Abstract
Among high-energy lasers, the short-pulsed infrared erbium-doped yttrium aluminium garnet (Er:YAG) laser has shown the best potential for precise cutting and rapid osseous healing. The objective of the present study was to analyse the healing of the bone tissue treated with short-pulsed infrared erbium-doped yttrium aluminium garnet laser in contact and non-contact mode and piezosurgery in relation to the circulatory leukotriene B4 and cysteinyl leukotrienes. Twenty-four 10-week-old adult male Wistar rats were used. Three osteotomies on the medial part of the tibia were performed on each animal: one on the right tibia and two ones on the left tibia. After surgery, six animals were immediately euthanized and the others were euthanized after 1, 2 and 3 weeks. The bone healing after osteotomy was analysed by a laser-based profilometry. After euthanasia, blood samples were taken from the abdominal aorta and collected for further analysis of the circulatory leukotriene B4 and cysteinyl leukotrienes. A decrease in the osteotomy volume resulted from bone healing during the 3-week period. After the third week, there was almost complete healing of the prepared osteotomies. The circulating levels of both LTB4 and Cys-LTs at 7 and 14 days after osteotomy, were significantly reduced in comparison with the initial levels (p < 0.001 for LTB4 and p < 0.05 for Cys-LTs). On the 21st day after surgical treatment, only the circulating levels of LTB4 were significantly decreased compared to those on day 7 (p < 0.001) and day 14 (p < 0.05). All osteotomies healed through a significant decrease in both examined circulatory LTs.
Introduction
Piezosurgery and various types of high-energy lasers have been investigated in bone surgery over the past few decades [Citation1,Citation2]. High-energy lasers have been used in health applications since the early 1960s. Among them, the short-pulsed infrared erbium-doped yttrium aluminium garnet (Er:YAG) laser has shown the best performance in clinical bone surgery [Citation3,Citation4]. Compared to conventional mechanical techniques, the above-mentioned techniques provide higher bactericidal and detoxification effects, less traumatisation, reduced bleeding and less invasiveness [Citation2,Citation3,Citation5]. Histologic and ultrastructural analyses of the efficacy of the mentioned techniques have shown precise cutting and rapid osseous healing [Citation6–9].
The immediate physiological response to bone injury is local and systemic inflammatory reaction. The importance of the early inflammatory response is widely recognised and many studies [Citation10–12] have demonstrated that arachidonic acid (AA) metabolism is of utmost importance in bone healing. AA is formed from cell membrane bound phospholipids, in response to different biological signals. The biologically relevant metabolites of AA are known as eicosanoids and comprise the families of prostaglandins (PGs), thromboxanes (TXs), leukotrienes (LTs), and lipoxins (LXs). Among them, LTs are the most prominent molecules with a wide variety of functions. For example, 5-lipoxygenase (5-LO) knockout mice demonstrated greater callous size and enhanced mechanical properties after fracture, although the mechanisms for these effects remained unclear [Citation13,Citation14]. The role of 5-LO is to catalyse the conversion of AA throughout a series of intermediate metabolites to the formation of two major groups of end LTs. These two groups consist of LTB4 and cysteinyl-LTs (LTC4, LTD4, LTE4 and LTF4). LTs in excess amounts can contribute to impaired healing of fracture by altering the inflammatory response [Citation15]. Also, Akino et al. [Citation16] reported that Cys-LTs negatively regulates the process of fracture healing.
In the literature, to the best of our knowledge, no reliable data on LTs metabolism during application of Er:YAG laser or piezosurgery have been presented so far. Therefore, we hypothesised that changes in circulatory LTs could be critical for the process of bone healing during this type of surgical treatment. In the present study, we aimed to obtain new evidence for the advantages of applying Er:YAG laser and piezosurgery in bone surgery. Therefore, the purpose of this study was to analyse the healing of tibia treated with Er:YAG laser in contact and non-contact mode and piezosurgery in relation to the circulatory LTB4 and Cys-LTs.
Materials and methods
Animals
Twenty-four 10-week-old adult male Wistar rats, each weighing 300–350 g, were obtained and maintained in a controlled pathogen-free environment (lights on 7 AM to 7 PM, 22 °C) with ad libitum access to standard rat chow and water during a 7-day adaptation period in the experimental room. The rats were randomly assigned into four experimental groups (six animals per group): group I, animals sacrificed immediately after surgery; group II, sacrificed 1 week after surgery; group III, sacrificed 2 weeks after surgery; group IV, sacrificed 3 weeks after surgery. All efforts were made to minimise animal suffering and to use only the necessary number of animals to produce reliable data.
Ethics of experimentation statement
This study was carried out in accordance with the recommendations in the Guide for Care and Use of Laboratory Animals of the EC Directive 86/609/EEC. All procedures carried out on animals were approved by the Ethics Committee at the University in Skopje.
Surgical protocols
The rats were anaesthetised by an initial intraperitoneal injection of thiopental sodium (Rhone-Poulenc Rorer Limited, Nenagh, Co, Tipperary, Ireland) of 50 mg/kg body weight. The animals were positioned in the dorsal decubitus position; the surgical sites on the two tibiae were shaved and cleaned with aqueous iodine solution. A linear incision (approximately 18 mm long) was made through the skin and periosteum to expose the bone. The soft tissue was gently elevated and retracted. Three osteotomies on the medial part of the tibia were performed on each animal, two in the right tibia and one in the left tibia (5 mm away from each other). The osteotomies were approximately 2 mm deep. The osteotomies were performed in the same sequence from proximal to distal as follows: right proximal using piezosurgery, right distal using the Er:YAG laser in contact mode, and left distal using the Er:YAG laser in digitally controlled non-contact mode X-runner hand piece). The piezosurgery was performed using a piezoelectric device (Piezomed, W&H Dental-werk Burmoos GmbH, Austria). In order to achieve reproducibility, one surgeon performed all the preparations, and the average pressure applied was approximately 15 N. Laser preparations were performed by Er:YAG laser (Light Walker, Fotona, Ljubljana, Slovenia) in contact and non-contact mode. The laser parameters were described in detail in our previous study [Citation16]. Biosecurity standards for personal protection during laser irradiation were applied.
In order to achieve similar osteotomy depths in each group, different irradiation times for contact/non-contact modes and for piezosurgery were used [Citation17]. The time required for each osteotomy technique was 10 seconds for laser contact mode, 5 s for laser non-contact mode and 30 s for piezosurgery. The precise time intervals were ensured by using a digital stopwatch (Motorola C 139, Schaumburg). All osteotomies were performed under continuous irrigation and cooling to avoid thermal damage of the bone. Incisions were closed using absorbable sutures (Vicryl 6-0; Ethicon, Inc, Somerville, NJ). After surgery, the animals from group I were immediately euthanized by overdose of thiopental sodium, while the animals from the other groups were treated with acetaminophen 1 mg/kg in water (ad libitum) and kept under the same laboratory conditions for an additional 1 week (group II), 2 weeks (group III) and 3 weeks (group IV).
Volume measurement and laser profilometry
Bone healing after osteotomy was analysed over a period of 3 weeks (on a weekly basis), using a three-dimensional laser scanning technique based on the optical triangulation principle (Customised laser-based profilometry; Ljubljana, Slovenia). The profilometric system measured 80 cross-sections per second with a precision of 0.02 mm. The preparation and analysis of the samples by laser profilometry were performed according to the methods described by Perhavec et al. [Citation18] and Baraba et al. [Citation19]. The volumes of the cross-sections of the osteotomies were calculated using a specially designed computer program with a precision of ≤5% [Citation17].
Blood collection
Blood samples were collected from the abdominal artery and placed into tubes containing 3.8% sodium citrate (0.9 mL of blood to 0.1 mL of sodium citrate). Plasma was prepared by centrifugation (2000g for 20 min), separated into aliquots, and stored at −80 °C until analyses were performed.
LTB4 and Cys-LTs immunoassays
LTB4 and Cys-LTs in the plasma were analysed by a newly developed enzyme-linked immunosorbent assay (ELISA) for quantitative analysis of LTB4 and Cys-LTs levels from Cayman Chemical Company. The total amounts of LTB4 and Cys-LTs were analysed after hydrolysis and extraction [Citation20–24]. The limits of detection of the assays were about 13 pg/mL for LTB4 and about 20 pg/mL for Cys-LTs. Inter-assay coefficients of variation were <7% for LTB4 and <9% for Cys-LTs.
Statistical analysis
The data were analysed by one-way analysis of variance (ANOVA), followed by Newman–Keuls multiple comparison test between all groups. The correlation between different parameters was assessed by Spearman’s test. Only two-tailed probabilities were used for testing statistical significance. Probability values of p < 0.05 were considered statistically significant. All analyses were performed with Graph Pad Prism 4.0 (San Diego, CA, USA).
Results and discussion
Volume of osteotomies
The mean values of the volume of osteotomies performed by three surgical techniques after 1, 2 and 3 weeks are presented in . The variations in the initial volumes after all three techniques were similar (within 10%). The reduction of the osteotomy volume resulted from bone healing during the 3-week period. After the third week, almost complete healing of the osteotomies was observed. The process of bone healing (osteotomy volume reduction) progressed in a time-dependent manner (approximately one third of the volume per osteotomy per week). Detailed results are presented in our previous pilot study on bone healing after osteotomies prepared with Er:YAG laser and piezosurgery [Citation17].
Table 1. Volumes of osteotomies* according to techniques of preparation, during three weeks of evaluation.
Levels of circulatory leukotrienes
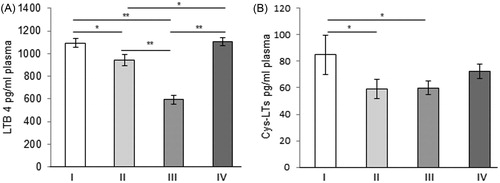
The circulating levels of both LTB4 and Cys-LTs at the 7th and 14th day after osteotomies were significantly reduced in comparison to the initial levels (p < 0.001 for LTB4 and p < 0.05 for Cys-LTs, respectively). On the 21st day after surgical treatment, only the circulating levels of LTB4 were significantly reduced compared to those on day 7 (p < 0.001) and day 14 (p < 0.05, ). Circulatory Cys-LTs after the 21st day were slightly increased compared to those on day 7 and day 14, but still did not reach the levels of the initial group (p = 0.112). According to the results obtained for LTB4 and Cys-LTs, significant healing of the rats’ tibiae, probably occurs somewhere between the second and the third week after the surgical treatments. It seems that the inhibitory mechanisms included in the control of the LTs expression reached their maximum somewhere about 2 weeks after the bone damage. This is in agreement with the data reported by Wixted et al. [Citation25] that bioactive lipids synthesised via 5-LO likely have multiple effects during tissue regeneration and repair [Citation26,Citation27]. The results obtained in the present study represent additional confirmation fact that circulatory LTs have a negative impact on the process of healing.
Figure 1. Plasma leukotriene B4 (A) and cysteynil leukotrienes (B), LTB4 and Cys-LTs (mean ± SE). Group I, immediately after surgery; Group II, 1 week after surgery; Group III, 2 weeks after surgery; Group IV, 3 weeks after surgery. *p < 0.05; **p < 0.001.
Further, the process of bone healing, was followed by a positive significant correlation between circulatory LTB4 and reduction in osteotomy volume on days 7 and 14 after piezosurgery (p = 0.049, ρ = 0.361 and p = 0.001, ρ = 0.605, respectively, ), after Er:YAG non-contact mode (p = 0.005, ρ = 0.428 and p = 0.001, ρ = 0.578, respectively, ). There was a positive significant correlation between LTB4 and reduction in osteotomy volume after Er:YAG contact mode application only after day 14 (p = 0.001, ρ = 0.837, ). The Cys-LT followed a similar course and showed a positive correlation to the reduction in osteotomy volume, only on day 7 (p = 0.001, ρ = 0.566, ), related to Er:YAG non-contact mode application. On day 14, the positive correlations between Cys-LTs and osteotomy volume reductions were significant for all three surgical treatments (p = 0.001, ρ = 0.514, related to piezosurgery; p = 0.001, ρ = 0.732 for Er:YAG contact mode and p = 0.001, ρ = 0.669, for Er:YAG non-contact mode, respectively, ). The obtained positive correlation between the decreased circulatory LTs and reduction in the volume of osteotomy during the process of bone healing corroborate that circulatory LTs have a negative impact on the process of healing. In consistence with these results, inhibition of the 5-LO with an orally delivered drug has been shown to accelerate and enhance fracture healing in the animal models [Citation28,Citation29].
Table 2. Correlation between osteotomy volumes measured for piezosurgery, Er:YAG in contact mode and Er:YAG in non-contact mode (x-Runner) and circulatory LTs, immediately after osteotomy and after 1 week, 2 weeks and 3 weeks.
This study also showed that on the 21st day after the applied surgical treatments, there were negative significant correlations between LTB4 and the reduction in osteotomy volume for all three surgical treatments (p = 0.012, ρ = −0.431, related to piezosurgery; p = 0.045, ρ = −0.398, related to Er:YAG contact mode and p = 0.033, ρ = −0.342 related to Er:YAG non-contact mode, ). The circulating Cys-LTs showed a negative significant correlation with the reduction in the osteotomy volume only after Er:YAG contact mode (p = 0.018, ρ = −0.456, ). The variations that appear in the circulating levels of both studied LTs between the days 7 and 14, could be a result of the different effects of the three different techniques applied to the bone tissues. We reported a stronger haemostatic effect and reduced blood clot formation for the Er:YAG laser in contact mode compared to the digitally controlled non-contact mode [Citation17]. This is in agreement with Yoshino et al. [Citation5], who compared bone healing after contact and non-contact Er:YAG laser and found that contact laser irradiation resulted in substantial ablation of the bone, whereas non-contact laser produced a slight removal of the tissue. Together with our findings for reduced LTs production, this opens the question of whether the applied surgical techniques enhanced the expression of some inhibitory mediators that could suppress LTs production, or cause direct LTs suppression. In the same context, we could speculate that the applied surgical techniques could have different effects on LTs/mRNA or even on 5-LO/mRNA suppression.
Overall, the accumulated evidence strongly suggests that the applied surgical techniques promote the mechanisms of LT suppression. Having in mind that different players (especially products of cyclooxygenases) are involved in the regulation of the LT production during the process of bone healing, further studies are needed to reveal the exact mechanisms involved in the different phases of the process of bone healing after Er:YAG or piezosurgery application. This can help in the development of new approaches that will target any particular LT and will effectively improve bone healing as a whole.
Limitations
The basic limitation of this study was that all osteotomies were performed on one animal, which was done in order to minimise animal suffering and use only the necessary number of animals to produce reliable scientific data. Our pilot study is limited in providing reliable data for the speed of bone healing depending on LTs suppression and applied surgical technique. Here, we can only address the negative regulatory effect of both examined LTs on the healing of the bone after application of the studied surgical procedures. Along these lines, it is necessary to carry out additional studies on a larger number of experimental animals, where the circulatory LTs associated with each osteotomy technique should be observed separately and compared between different techniques.
This study did not take into account the influence of accompanying production of other LTs and inflammatory mediators during vascular and soft tissue surgery. For instance, Cys-LT reportedly rises shortly after vascular surgery and returns to the preoperative level on the second day after [Citation29,Citation30]. Thus, we could conclude that such a pattern of biosynthesis is different from patterns induced during bone surgery.
Conclusions
The obtained results showed that the applied surgical techniques (Er:YAG laser or piezosurgery) promote the mechanisms of LTs suppression. The examined LTs expressed negative regulatory effect on the healing of the bone after application of the studied surgical procedures.
| Abbreviations | ||
| Er:YAG laser | = | erbium-doped yttrium aluminium garnet laser |
| LTB4 | = | leukotriene B4 |
| Cys-LTs | = | cysteinyl leukotrienes |
| LT | = | leukotrienes |
Acknowledgement
MM acknowledges support by BIOCOMPMAT project.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study was supported by the Russian Science Foundation under grant number 16-14-10372.
References
- Romeo U, Del Vecchio A, Palaia G. Bone damage induced by different cutting instruments-an in vitro study. Braz Dent J. 2009;20:162–168.
- de Mello DE, Pagnoncelli MR, Munin E. Comparative histological analysis of bone healing of standardized bone defects performed with the Er:YAG laser and steel burs. Lasers Med Sci. 2008;23:253–260.
- Papadaki M, Doukas A, Farinelli AW. Vertical ramus osteotomy with Er:YAG laser: a feasibility study. Int J Oral Maxillofac Surg. 2007;36:1193–1197.
- Steubinger S, von Rechenberg B, Zeilhofer FH, et al. Er:YAG laser osteotomy for removal of impacted teeth: clinical comparison of two techniques. Lasers Surg Med. 2007;39:583–588.
- Yoshino T, Aoki A, Oda S, et al. Long-term histologic analysis of bone tissue alteration and healing following Er:YAG laser irradiation compared to electrosurgery. J Periodontol. 2009;80:82–92.
- Gabric-Panduric D, Bago I, Katanec D, et al. Comparison of Er:YAG laser and surgical drill for osteotomy in oral surgery: an experimental study. J Oral Maxillofac Surg.. 2012;70:2515–2621.
- Gabric-Panduric D, Bago-Juric I, Music S, et al. Morphological and ultrastructural comparative analysis of bone tissue after Er:YAG laser and surgical drill osteotomy. Photomed Laser Surg. 2014;32:401–408.
- Sasaki MK, Aoki A, Ichinose S, et al. Ultrastructural analysis of bone tissue irradiated by Er:YAG laser. Lasers Surg Med. 2002;31:322–332.
- Sasaki MK, Aoki A, Ichinose S, et al. Scanning electron microscopy and Fourier transformed infrared spectroscopy analysis of bone removal using Er:YAG and CO2 lasers. J Periodontol. 2002;73:643–652.
- Bergenstock M, Min W, Simon MA, et al. A comparison between the effects of acetaminophen and celecoxib on bone fracture healing in rats. J Orthop Trauma. 2005;19:717–723.
- Gerstenfeld CL, Al-Ghawas M, Alkhiary YM, et al. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am. 2007;89:114–125.
- Simon AM, O'Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg Am. 2007;89:500–511.
- Manigrasso BM, O’Connor PJ. Accelerating fracture healing by manipulating arachidonic acid metabolism. ORS. 2006;31:0070.
- O'Connor JP, Manigrasso MB, Kim BD, et al. Fracture healing and lipid mediators. Bonekey Rep. 2014;3:517.
- Byrum SR, Goulet LJ, Griffiths JR, et al. Role of the 5-lipoxygenase-activating protein (FLAP) in murine acute inflammatory responses. J Exp Med. 1997; 185:1065–1075.
- Akino K, Mineda T, Mori N, et al. Attenuation of cysteinyl leukotrienes induces human mesenchymal stem cell differentiation. Wound Repair Regen. 2006;14:343–349.
- Gabric D, Blashkovic M, Gjorgievska E, et al. Evaluation of bone healing after osteotomies prepared with Er:YAG laser in contact and non-contact modes and piezosurgery—an animal study. Int J Oral Maxillofac Surg. 2016;74:18–28.
- Perhavec T, Gorkic A, Bracun D, et al. A method for rapid measurement of laser ablation rate of hard dental tissue. Opt Laser Technol. 2009;41:397–402.
- Baraba A, Miletic I, Krmek JS, et al. Ablative potential of the erbium-doped yttrium aluminium garnet laser and conventional handpieces: a comparative study. Photomed Laser Surg. 2009;27:921–927.
- Mladenov M, Tanska V, Vitkovska T, et al. Evidence for the influence of vitamin C on age- and heat exposure-dependent deterioration of biochemical function in rat's liver and kidney. J Therm Biol. 2008;33:431–436.
- Hadzi-Petrushev N, Mladenov K, Sopi R, et al. Enhanced lipid peroxidation and inflammation during heat exposure in rats of different ages: role of α-tocopherol. J Therm Biol. 2013;38:474–479.
- Hadzi-Petrushev N, Stojkovski V, Mitrov D, et al. D-galactose induced inflammation lipid peroxidation and platelet activation in rats. Cytokine 2014; 69:150–315.
- Mitrov D, Hadzi-Petrushev N, Stojkovski V, et al. Influence of chronic chromium exposition on the processes of lipid peroxidation inflammation and platelet activation in rats. J Biol Regul Homeost Agents. 2014;28:531–535.
- Mitrokhin V, Gorbacheva L, Mladenov M, et al. IL-2 induced NF-κB phosphorylation upregulates cation nonselective conductance in human cardiac fibroblasts. Int Immunopharmacol. 2018;64:170–174.
- Wixted JJ, Fanning P, Rothkopf I, et al. Arachidonic acid, eicosanoids, and fracture repair. J Orthop Trauma. 2010;24:539–542.
- Wixted JJ, Fanning PJ, Gaur T, et al. Enhanced fracture repair by leukotriene antagonism is characterized by increased chondrocyte proliferation and early bone formation: a novel role of the cysteinyl LT-1 receptor. J Cell Physiol. 2009; 221:31–39.
- Lin H-N, O’Connor JP. Immunohistochemical localization of key arachidonic acid metabolism enzymes during fracture healing in mice. PLoS One. 2014;9:e88423.
- Cottrell JA, OʼConnor JP. Pharmacological inhibition of 5-lipoxygenase accelerates and enhances fracture-healing. J Bone Joint Surg. 2009;91:2653–2665.
- Chen Y-C, Lin Y-H, Wang S-H, et al. Monitoring tissue inflammation and responses to drug treatments in early stages of mice bone fracture using 50 MHz ultrasound. Ultrasonics. 2014;54:177–186.
- Szczeklik W, Gorka J, Kozka M. Leukotrienes biosynthesis in vascular surgery patients during perioperative period. J Physiol Pharm. 2014;65:705–708.
