Abstract
Legumes are one of the world's leading sources of nutrition, providing sustainable agriculture. They require minimal amounts of soil improvers (fertilizers) due to the ability to absorb nitrogen through symbiotic interactions with nitrogen-fixing soil microorganisms. The trend for human population growth requires an adequate growth in crop production. This directly depends on the seed size and number as well as on the leaf biomass. In turn, seed size has been the subject of selection programmers for all crops. In this study, we explored the relation between the function of the gene coding Zinc finger CCHC-type protein and flower morphology and seed size in the model legume species Medicago truncatula. M. truncatula lines with modified level of Zinc finger CCHC transcript and transcriptional reporters were developed and analysed by real-time polymerase chain reaction and expression of the GUS (β-glucuronidase) and green fluorescent protein (GFP) reporter genes. A tissue-specific GFP signal was detected in the anthers from overexpessing M. truncatula lines. The M. truncatula lines with knockdown expression showed direct relation between low transcription level of Mt-Zn-CCHC gene and strongly reduced seed size accompanied with short stems length and internodes.
Introduction
Seed size is determined by the complex relationship between the endosperm and the integuments during the early stages of seed growth. Despite the huge importance of seeds, there is still a gap in the knowledge about the genetic mechanisms determining their size. Li and Li [Citation1] reviewed some aspects of seed-control signalling pathways such as G-protein pathway, MAPK signalling pathway, ubiquitin-proteasome pathway, IKU signalling pathway, phytohormones and transcription regulators. Studies have focused on the molecular and epigenetic regulations and functions of LEC1, ABI3, FUS3 and LEC2 master transcriptional regulators which control seed development in Arabidopsis and soybean (reviewed in [Citation2,Citation3]).
In the model legume Medicago truncatula, transcriptional profiling of the seed development stages has identified six transcription factors that are expressed in the seed coat or in the endosperm [Citation4]. These transcription factors possess zinc finger domains, which are small, functional, freely folded domains that need incorporation of one or more zinc ions to stabilize their structure [Citation5].
The proteins with CCHC type of Zn finger domains are widely distributed in the plant genomes. They have various functions such as DNA recognition, RNA packaging, activation of transcription, regulation of apoptosis and lipid binding [Citation5]. Zinc-finger proteins regulate the function of nucleic acids or participate in transcriptional or translational regulation [Citation6]. There are nine types of Zinc finger domains based on their structure and functions: C2H2, C8, C6, C3HC4, C2HC, C2HC5, C4, C4HC3 and CCCH (C and H represent cysteine and histidine, respectively) [Citation7–11].
Two of the genes described by Verdier et al. [Citation4] encoding CCHC (CxxCxxxxHxxxxC) domain are expressed during the early stages of seed development, when the seed coat and the endosperm are active in the delivery of nutrients to the developing embryo. In a Tnt1 insertion mutant collection of M. truncatula developed in AgroBioInstitute (Sofia, Bulgaria), sequence analysis of the M. truncatula mutant line So5823,insertion 9 revealed similarity with a gene encoding a Zn finger CCHC-type protein (ABE91952.1) [Citation12].
In order to study the function of the Mt-Zn-CCHC protein and its potential association with seed size; here, we cloned the gene in plant transformation vectors and developed M. truncatula plants with modified expression of the gene and transcriptional reporters.
Materials and methods
Cloning and transformation procedures
Plant transformation vectors were created according to Revalska et al. [Citation13]. Briefly, the Gateway system [Citation14] (Invitrogen Life Technologies) was applied for recombinant plasmid development. The open reading frame of the Mt-Zn-CCHC gene was amplified and cloned into the donor vector pDONR221. After sequencing, the positive clones were transferred into pK7WG2 or pK7FWG2 destination vectors for plant transformation and development of plants overexpressing gene Mt–Zn CCHC. The vector pK7FWG2 possesses a green fluorescent protein (GFP) reporter gene. Both vectors are under the control of CaMV 35S promoter and have neomycin phosphotransferase (nptII) gene for plant selection [Citation15]. RNA interference method [Citation16] and pK7GWIWG2D(II) hairpin RNA expression vector were used for production of plants with silenced gene function. In silico predicted fragment of 153 bp from the ORF of Mt-Zn-CCHC was optimal for sufficient gene silencing. This fragment is located between 729 and 882 bp of the ORF. Additionally, the promoter sequence of the gene Mt-Zn-CCHC was cloned into the vector pExK7SWFm14GW caring both reporter genes – GUS and GFP. Agrobacterium tumefaciens strain C58C1 was used for M. truncatula transformation.
Gene transfer experiments were performed as described in [Citation13] using leaf and petiole explants of M. truncatula cv. ‘Jemalong 2HA’ [Citation17]. The transgenic nature of mutant T0 lines was assessed by rooting on Km selection medium and positive npt II amplification of a 550 bp fragment using the following primers: FW 5′-GAACAAGATGGATTGCACGC-3′ and REV 5′- GAAGAACTCGTCAAGAAGGC-3′. Positive plants were transferred to the greenhouse for seed production. The obtained T1 seeds were screened on Km medium and positive plants were grown for T2 seeds production.
Histochemical and fluorescent detection of GUS and GFP reporter genes
Histochemical study of the tissue-specific localization of GUS (β-glucuronidase) activity was conducted according to Jefferson et al. [Citation18]. Samples were collected under 90% acetone for 30 min at 4 °C, washed with phosphate buffer at room temperature and incubated in GUS solution at 37 °C overnight [Citation19]. The images were taken with binocular microscope (MZ16, Leika) and camera (Nikon). Images of plants expressing GFP were collected using a SZX7 fluorescence stereomicroscope (460–490 nm excitations and 510–550 nm emission) with a DP73 digital camera (Olympus).
Expression analysis
Total RNA was extracted from leaf tissue of T0 plants and 18-day-old seedlings – T1 and T2 progeny. Additionally, total RNA was extracted from green seeds 10 days after pollination and mature/dry seeds. The manufacturer’s instructions for RNeasy Plant Mini Kit (EurEx) were followed. Extracted RNA was quantified with a Nanodrop2000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA) and treated with 10 U DNaseI according to the protocol (Fermentas). One microgram of RNA was used for cDNA synthesis following the protocol of iScript cDNA Synthesis Kit (BIO-RAD). The transcription level of the gene M -Zinc finger CCHC type was analysed by real-time polymerase chain reaction (PCR) using iTaqUniversal SYBR green Super mix including ROX and fluorescein (BIO-RAD). The analyses were performed on 7300 Real-Time PCR System (Applied Biosystems). Five microliters of 5x diluted first-strand cDNA were used for each amplification reaction in a final volume of 20 µL. Gene-specific primers were as follows: Forward 5′-CCCCATCAACAACAAATTCC-3′ and Reverse 5′-TAGGTGGGGTTGGGTTATGT-3′, in a concentration of 0.25 µmol/L. The actin housekeeping gene was used as a reference gene for data normalization by the following primers: F – 5′-TCAATGTGCCTGCCATGTATGT-3′ and R – 5′-ACTCACACCGTCACCAGAATCC-3′.
The signal was monitored by recording the dissociation curve detected during every PCR experiment corresponding to the synthesis of one PCR product (with respective primer pairs). Data were analysed by application of qBase 1.3.5 software.
Phenotypic characterization
Positive OE and RNAi M. truncatula lines selected on the basis of the real-time PCR data were grown in pots in open air for phenotyping and seeds production. The growth characteristics as percentage of germination; period between germination and beginning of flowering; duration of the flowering and morphometric parameters – length of the longest stem; number of branches; number of pods; number of seeds per pod, weight of the seeds were monitored and compared with control plants – cv. Jamalong 2HA of M. truncatula, according to UPOV criteria.
Statistical analysis
All experiments were performed in triplicate. The results were analysed with one-way analysis of variance (ANOVA) using GraphPad Prism Software v. 4.03. The differences were considered statistically significant at P-value < 0.05.
Results and discussion
In this study, a new gene encoding a Zinc finger CCHC-type protein was described. The gene was selected because of similarity detected with the exon insertion 9 of a Tnt mutant line. To the best of our knowledge, this is the first investigation of this gene in the model legume M. truncatula. The predicted cDNA of the gene was used for cloning and construction of the expression vectors for obtaining stable trangenic plants of M. truncatula with modified expression.
Quantitative real-time PCR analysis of modified M. truncatula lines
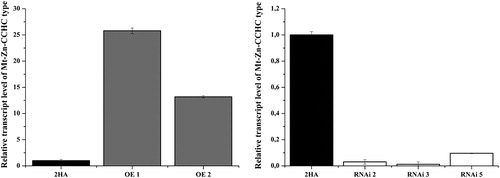
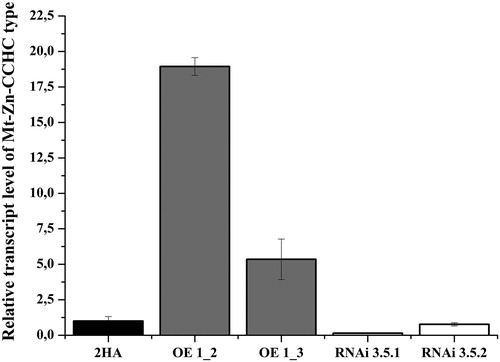
Using real-time PCR, we selected three RNAi M. truncatula lines (RNAi 2, 3 and 5) with knockdown expression level of the gene Mt-Zn-CCHC and five overexpressed lines (OE 1, 2, 4, 5 and 11). presents the transcript level of the Mt-Zn-CCHC gene in T0 lines, normalized according to the transcript level of the house-keeping gene actin. The transcript value in T1 and T2 progeny is also demonstrated ().
Histochemical and fluorescent analyses of the reporter genes, GUS and GFP
We obtained and analysed transgenic M. trucatula plants carrying the promoter sequence pMt-Zn-CCHC- of the gene fused to reporter genes GUS and GFP. The signals corresponding to the β-glucoronidase reporter gene were detected in the vascular system of the leaves (, panels A, B), in the stipule (, A), in the developing shoots (, C) and axillary buds (, D). The specific tissue localization of GFP was strictly in the anthers of OE M. truncatula lines (, A,B). There was no signal in the stamen holder or in the other parts of the flowers ().
Figure 3. Histochemical GUS signal localization in м. truncatula – pZn-CCHC::GUS-GFP transcriptional reporter plants. Signal detection in leaves (A), in stipule (B), in developing shoot (C) and in axillary buds (D).
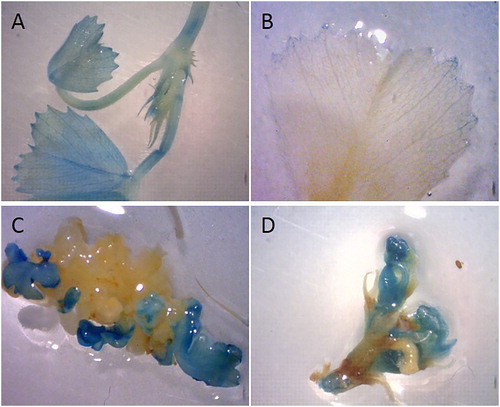
Figure 4. GFP localization in the anthers from M. truncatula OE lines: OE line 1 (A) and OE line 2 (B).
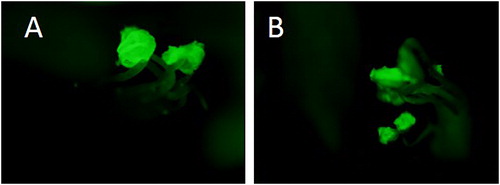
The localization of the signal in the actively dividing zones: axilary buds, vascular system of the leaves and stipule, indicated that the function of the Mt-Zn-CCHC gene is associated with the procesess of plant development. A specific relation with flower and seed development was demostrated by strong, tissue-specific GFP signal detected in the anthers of OE M. truncatula lines.
Similar results were reported for cold shock protein encoded by the AtCSP2 gene studied in Arabidopsis thaliana [Citation20–22]. Cold shock domain proteins have similar structure to the gene presented in this study. They possess CCHC type of Zinc finger domains and nucleic acid-binding domains capable of binding RNA, ssDNA and dsDNA. The AtCSP2 gene is highly expressed in meristematic and developing tissues [Citation20–22]. Altogether these data suggest that the group of genes encoding cold shock proteins and the Mt-Zn-CCHC gene might function in cell division and proliferation.
Morphometric characterization of modified M. truncatula plants
We evaluated the morphometric parameters and plant development of the T1 and T2 progeny of the selected RNAi and OE M. truncatula lines grown in pots. The RNAi plants had low seed germination percentage (17.6%) as compared to the control (33.3%). The seed germination percentage of the OE plants was 66.6%. Additionally, the RNAi plants showed reduced potential for growth: they had a shortened stem and a low number of branches (). The RNAi plants had a prolonged flowering period of 45 days, whereas in the control plants, this period took 34 days. The long flowering period in the RNAi plants was accompanied with formation of more flowers but smaller in size. These plants also formed less pods: a mean of 11.2 pods per plant in comparison with a mean of 28.5 pods per plant in the control (). These results correlated with the deviation in the flower morphology observed in RNAi plants (). Additionally, the weight of 1000 seeds () showed that the plants from the RNAi line formed lighter seeds (1.056 g) in comparison with the control plants (2.949 g). Taken together, these results indicated that the plants with knockdown expression of the Mt-Zn-CCHC gene had reduced capacity to form seeds. In the case of successful seed formation, the produced seeds had reduced size. presents pods and seeds harvested from RNAi and OE lines. All data confirmed that the process of flower morphology and seed formation was strongly disturbed in the lines with knockdown function of the Mt-Zn-CCHC gene. The parameters measured in OE plants show values close to the control and two times higher in comparison with RNAi plants.
Figure 5. Flower morphology in M. truncatula lines RNAi 3 (A) and RNAi 2 (B), OE line 5 (C) and control plant (D).

Figure 6. Seeds and pods collected from M. truncatula lines: control plant (а), RNAi 3 (B), line ое 1 (С) and ое 11 (D).

Table 1. Morphometric parameters of the T1 and T2 generation of RNAi and OE lines of M. truncatula.
The mophological characteristics of M. truncatula OE and RNAi lines confirmed the connection between gene function and plant development, especially with flower and seeds development. These results are in agreement with previous reports. Fusaro et al. [Citation21] demonstrated that AtCSP2 regulates flowering time, stamen number and seed development in RNAi knock-down transgenic plants. Additionally, overexpression of AtCSP2 affects flowering time and silique length [Citation23].
Mt-Zn-CCHC transcript accumulation in M. truncatula seeds
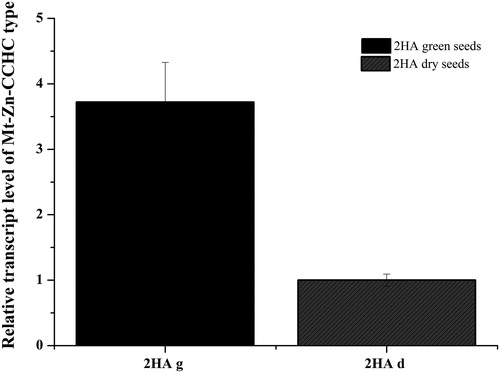
To obtain experimental evidence of the role of the Mt Zn-CCHC gene in seed development, we determined the relative expression levels of Mt-Zn-CCHC in the green and dry/mature seeds of M. truncatula control plants. The results presented in show that the Mt-Zn-CCHC transcript level was more than 3.5-fold higher in the expression profile of the green seeds than in dry seeds. These results suggest that the studied gene is more active during the early stage of seed development and its activity decreases in mature seeds.
Figure 7. Relative expression level of Mt-Zn-CCHC gene in green and dry seeds harvested from control plants of M. truncatula; g – green seeds; d – dry/mature seeds.
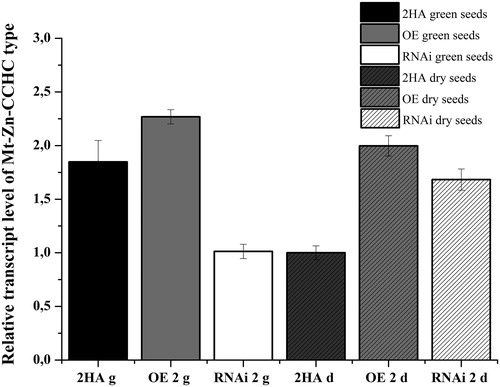
Next, we compared the transcription level in the profiles of green and mature/dry T1 seeds harvested from OE and RNAi lines. The results showed that the transcription level of the gene in the green seeds of the OE line was higher than in the control. The signal accumulated in the green seeds harvested from the RNAi line was lower in comparison with the control and the OE line (). Similar to its expression level in the control, gene Mt-Zn-CCHC showed a low transcription level in the dry seeds pattern of the OE line. The signal was 2-fold higher in comparison with the control. The accumulation of the Mt-Zn-CCHC transcript in the dry seeds collected from the RNAi line was higher in comparison with the control (), which could be explained with the heterozygosity of the T1 seeds.
Figure 8. Relative expression level of Mt-Zn-CCHC gene in green and dry T1 seeds collected from T0 M. truncatula lines with modified expression and control.
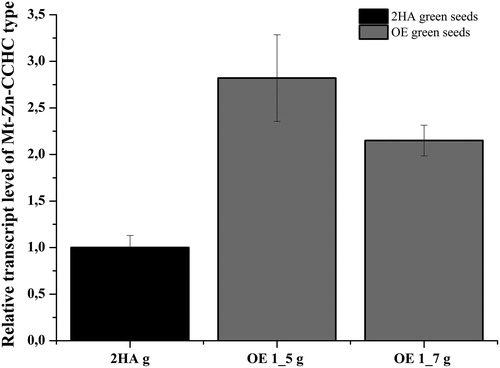
An additional experiment was conducted with green seeds harvested from progeny plants of the OE1 line, OE1.5 and OE1.7. The results presented in show that although the plants are heterozygous, the detected signal was significantly higher (P < 0.05) than that detected from the control green seeds.
Figure 9. Relative expression level of Mt-Zn-CCHC gene in green T2 seeds from T1 progeny of M. truncatula OE 1 line, OE 1_5 and OE 1_7.
According to Sun et al. [Citation24], Xiao et al. [Citation25] and Li and Li [Citation26], transcription factors have important roles in the seed size control processes in Arabidopsis and rice. In Arabidopsis, these genes are TRANSPARENT TESTA GLABRA2 (TTG2) and APETALA2 (AP2) [Citation1]. Zhang et al. [Citation27] reported that NGATHA-like B3 domain transcriptional repressor (NGAL2)/SUPPRESSOR OF DA1 (SOD7) and its homologue NGAL3/DEVELOPMENT-RELATED PCG TARGET IN THE APEX 4 (DPA4) act by restriction of seed growth in Arabidopsis. NGAL2/SOD7 directly binds to the promoter of KLUH (KLU)/CYP78A5 and represses the transcription of KLU, which regulates seed size by promoting cell proliferation in maternal integuments [Citation27]. A KLU homologue in rice (OsCYP78A13/GIANT EMBRYO, GE) also promotes grain growth [Citation28,Citation29]. In our study, high level of Mt-Zn-CCHC transcript accumulation in the control green seeds was obtained in comparison with the dry/mature seeds. This result was confirmed by data demonstating a higher level of gene expression in the green T1 seeds harvested from the OE line and low transcript level in the green T1 seeds from the RNAi line. These results sugest that the function of the Mt-Zn-CCHC gene is most probaby related to the early stage of the seed development similary to the results reported by Verdier et al. [Citation4].
Conclusions
The results presented in this article demonstarted relation between the accumulation of Mt-Zn-CCHC transcripts and seed size in M. trucatula. Additional studies need to explore the putative interactor genes of this newly studied transcription factor. The major challenge in the future investigations will be to transfer the knowledge obtained by model legume M. truncatula to the crop legumes Glycine max and M. sativa.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study was supported by the Bulgarian National Science Fund, Ministry of Education and Science, under grant number DH 16/10 dated 20.12.2017.
References
- Li N, Li Y. Signaling pathways of seed size control in plants. Curr Opin Plant Biol. 2016; 33:23–32.
- Lepiniec L, Devic M, Roscoe TJ, et al. Molecular and epigenetic regulations and functions of the LAFL transcriptional regulators that control seed development. Plant Reprod. 2018; 31:291–307.
- Manan S, Ahmad MZ, Zhang G, et al. Soybean LEC2 regulates subsets of genes involved in controlling the biosynthesis and catabolism of seed storage substances and seed development. Front Plant Sci. 2017; 8:1604.
- Verdier J, Kakar K, Gallardo K, et al. Gene expression profiling of M. truncatula transcription factors identifies putative regulators of grain legume seed filling. Plant Mol Biol. 2008; 67:567–580.
- Laity JH, Lee BM, Wright PE. Zinc finger proteins: New insights into structural and functional diversity. Curr Opin Struct Biol. 2001;11:39–46.
- Klug A. Towards therapeutic applications of engineered zinc finger proteins. FEBS Lett. 2005; 579:892–894.
- Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996; 271:1081–1085.
- Jenkins TH, Li J, Scutt CP, et al. Analysis of members of the Silene latifolia Cys2/His2 zinc-finger transcription factor family during dioecious flower development and in a novel stamen-defective mutant ssf1. Planta. 2005; 220:559–571.
- Ori N, Eshed Y, Paran I, et al. The I2C family from the wilt disease resistance locus I2 belongs to the nucleotide binding, leucine-rich repeat superfamily of plant resistance genes. Plant Cell. 1997; 9:521–532.
- Schumann U, Prestele J, O'Geen H, et al. Requirement of the C3HC4 zinc RING finger of the Arabidopsis PEX10 for photorespiration and leaf peroxisome contact with chloroplasts. Proc Natl Acad Sci USA.. 2007; 104:1069–1074.
- Takatsuji H. Zinc-finger proteins: The classical zinc finger emerges in contemporary plant science. Plant Mol Biol. 1999; 39:1073–1078.
- Iantcheva A, Vassileva V, Ugrinova M, et al. Development of functional genomic platform for model legume Medicago truncatula in Bulgaria. Biotechnol Biotechnol Equip. 2009;23:1440–1443.
- Revalska M, Vassileva V, Zechirov G, et al. Is the auxin influx carrier LAX3 essential for plant growth and development in the model plants Medicago truncatula, Lotus japonicas and Arabidopsis thaliana? Biotechnol Biotechnol Equip. 2015;29:786–797.
- Karimi M, Inze D, Depicker A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002;7:193–195.
- Karimi M, Bleys A, Vanderhaeghen R, et al. Building blocks for plant gene assembly. Plant Physiol. 2007;145:1183–1191.
- Limpens E, Ramos J, Franken C, et al. RNA interference in Agrobacterium rhizogenes-transformed roots of Arabidopsis and Medicago truncatula. J Exp Bot. 2004;55:983–992.
- Nolan KE, Rose RJ, Gorst JR. Regeneration of Medicago truncatula from tissue culture: increased somatic embryogenesis using explants from regenerated plants. Plant Cell Rep. 1989;8:278–281.
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. Embo J. 1987;6:3901–3907.
- Revalska M, Vassileva V, Zehirov G, et al. Analyzing the function and the expression pattern of auxin response factor B3 from Madicago truncatula in the model plant Lotus japonicas. Bulg J Agric Sci. 2016; 2: 253–261.
- Sasaki K, Kim M, Imai R. Arabidopsis cold shock domain protein 2 is an RNA chaperone that is regulated by cold and developmental signals. Biochem Biophys Res Commun. 2007; 364:633–638.
- Fusaro AF, Bocca SN, Ramos RLB, et al. AtGRP2, a cold-induced nucleo-cytoplasmic RNA-binding protein, has a role in flower and seed development. Planta. 2007; 225:1339–1351.
- Nakaminami K, Hill K, Perry SE, et al. Arabidopsis cold shock domain proteins: relationships to floral and silique development. J Exp Bot.. 2009; 60:1047–1062.
- Sasaki K, Kim MH, Imai R. Arabidopsis cold shock domain protein 2 is a negative regulator of cold acclimation. New Phytol. 2013; 198:95–102.
- Sun X, Shantharaj D, Kang X, et al. Transcriptional and hormonal signaling control of Arabidopsis seed development. Curr Opin Plant Biol. 2010; 13:611–620.
- Xiao W, Brown RC, Lemmon BE, et al. Regulation of seed size by hypomethylation of maternal and paternal genomes. Plant Physiol. 2006; 142:1160–1168.
- Li N, Li Y. Maternal control of seed size in plants. J Exp Bot. 2015; 66:1087–1097.
- Zhang Y, Du L, Xu R, et al. Transcription factors SOD7/NGAL2 and DPA4/NGAL3 act redundantly to regulate seed size by directly repressing KLU expression in Arabidopsis thaliana. Plant Cell. 2015; 27:620–632.
- Yang W, Gao M, Yin X, et al. Control of rice embryo development, shoot apical meristem maintenance, and grain yield by a novel cytochrome p450. Mol Plant. 2013; 6:1945–1960.
- Xu F, Fang J, Ou S, et al. Variations in CYP78A13 coding region influence grain size and yield in rice. Plant Cell Environ. 2015; 38:800–811.