN57 containing total bases of 2.6 Giga generated by NGS were uploaded to a browser-accessible bioinformatics tool called Mutation discovery (Mudi; http://naoii.nig.ac.jp/mudi_top.html) [Citation19] as the mutant fastq.gz files and the parental fastq.gz files, respectively, by choosing S. cerevisiae as a reference genome. The fastq.gz files of the MLT2-1 Mix and the fastq.gz files of YN101 containing total bases of 2.7 Giga generated by NGS were similarly uploaded to Mudi as the mutant fastq.gz files and the parental fastq.gz files, respectively. Then, mutations in nuclear DNA that were commonly detected more than 20 times in both output data, but had no different variants in the same position as the NGS data for MLT2-1 Mix or in the same gene as the NGS data for YN57 or YN101, were listed as candidates for the multistress-tolerant mutation.
Substitution of CDC25 in the parental strain to CDC25-P1306L
Substitution of CDC25 in the parental strain YN57 to CDC25-P1306L was performed by two-step gene replacement method [Citation16] as follows. The 3’-terminal part of the CDC25-P1306L allele containing the mutation point was amplified by PCR using the chromosomal DNA of the multistress-tolerant strain JSEOA8 as a template and oligonucleotides 5′-CTCGAATTCCCGTGTGGTCAACATTATGAGA-3′ (EcoRI_CDC25 + 3582) and 5′-CTCGTCGACCTTGGATCGATAACTTAACTGG-3′ (SalI_CDC25 + 5020c) as the forward and reverse primers, respectively. The underlined sequences in the oligonucleotides EcoRI_CDC25 + 3582 and SalI_CDC25 + 5020c indicate the restriction sites of EcoRI and SalI, respectively. The amplified product was doubly digested with EcoRI and SalI, and cloned into the EcoRI-SalI gap in YIp5 [Citation20] to obtain YIp5+CDC25-P1306L. Then, the YIp5+CDC25-P1306L was cleaved at the HindIII site in the CDC25-P1306L and integrated into the CDC25 locus of YN57 by transformation, resulting in an uracil prototrophic transformant carrying a duplication of the CDC25 region in which one duplicate is CDC25 and the other is CDC25-P1306L with the plasmid sequences in between. Strains that excised the plasmid by homologous crossovers between the CDC25 and the CDC25-P1306L were selected using 5-FOA medium. The 5-FOA-resistant strains with uracil auxotrophy were analyzed by direct sequencing of the CDC25 region amplified by genomic PCR using oligonucleotides 5′-CCGTGTGGTCAACATTATGAGA-3′ (CDC25 + 3582) and 5′-CGGTAGATTGGGGAGGAATA-3′ (CDC25 + 4232c) as the primers. Then, the strain that was verified to possess the CDC25-P1306L allele, but not the wild-type CDC25 allele, was selected as the YN57 CDC25-P1306L strain (), which is isogenic to YN57, except for CDC25-P1306L.
Results and discussion
Identification of candidates for the multistress-tolerant mutation MLT2-1 by a combination of genetics and NGS technology
A multistress-tolerant semidominant mutant strain JSEOA8 was previously isolated by using the LCH method in EMS-mutagenised cells of a laboratory S. cerevisiae strain YN57 with a W303-1A background [Citation14]. Recently, NGS technology has become a powerful tool for finding mutated genes in mutant strains. However, it is difficult to identify the causal mutated gene responsible for the phenotypes of mutant strains treated with mutagen, because these strains have many other mutated genes unrelated to the phenotypes [Citation19]. On the other hand, S. cerevisiae is an ideal model organism to use classical genetics because it can proliferate as haploid cells (mating type a or α), can be easily manipulated to mate between opposite mating type cells and sporulate to produce ascospores for tetrad analysis [Citation16]. Therefore, to identify candidates for the multistress-tolerant mutation MLT2-1, we applied a combination of genetics and NGS technology (). First, we mated JSEOA8 with the wild-type strain YN101 of the opposite mating type. Then, cells of the resulting diploid strain were sporulated and the tetrads were analyzed. The 20 segregants which exhibited multistress tolerance were selected as multistress-tolerant progeny strains and cultivated independently in YPDA broth. Then each cultivated broth was mixed with the others and the cells in the mixture were collected and subjected to chromosomal DNA extraction. Chromosomal DNAs designated as MLT2-1 Mix were subjected to NGS. As a result, 2.1 Gb of DNA sequence of MLT2-1 Mix, which is more than 161-fold of the genome size (13 Mb) of laboratory haploid S. cerevisiae strain [Citation21], was obtained. On the other hand, 2.6 Gb of genomic DNA sequence of the wild-type strain YN57 and 2.7 Gb of genomic DNA sequence of YN101 were also obtained by NGS. These NGS data were uploaded to Mudi, a browser-accessible bioinformatics tool, and the candidates for the multistress-tolerant mutation MLT2-1 were listed () using the output data as described in ‘Materials and methods’. As a result, one missense mutation was detected in the CDC25 gene. The other mutations were outside of the coding regions. The missense mutation in CDC25 was a G-to-A base substitution at position 753077 of chromosome XII and was predicted to result in a single amino acid substitution from proline to leucine at position 1306 in the Cdc25p protein. Therefore, we designated the mutant allele of CDC25 as CDC25-P1306L. The CDC25-P1306L allele was detected 86 times, but a wild-type CDC25 allele was not detected in the NGS data of the MLT2-1 Mix (), suggesting that all of the progeny strains showing multistress tolerance have a CDC25-P1306L allele instead of a wild-type CDC25 allele.
Figure 1. Procedure for identification of candidates for the multistress-tolerant mutation MLT2-1 by a combination of genetics and NGS technology. JSEOA8 strain possesses a chromosomal semidominant single-gene mutation designated as MLT2-1, which is responsible for the multistress-tolerant phenotype and has not been identified yet [Citation14]. WT indicates wild-type.
![Figure 1. Procedure for identification of candidates for the multistress-tolerant mutation MLT2-1 by a combination of genetics and NGS technology. JSEOA8 strain possesses a chromosomal semidominant single-gene mutation designated as MLT2-1, which is responsible for the multistress-tolerant phenotype and has not been identified yet [Citation14]. WT indicates wild-type.](/cms/asset/0fb066fd-6957-4440-bc6b-3ba4ff613258/tbeq_a_1559094_f0001_b.jpg)
Table 2. Candidates for the multistress-tolerant mutation MLT2-1 identified by a combination of genetics and NGS technology.
Identification of CDC25-P1306L as the multistress-tolerant mutation MLT2-1
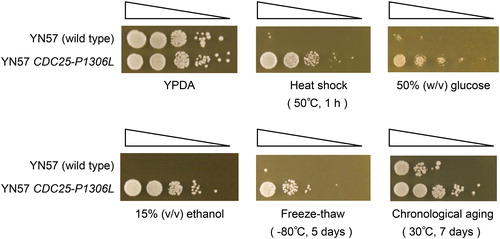
Among the candidates for the MLT2-1 mutation listed in , a missense mutation in CDC25 designated as CDC25-P1306L seemed to be most probable because the other mutations were outside of any coding regions. To elucidate whether CDC25-P1306L is the causal mutation for multistress tolerance of the JSEOA8 strain, we substituted CDC25 in the parental wild-type strain YN57 to CDC25-P1306L by a two-step gene replacement method. The substituted strain was designated as YN57 CDC25-P1306L strain and was analyzed for stress tolerance by spot assays (). The YN57 CDC25-P1306L cells grew well, like the YN57 cells on YPDA medium as no-stress controls. On the other hand, the YN57 CDC25-P1306L cells grew much better than the YN57 cells on the YPDA media after heat shock stress (50 °C, 1 h), freeze-thaw stress (−80 °C, 5 days), and chronological aging stress (30 °C, 7 days). Furthermore, the YN57 CDC25-P1306L cells also grew much better than YN57 cells on the YPDA media containing 50% (w/v) glucose and 15% (v/v) ethanol. From the results described above, CDC25-P1306L was demonstrated to be the causal mutation for the multistress tolerance of the JSEOA8 strain, namely MLT2-1.
Figure 2. Spot assays for analyzing stress tolerance of the wild-type YN57 strain and the YN57 CDC25-P1306L strain. Five microliters of the cell suspensions of each strain serially diluted 10-fold were spotted on the solid media for analyzing stress tolerance as described in ‘Materials and methods’.
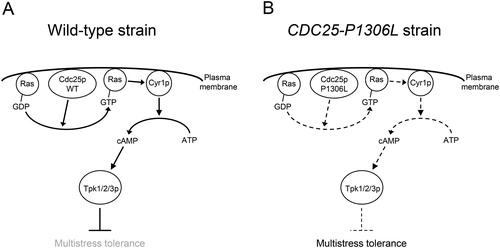
How did the CDC25-P1306L strain, YN57 CDC25-P1306L, exhibit multistress tolerance? Cdc25p protein, the product of CDC25, is a Ras guanine nucleotide exchange factor (rasGEF), which promotes exchange of GDP bound to Ras1/2p (Ras) to GTP, and activates the cAMP-protein kinase A (PKA) pathway [Citation22]. Cdc25p is a 1589-amino-acid-long protein and has a Src homology 3 (SH3) domain (65 to 129 aa region) [Citation23], a cyclin destruction box (CDB) motif (149 to 157 aa region) [Citation24] and a C-terminal catalytically active region (amino acids 1084 to 1589 aa region) [Citation25] which contains three structurally conserved regions (SCRs) between rasGEFs of various species: SCR1 (1301 to 1324 aa region), SCR2 (1374 to 1416 aa region) and SCR3 (1454 to 1475 aa region) ( ) [Citation26]. Interestingly, the mutated proline residue at position 1306 in Cdc25p, which is changed to leucine in the CDC25-P1306L strain, is located in the SCR1 region and conserved in rasGEFs of various species [Citation26,Citation27] such as Ste6 of Schizosaccharomyces pombe, protein Son of sevenless (Sos) of Drosophila melanogaster, Son of sevenless homologues of Caenorhabditis elegans (CeSos1), Mus musculus (MmSos1) and Homo sapiens (HsSos1) (). In S. cerevisiae, decreased activity of the cAMP-PKA pathway increases the stress tolerance of the cells [Citation28,Citation29]. Therefore, we propose the following mechanism of multistress tolerance of the CDC25-P1306L strain (). In the wild-type CDC25 strain (), Cdc25p promotes exchange of GDP bound to Ras to GTP. GTP-bound Ras (Ras-GTP), an active form of Ras, activates adenylate cyclase Cyr1p which synthesizes cAMP from ATP. cAMP activates catalytic subunits of PKA (Tpk1/2/3p) by binding to regulatory subunits of PKA, resulting in inhibition of the stress tolerance of the cells [Citation28,Citation29]. On the other hand, in the CDC25-P1306L strain (), the mutated variant of the Cdc25p protein, Cdc25p P1306L, which probably has lower catalytic activity for the exchange of GDP bound to Ras to GTP than the wild-type Cdc25p, confers multistress tolerance on the S. cerevisiae strain probably by decreasing the activity of the cAMP-PKA pathway.
Figure 3. Schematic diagram of Cdc25p (A) and alignment of SCR1 region in the rasGEFs of various species and a single amino acid substitution caused by CDC25-P1306L mutation (B). (A) A SH3 domain, a CDB motif and three structurally conserved regions between rasGEFs (SCR1, SCR2 and SCR3) are indicated by shaded boxes. The numbers indicate amino acid positions. (B) The UniProtKB accession numbers of rasGEFs are indicated in parentheses. The numbers indicate amino acid positions. The amino acid residues conserved in all the aligned sequences are written in boldface and indicated by asterisks. The conserved proline residue at position 1306 in Cdc25p, which is changed to leucine by the CDC25-P1306L mutation, is indicated by a grey box with those of the other rasGEFs.
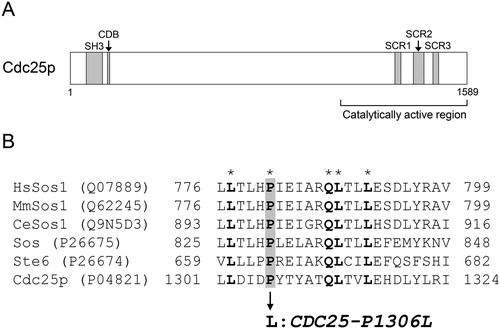
Figure 4. Presumed mechanism of multistress tolerance conferred by the CDC25-P1306L mutation allele. The arrows and blunt arrows with straight lines indicate positive and negative interactions, respectively. The arrows with curved lines indicate conversions. The bold and dashed lines indicate strong and weak interactions/conversions, respectively. The text in grey typeface indicates the inhibited phenotype. WT indicates wild type. See ‘Results and discussion’ for details.
Previously, Folch-Mallol et al. [Citation30] isolated heat-shock resistant mutants from a laboratory S. cerevisiae strain and found that the mutant strain harbouring cdc25-21, a mutant allele of CDC25 predicted to encode a fusion protein containing the N-terminal 977 amino acids of Cdc25p fused to 12 non-native amino acids at the C-terminus, or cdc25-22, a mutant allele of CDC25 predicted to result in a single amino acid substitution from histidine to proline at position 1363 in the protein, showed tolerance to heat shock, H2O2, high concentrations of sorbitol and LiCl and resistance to lyticase digestion. Recently, Satomura et al. [Citation31] isolated thermotolerant mutant strains from a laboratory S. cerevisiae strain by adaptation experiments under heat stress and found four kinds of one-point mutations in CDC25, designated as CDC25 (T943P), CDC25 (N1393T), CDC25 (W1416C) and CDC25 (G1459C), among the mutant strains. All the reconstructed mutant strains harbouring each one-point mutation exhibited thermotolerance [Citation31], indicating that these mutant alleles of CDC25 confer thermotolerance on the S. cerevisiae strain. In this study, we identified CDC25-P1306L, a novel mutant allele of CDC25, as the causal mutated gene for multistress tolerance of the multistress-tolerant semidominant mutant of S. cerevisiae isolated by the LCH method using lethal concentration of H2O2 for the first screening. To the best of our knowledge, this is the first report showing that the specific allele of CDC25 confers stress tolerance to ethanol, freeze-thaw, chronological aging and high concentrations of glucose. It is interesting to know whether the alleles of CDC25 other than CDC25-P1306L identified in this study confer stress tolerance to ethanol, freeze-thaw, chronological aging and high concentrations of glucose, which are industrially important stresses for the production of foods, such as bread, Japanese sake and wine.
In the breeding of microorganisms for the food industry, mutation breeding is a useful procedure because gene-recombination technologies, which are unfavourable for food production, are not required for isolation of mutant strains [Citation32]. If the LCH method is applied to the breeding of industrial yeast strains such as baker’s yeast and wine yeast, identification of the causal mutated gene by a combination of genetics and NGS technology would be difficult because most of these strains have poor sporulation and low spore viability [Citation33]. In this study, CDC25-P1306L was identified for the first time as the causal mutated gene for multistress tolerance of the multistress-tolerant mutant isolated by using the LCH method from the laboratory S. cerevisiae strain, which is easy to perform genetic analysis on. This finding would accelerate the identification of previously unknown causal mutated genes for stress tolerance in the stress-tolerant industrial mutant stains for food production obtained by mutation breeding such as the LCH method. Furthermore, this finding would also be useful for breeding multistress-tolerant and genetically stable diploid industrial strains of S. cerevisiae for food production harbouring homozygous mutated alleles.
Conclusions
To our knowledge, this is the first report on the identification of the causal mutated gene for multistress tolerance of a multistress-tolerant mutant of S. cerevisiae isolated using the LCH method. The identified CDC25-P1306L gene, a novel mutant allele of CDC25, was shown to confer stress tolerance to ethanol, heat shock, freeze-thaw, chronological aging and high concentrations of glucose on a S. cerevisiae strain. Therefore, CDC25-P1306L is promising for breeding multistress-tolerant S. cerevisiae strains for production of foods, such as bread, Japanese sake and wine.
Acknowledgements
We would like to thank Dr. Naoko Iida (National Institute of Genetics, Mishima, Japan) for the advice on sample preparation for NGS. We also would like to thank Ms. Momomi Tanaka of our laboratory for her technical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was partially supported by JSPS KAKENHI Grant Number JP17K07715.
References
- Attfield PV. Stress tolerance: the key to effective strains of industrial baker's yeast. Nat Biotechnol. 1997;15:1351–1357.
- Marullo P, Mansour C, Dufour M, et al. Genetic improvement of thermo-tolerance in wine Saccharomyces cerevisiae strains by a backcross approach. FEMS Yeast Res. 2009;9:1148–1160.
- Nakagawa Y, Ogihara H, Mochizuki C, et al. Development of intra-strain self-cloning procedure for breeding baker's yeast strains. J Biosci Bioeng. 2017;123:319–326.
- Erasmus DJ, van der Merwe GK, van Vuuren HJ. Genome-wide expression analyses: metabolic adaptation of Saccharomyces cerevisiae to high sugar stress. FEMS Yeast Res. 2003;3:375–399.
- Lin X, Zhang CY, Bai XW, et al. Improvement of stress tolerance and leavening ability under multiple baking-associated stress conditions by overexpression of the SNR84 gene in baker’s yeast. Int J Food Microbiol. 2015;197:15–21.
- Picazo C, Orozco H, Matallana E, et al. Interplay among Gcn5, Sch9 and mitochondria during chronological aging of wine yeast is dependent on growth conditions. PLoS One. 2015;10:e0117267. DOI:10.1371/journal.pone.0117267
- Sauer M, Mattanovich D. Non-genetic impact factors on chronological lifespan and stress resistance of baker’s yeast. Microb Cell. 2016;3:232–235.
- Watanabe M, Tamura K, Magbanua JP, et al. Elevated expression of genes under the control of stress response element (STRE) and Msn2p in an ethanol-tolerance sake yeast Kyokai no. 11. J Biosci Bioeng. 2007;104:163–170.
- Matallana E, Aranda A. Biotechnological impact of stress response on wine yeast. Lett Appl Microbiol. 2017;64:103–110.
- Akada R. Genetically modified industrial yeast ready for application. J Biosci Bioeng. 2002;94:536–544.
- Ando A, Suzuki C, Shima J. Survival of genetically modified and self-cloned strains of commercial baker’s yeast in simulated natural environments: environmental risk assessment. Appl Environ Microbiol. 2005;71:7075–7082.
- Ramirez M, Vinagre A, Ambrona J, et al. Genetic instability of heterozygous, hybrid, natural wine yeasts. Appl Environ Microbiol. 2004;70:4686–4691.
- Takashita H, Kajiwara Y, Shimoda M, et al. Genetic instability of constitutive acid phosphatase in shochu and sake yeast. J Biosci Bioeng. 2013;116:71–78.
- Nakagawa Y, Seita J, Komiyama S, et al. A new simple method for isolating multistress-tolerant semidominant mutants of Saccharomyces cerevisiae by one-step selection under lethal hydrogen peroxide stress condition. Biosci Biotechnol Biochem. 2013;77:224–228.
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2001.
- Amberg DC, Burke DJ, Strathern JN. Methods in yeast genetics: a cold spring harbor laboratory course manual. 2005 ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2005.
- Akada R, Kitagawa T, Kaneko S, et al. PCR-mediated seamless gene deletion and marker recycling in Saccharomyces cerevisiae. Yeast. 2006;23:399–405.
- Hereford L, Fahrner K, Woolford J, Jr, et al. Isolation of yeast histone genes H2A and H2B. Cell. 1979;18:1261–1271.
- Iida N, Yamao F, Nakamura Y, et al. Mudi, a web tool for identifying mutations by bioinformatics analysis of whole-genome sequence. Genes Cells. 2014;19:517–527.
- Struhl K, Stinchcomb DT, Scherer S, et al. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci USA. 1979;76:1035–1039.
- Goffeau A, Barrell BG, Bussey H, et al. Life with 6000 genes. Science. 1996;274:546, 563–547.
- Jones S, Vignais ML, Broach JR. The CDC25 protein of Saccharomyces cerevisiae promotes exchange of guanine nucleotides bound to Ras. Mol Cell Biol. 1991;11:2641–2646.
- Rodaway AR, Sternberg MJ, Bentley DL. Similarity in membrane proteins. Nature. 1989;342:624.
- Kaplon T, Jacquet M. The cellular content of Cdc25p, the Ras exchange factor in Saccharomyces cerevisiae, is regulated by destabilization through a cyclin destruction box. J Biol Chem. 1995;270:20742–20747.
- Lai CC, Boguski M, Broek D, et al. Influence of guanine nucleotides on complex formation between Ras and CDC25 proteins. Mol Cell Biol. 1993;13:1345–1352.
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654.
- Boriack-Sjodin PA, Margarit SM, Bar-Sagi D, et al. The structural basis of the activation of Ras by Sos. Nature. 1998;394:337–343.
- Thevelein JM, de Winde JH. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1999;33:904–918.
- Conrad M, Schothorst J, Kankipati HN, et al. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 2014;38:254–299.
- Folch-Mallol JL, Martinez LM, Casas SJ, et al. New roles for CDC25 in growth control, galactose regulation and cellular differentiation in Saccharomyces cerevisiae. Microbiology. 2004;150:2865–2879.
- Satomura A, Miura N, Kuroda K, et al. Reconstruction of thermotolerant yeast by one-point mutation identified through whole-genome analyses of adaptively-evolved strains. Sci Rep. 2016;6:23157.
- Kotaka A, Sahara H, Hata Y. The construction and application of diploid sake yeast with a homozygous mutation in the FAS2 gene. J Biosci Bioeng. 2010;110:675–678.
- Oda Y, Ouchi K. Genetic analysis of haploids from industrial strains of baker’s yeast. Appl Environ Microbiol. 1989;55:1742–1747.
