Abstract
Elaeis guineensis is a tropical crop with high oil content, and the nutritional value of oil is very high. Amino acids not only affect the growth and development of plants but also act as intermediate metabolites of oils, which determine the final quality of oils. In this study, eleven amino acid permease genes (EgAAPs) in E. guineensis were identified from the amino acid transporter family. Real-time PCR results showed that seven EgAAPs (EgAAP1, EgAAP2, EgAAP3, EgAAP4, EgAAP6 EgAAP9, and EgAAP10) played an important role in vegetative growth because of their higher expression levels in roots and leaves, but EgAAP5, EgAAP7, EgAAP8 and EgAAP11 were also important for their higher expression levels in flowers or fruits. E. guineensis seedlings were treated with 20% PEG-6000 and 4 °C to induce drought stress and cold stress, respectively. The expression of six EgAAPs (EgAAP1, EgAAP2, EgAAP4, EgAAP6, EgAAP8 and EgAAP11) was decreased in both roots and leaves during cold treatment, and only the expression of EgAAP5 was increased in both roots and leaves after 6 h of cold treatment. The expression of six EgAAPs (EgAAP2, EgAAP4, EgAAP7, EgAAP8, EgAAP10 and EgAAP11) was decreased in roots but increased in leaves under PEG treatment, indicating an opposite pattern of expression levels of these EgAAPs in roots and leaves. However, only EgAAP5 had a similar pattern of expression levels between roots and leaves under PEG treatment. The findings provide information on how EgAAPs in E. guineensis are regulated during growth and development, and under various environmental stresses.
Introduction
Elaeis guineensis is an ancient tropical crop with high oil content, and its oil is rich in palmitic acid, β-carotene and vitamin E [Citation1]. It has been reported that fertilization could promote the growth of E. guineensis under good watering conditions [Citation2]. Nitrogen, including inorganic nitrogen and organic nitrogen, has important effects on plant growth and development and nutrition quality. Amino acids, as the most important form of organic nitrogen, are transported and utilized by plants and determine the final quality of oils.
Amino acids are transported from source to sink organs [Citation3,Citation4]. Amino acid transporters (AATs) are cellular membrane proteins that can transport amino acids. AATs play an important role in various plant processes, such as seedling development and responses to pathogen and abiotic stresses [Citation5–7]. More than 60 AtAATs in Arabidopsis [Citation4,Citation8] and more than 80 OsAATs in rice [Citation9,Citation10] have been identified in model plants. Furthermore, AAT families have also been identified in poplar [Citation11], Solanum tuberosum L. [Citation12] and Glycine max L. [Citation13]. Importantly, studies have shown that amino acid permeases (AAPs), which belong to the AAT family [Citation14–16], played an important role in loading amino acids for nitrogen sink and supply [Citation17].
Although the functions of many AAPs in Arabidopsis have been identified, few studies have investigated AAP family members in tropical crop E. guineensis. In this study, we identified 11 amino acid permease genes in E. guineensis and showed that these genes had differential expression patterns in various tissues. Furthermore, they had differential expression patterns in roots and leaves under cold and PEG stress. These data might provide insight for further understanding their roles in the growth and development of E. guineensis and give clues to understand how E. guineensis utilizes the expression of these amino acid permease genes to adapt to environmental stress.
Materials and methods
Identification of amino acid permease genes in E. guineensis
The EgAAP gene sequences in E. guineensis were acquired from the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/), and the gene structures were analyzed. Protein structures were analyzed using Protparam (http://web.expasy.org/protparam/). Their subcellular localization was predicted using Wolf PSORT (http://psort.hgc.jp/), and transmembrane domains were analyzed using TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/). A phylogenetic tree of EgAAPs based on the amino acid sequence was drawn according to the results generated by MEGA4.0 analysis using the neighbour-joining method with an amino acid and the Poisson correction model. Bootstrap values calculated for 1000 replicates are indicated at corresponding nodes. The promoter sequence analysis of EgAAPs was conducted using Plant CARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
E. guineensis materials and treatments
For different tissue expression analyses of the EgAAP genes, male flower, female flower, lateral root, primary root, fruit and leaf tissues were sampled from African oil palm (pisifera, thin-shelled). Each type of tissue was collected on the same day from 15-year-old oil palms from the same estate in the germplasm nursery of oil palms at the Coconut Research Institute, CATAS, China. Nine-week-old seedlings were grown in an artificial growth chamber with a constant photoperiod (16 h light/8 h darkness) and an average temperature of approximately 26 °C. The seedlings were treated with 20% PEG-6000 and 4 °C to induce drought stress and cold stress, respectively. To obtain reliable experimental data and reduce experimental error, for each sample, we executed three repeated trials using the same stressor and carried out three biological replicates of expression analysis. For each induction treatment, spear leaves and roots were sampled at six time points (0, 1, 3, 6, 12, 24 and 48 h) and immediately stored at –80 °C for RNA extraction. In addition, untreated plant materials (0 h) were used as the control group.
RNA extraction and real-time polymerase chain reaction (RT-qPCR) analysis
Approximately, 100 mg of tissue was utilized to extract total RNA using a MiniBEST plant RNA extraction kit (Takara, Japan). The RNA quantity was determined by a NanoDrop-2000 spectrophotometer (ThermoFisher, USA), and RNA integrity was confirmed by electrophoresis. The RNA from all tissues was used for reverse transcription (RT) using the PrimeScripttm RT reagent kit with genomic DNA eraser (Takara). The RT-qPCR mixture contained 1 μL diluted cDNA, 5 μL of 2 × PowerUp SYBR Green Master mix (Applied Biosystems, USA) and 0.5 μL of each gene-specific primer (10 μmol/L) in a final volume of 10 μL. All PCRs were performed using an ABI QuanStudio 6 machine under the following conditions: 10 min at 95 °C and 40 cycles of 5 s at 95 °C, 15 s at 55 °C and 20 s at 60 °C in 384-well clear optical reaction plates (Applied Biosystems, USA). The procedure ended with a melt curve ramping from 60 °C to 95 °C for 20 min to verify the PCR specificity. All qPCRs were carried out in biological and technical triplicate. The final Ct values were the means of nine measurements. Oil palm actin (GenBank accession number XP_010936865.1) was used as an internal control to normalize the expression of other genes. Gene-specific primers were designed with Primer Premier 5.0 ().
Table 1. Primers used for qRT-PCR of EgAAPs and Actin genes.
Results and discussion
Identification of amino acid permease genes in E. guineensis
Amino acid permease can transport a range of different types of amino acids in the roots directly [Citation18,Citation19] or indirectly via other amino acids that are transferred from ammonium to glutamine by glutamine synthetase [Citation20]. The amino acid permease genes in E. guineensis were identified by searching the sequences from NCBI. Eleven putative amino acid permease genes were found ( 2), and the exon number of the 11 EgAAP genes varied from 3 to 8 (2). Furthermore, the results demonstrate that the corresponding proteins for 11 EgAAP genes had 457–513 amino acid residues (3), indicating that these proteins are relatively similar in size. In addition, these proteins were predicted to all be localized at the membrane and to contain 9–11 transmembrane helices (3). Although the number of amino acid residues was found to be similar to the predicted value, the number and arrangement of transmembrane helices were different (). Many proteins did not have transmembrane helices in a large region after the fourth or fifth transmembrane helices (). To further confirm the phylogenetic relationship of the proteins encoded by the 11 EgAAP genes, we compared their amino acid sequences, and the results showed that EgAAP1/EgAAP6, EgAAP2/EgAAP3/EgAAP4, EgAAP5/EgAAP11, EgAAP7/EgAAP9 and EgAAP8/EgAAP10 were clustered (). Furthermore, we analyzed the promoter sequences of all EgAAPs and found that all the promoters of these genes have many light-responsive elements and enhancer regions (4). In addition, the promoters of four genes (EgAAP1, EgAAP7, EgAAP9 and EgAAP10) have low temperature-responsive elements (4). Almost all promoters of EgAAPs have abscisic acid-responsive elements except EgAAP3 (4).
Figure 1. Transmembrane helices of EgAAPs (A–K) in E. guineensis based on amino acid sequences. The transmembrane helices were acquired from TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/). The red line represents the transmembrane, the blue line represents the inside, and the pink line represents the outside of the transmembrane helices.
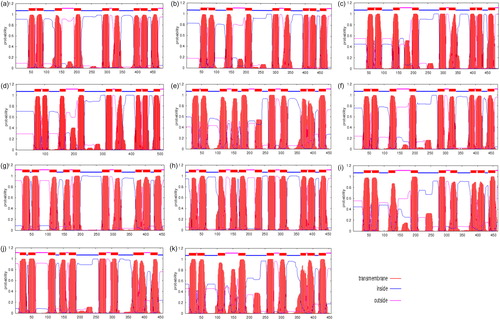
Figure 2. Phylogenetic tree of EgAAPs in E. guineensis based on amino acid sequences. The tree was drawn according to results generated by MEGA4.0 analysis using the neighbour-joining method with amino acids and the Poisson correction model. Bootstrap values calculated for 1000 replicates are indicated at corresponding nodes. Locus IDs of EgAAP in E. guineensis genes are given in .
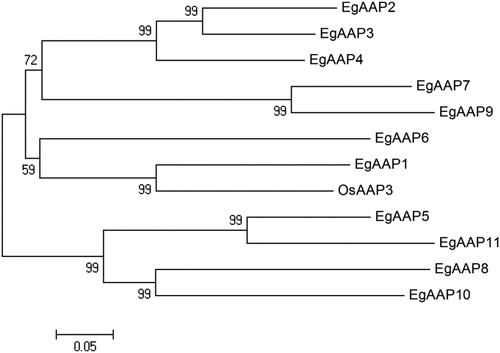
Table 2. Gene information of the amino acid permease genes in E. guineensis.
Table 3. Protein information and transmembrane helices of the amino acid permease genes in E. guineensis.
Table 4. Promoter cis-element analysis of the amino acid permease genes in E. guineensis.
Different tissue expression profiles of amino acid permease genes in E. guineensis
Expression profiles can reflect the activity of genes in tissues, and the expression profiles of AATs have been identified in Arabidopsis [Citation21]. In the present study, we used qRT-PCR to monitor the expression levels of different tissues in an important oil crop, E. guineensis (). Since the root is one of the main sources of nutritional uptake, the results indicate that the expression of EgAAP1 was higher in lateral roots than in other tissues (). It was reported that AtAAP1 transports amino acids at the roots of Arabidopsis [Citation22] and regulates amino acids in developing embryos [Citation23]. In our study, only the expression of EgAAP5 in lateral roots was very low (), and the expression of seven EgAAPs (EgAAP3, EgAAP4, EgAAP5, EgAAP6, EgAAP9, EgAAP10 and EgAAP11) was higher in the primary root (), indicating that most EgAAPs played an important role in the amino acid transport in roots. Moreover, our results showed that none of the EgAAP genes had very low expression in the primary root ().
Figure 3. Different tissue expression patterns of EgAAPs (A–K) in E. guineensis under normal conditions. Error bars are the standard error (SE) of three replicates.
The leaves are the primary tissue that is a rich source of nitrogen and that remobilizes nutrients to developing seeds or fruits. Free amino acids can be transported from leaves to sink tissues [Citation23]. The expression of amino acid permease genes is essential for efficient nutrient transportation to developing fruits. A previous study reported that rice OsAAP5 is highly expressed in the leaves [Citation10]. Our results indicated that the expression levels were different among these EgAAP genes and that the expression levels of EgAAP2, EgAAP7, EgAAP8, EgAAP9, EgAAP10 and EgAAP11 were higher in the leaves of E. guineensis (). However, few genes were expressed at a higher level in female flowers, except EgAAP11 (3K). EgAAP7, EgAAP8 and EgAAP11 were expressed at slightly higher levels in male flowers than in other tissues (). Furthermore, the expression of EgAAP1, EgAAP4, EgAAP6, EgAAP9 and EgAAP10 was lower than that of other tissues (), but the expression of four genes (EgAAP5, EgAAP7, EgAAP8 and EgAAP11) was highly expressed in the fruits (). These findings suggest that different EgAAPs play different roles during E. guineensis growth and development.
Cold stress effects on the expression profiles of amino acid permease genes in E. guineensis
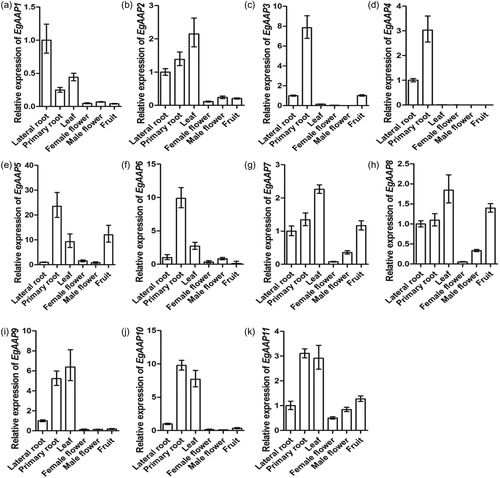
Tropical crops are affected negatively by climate change [Citation24], and the fresh fruit of palm oil is easily affected by temperature [Citation25]. The best average temperature for oil palm is approximately 27 °C, with a minimal growth temperature of 15 °C [Citation26]. Low temperature is an important ecological factor that affects the distribution and cultivation of oil palms [Citation27]. It was revealed that the C-repeat binding factor (CBF) mediated the gene expression pattern in E. guineensis under cold stress via RNA-Seq analysis [Citation26]. Other reports indicated that the PtAAT genes might play a critical role in the abiotic stress signaling in Populus because their expression was either increased or repressed after the cold and PEG treatments [Citation11]. However, little is known about the role of EgAAPs in response to environmental signals. E. guineensis is an economic crop in the tropics, and its growth and oil quality are affected when it suffers from cold stress. The comprehensive roles of the amino acid permease genes in E. guineensis in response to cold stress were determined by investigating their expression patterns by using qRT-PCR (). The results showed that the expression level of seven EgAAPs (EgAAP1, EgAAP2, EgAAP4, EgAAP6, EgAAP8, EgAAP9 and EgAAP11) was decreased during the cold treatment duration and that the expression level of EgAAP5 was increased after 6 h of cold treatment (). At 12 h of cold treatment, only one gene, EgAAP7, was expressed at a level similar to that with no treatment, but it was down-regulated at 24 h in both roots and leaves (). It has been reported that AAPs are highly regulated by environmental signals [Citation28], and the promoters of four genes (EgAAP1, EgAAP7, EgAAP9 and EgAAP10) have a low temperature-responsive element (). Here, we also found that the expression of most EgAAPs was affected by cold treatment.
Figure 4. Root and leaf expression patterns of EgAAPs (A–K) in E. guineensis under cold treatment. The Y-axis and X-axis indicate relative expression levels and the time courses of stress treatments, respectively. Error bars are the standard error (SE) of three replicates.
Leaves subjected to cold treatment showed the downregulation of eight amino acid permease genes (EgAAP1, EgAAP2, EgAAP4, EgAAP6, EgAAP7, EgAAP8, EgAAP10 and EgAAP11), and only two amino acid permease genes (EgAAP5 and EgAAP9) showed upregulation in response to cold stress (). Interestingly, the expression of EgAAP3 was almost unchanged at the beginning but decreased at 6 h and increased at 24 h after cold treatment (). However, the expression of EgAAP9 was upregulated after cold treatment at 3 and 6 h but downregulated after cold treatment at 12 and 24 h ().
PEG stress effects on the expression profiles of amino acid permease genes in E. guineensis
E. guineensis requires sufficient sunlight and water for growth and production. Breeding drought tolerant varieties of E. guineensis is the goal of breeders [Citation29]. Previous research revealed that the proline content increased in vegetative tissues in response to drought [Citation30], while the total soluble protein content was affected by increasing drought severity [Citation31]. Previous results showed that boron (B) and silicon (Si) application could induce physiological resistance of oil palm seedlings to drought stress [Citation32]. The genes involved in cellular nitrogen compound metabolic processes and transmembrane transport are differentially expressed during drought treatments [Citation33]. Therefore, we investigated the expression pattern of the amino acid permease genes in the root and leaf tissues in E. guineensis in response to PEG treatment by using qRT-PCR (). When the expression of the amino acid permease genes was compared, there was a lower expression level of EgAAP2, EgAAP3 and EgAAP6 in the root (). No gene was expressed at a level similar to that in the non-treatment group (). EgAAP5, EgAAP9 and EgAAP10 showed higher expression in the root under PEG treatment (). In leaves subjected to PEG treatment, the expression of five EgAAPs (EgAAP1, EgAAP4, EgAAP7, EgAAP8 and EgAAP11) was downregulated in the first few hours and was then subsequently further downregulated with treatment duration. Surprisingly, the results indicate that the expression of amino acid permease genes did not increase at the beginning of the experiment and then decreased later (). It was reported that water stress inhibited the growth of oil palm seedlings [Citation34]; similarly, we hypothesize that simulated drought treatment with PEG affected the expression of most EgAAP genes, thereby affecting nutrient transport.
Figure 5. Root and leaf expression patterns of EgAAPs (A–K) in E. guineensis under PEG treatment. The Y-axis and X-axis indicate relative expression levels and the time courses of stress treatments, respectively. Error bars are the standard error (SE) of three replicates.
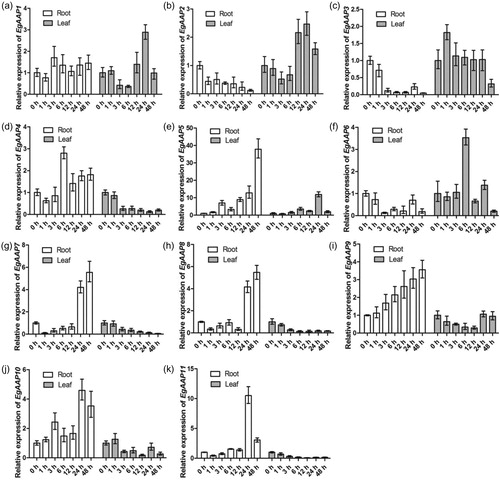
The expression of EgAAP4, EgAAP7, EgAAP8 and EgAAP11 decreased in the leaf during the PEG treatment from 0 to 48 h (). No elevated gene expression was observed under PEG treatment. The expression of EgAAP1, EgAAP2 and EgAAP9 decreased at the early stage of PEG treatment and then increased at the late stage (). However, the expression of three genes, EgAAP3, EgAAP6 and EgAAP10, increased at the beginning of PEG treatment but then decreased when PEG treatment continued ().
In plants, many stress-related genes generated a series of stress responses to meet the adverse environmental condition during growth and development. Overall, our data showed that the eleven identified EgAAP genes exhibited differential expression in adapting to developmental conditions and environmental stress. These data might provide insight for further understanding the functions of EgAAP genes and their roles in E. guineensis growth and development.
Conclusions
We performed qRT-PCR to investigate the expression patterns of EgAAP genes under PEG and cold treatment. The EgAAP members showed significantly differential expression patterns under the two abiotic stresses examined. Most EgAAP genes were upregulated by the two abiotic stress treatments, suggesting that EgAAP genes may play crucial roles in abiotic stress responses in E. guineensis. For instance, EgAAP5 was highly expressed (over 40-fold that of the control level) in roots under PEG (drought) stress treatment. Furthermore, two paralogous pairs (EgAAP1/EgAAP2, EgAAP8/EgAAP11) had similar expression levels and behaviour under cold and PEG treatment. These results might suggest that homologous genes had similar putative functions in the processes of plant growth and development.
This work was supported by the Central Public-interest Scientific Institution Basal Research Fund for Innovative Research Team Program of CATAS under grant number 17CXTD-13 (1630152017012).
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
References
- Mba OI, Dumont MJ, Ngadi M. Palm oil: processing, characterization and utilization in the food industry – a review. Food Biosci. 2015;10:26–41.
- Sun CX, Cao HX, Shao HB, et al. Growth and physiological responses to water and nutrient stress in oil palm. Afr J Biotechnol. 2011;10:10465–10471.
- Coruzzi G, Bush DR. Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiol. 2001;125:61–64.
- Tegeder M. Transporters for amino acids in plant cells: some functions and many unknowns. Curr Opin Plant Biol. 2012;15:315–321.
- Paungfoo-Lonhienne C, Lonhienne TG, Rentsch D, et al. Plants can use protein as a nitrogen source without assistance from other organisms. Proc Natl Acad Sci USA.. 2008;105:4524–4529.
- Näsholm T, Kielland K, Ganeteg U. Uptake of organic nitrogen by plants. New Phytol. 2009;182:31–48.
- Schulze W, Frommer WB, Ward JM. Transporters for ammonium, amino acids and peptides are expressed in pitchers of the carnivorous plant Nepenthes. Plant J. 1999;17:637–646.
- Andrews M, Lea PJ, Raven JA, et al. Nitrogen use efficiency. 3. Nitrogen fixation: genes and costs. Ann Appl Bio. 2009;155:1–13.
- Lu Y, Song Z, Kai L, et al. Molecular characterization, expression and functional analysis of the amino acid transporter gene family (OsAATs) in rice. Acta Physiol Plant. 2012;34:1943–1962.
- Zhao H, Ma H, Yu L, et al. Genome-wide survey and expression analysis of amino acid transporter gene family in rice (Oryza sativa L.). Plos One. 2012;7:e49210
- Wu M, Wu SN, Chen Z, et al. Genome-wide survey and expression analysis of the amino acid transporter gene family in poplar. Tree Genet Genomes. 2015;11:83.
- Ma HL, Cao XL, Shi SD, et al. Genome-wide survey and expression analysis of the amino acid transporter superfamily in potato (Solanum tuberosum L. ). Plant Physiol Bioch. 2016;107:164–177.
- Cheng L, Yuan HY, Ren R, et al. Genome-wide identification, classification, and expression analysis of amino acid transporter gene family in Glycine Max. Front Plant Sci. 2016; [cited 2018 Oct 25]7:515. [14 p.]
- Fischer WN, André B, Rentsch D, et al. Amino acid transport in plants. Trends Plant Sci. 1998;3:188–195.
- Gillissen B, Bürkle L, André B, et al. A new family of high-affinity transporters for adenine, cytosine, and purine derivatives in Arabidopsis. Plant Cell. 2000;12:291–300.
- Ortiz-Lopez A, Chang H, Bush DR. Amino acid transporters in plants. Biochim Biophys Acta.. 2000;1465:275–280.
- Tegeder M, Ward JM. Molecular evolution of plant AAP and LHT amino acid transporters. Front Plant Sci. 2012;3:21– 11.
- Miller AJ, Fan X, Shen Q, et al. Amino acids and nitrate as signals for the regulation of nitrogen acquisition. J Exp Bot. 2008;146:S238–S238.
- Svennerstam H, Ganeteg U, Näsholm T. Root uptake of cationic amino acids by Arabidopsis depends on functional expression of amino acid permease 5. New Phytol. 2008;180:620–630.
- Sonoda Y, Ikeda A, Saiki S, et al. Feedback regulation of the ammonium transporter gene family AMT1 by glutamine in rice. Plant Cell Physiol. 2003;44:1396–1402.
- Fischer WN, Kwart M, Hummel S, et al. Substrate specificity and expression profile of amino acid transporters (AAPs) in Arabidopsis. J Biol Chem. 1995;270:16315–16320.
- Lee YH, Foster J, Chen J, et al. AAP1 transports uncharged amino acids into roots of Arabidopsis. Plant J. 2007;50:305–319.
- Sanders A, Collier R, Trethewy A, et al. AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J. 2009;59:540–552.
- Paterson RRM, Lima N. Climate change affecting oil palm agronomy, and oil palm cultivation increasing climate change, require amelioration. Ecol Evol. 2018;8:452–461.
- Keshvadi A, Endan JB, Harun H, et al. The effect of high temperature on viscosity of palm oil during the ripening process of fresh fruits. IJSER. 2011;2:139–153.
- Lei XT, Xiao Y, Xia W, et al. RNA-seq analysis of oil palm under cold stress reveals a different C-repeat binding factor (CBF) mediated gene expression pattern in elaeis guineensis compared to other species. Plos One. 2014;9:e114482. [cited 2018 Oct 25][20 p.]
- Xiao Y, Zhou L, Lei X, et al. Genome-wide identification of WRKY genes and their expression profiles under different abiotic stresses in Elaeis guineensis. Plos One. 2017;12:e0189224. [cited 2018 Oct 25] p.]
- Grallath S, Weimar T, Meyer A, et al. The AtProT family. Compatible solute transporters with similar substrate specificity but differential expression patterns. Plant Physiol. 2005;137:117–126.
- Ubara UE, Agho CA, Aye AI, et al. Identification of drought tolerant progenies in oil palm (Elaeis guineensis Jacq. ). Int J Adv Res Biol Sci. 2017;4:120–127.
- Chaum S, Takabe T, Kirdmanee C. Physio-biochemical responses of oil palm (Elaeis guineensis Jacq.) seedlings to mannitol and polyethylene glycol induced iso-osmotic stresses. Plant Prod Sci. 2012;15:65–72.
- Azzeme AM, Siti Nor AA, Aziz MA, et al. Oil palm leaves and roots differ in physiological response, antioxidant enzyme activities and expression of stress-responsive genes upon exposure to drought stress. Acta Physiol Plant. 2016; 38:1–12.
- Putra ETS, I, T, et al. Physiological responses of oil palm seedlings to the drought stress using boron and silicon applications. J of Agronomy.. 2015;14:49–61.
- Zhang XB, Lei L, Lai JS, et al. Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biol. 2018; [cited 2018 Oct 25]18:68. [16 p.]
- Cao HX, Sun CX, Shao HB, et al. Effects of low temperature and drought on the physiological and growth changes in oil palm seedlings. Afr J Biotechnol. 2016;10:2630–2637.
