Abstract
Interactions between oral microorganisms contribute to formation of polymicrobial communities on surfaces and prosthetics. Since streptococci are early colonizers, the ability of Candida albicans to adhere to streptococci paves the way for an additional surface for candidal colonization and propagation. This study aimed to investigate the molecular response of C. albicans to two species of streptococci, i.e. Streptococcus mitis and Streptococcus sanguinis in in vitro salivary biofilms. Single, dual and mixed species salivary biofilms were formed for 24 h. Biofilm biomass and cellular metabolic activity of microbial species were assessed by crystal violet (CV) staining and XTT reduction assay, respectively, followed by scanning electron microscopy. ALS1, ALS2 and ALS3 genes were screened and quantified using qPCR. CV and XTT analysis showed a significant increase in biofilm biomass and cellular metabolic activity of two dual species and mixed species in comparison to single species biofilm. SEM analysis revealed formation of candidal hyphae under influence of streptococci. ALS1 and ALS3 were significantly overexpressed in a mixed species biofilm, in comparison to dual species and single species biofilms. Presence of S. mitis and S. sanguinis assisted in the proliferation of C. albicans which may augment tissue invasion. This study proposes substantial contribution of bacteria in propagation of C. albicans biofilm. Hence, in oral fungal conditions, promotion of the infection may be contingent upon the bacterial constituent, a prospect which is frequently neglected and requires more research.
Introduction
Candida albicans is a pleomorphic yeast that is a part of commensal microbial flora in the oral cavity, gastrointestinal (GI) and genitourinary mucosal surfaces of 60% of healthy individuals [Citation1, Citation2]. Commensal microorganisms colonize oral hard and soft tissues, e.g. teeth, mucosa, tongue and even prosthetic devices like implants and dentures to form complex polymicrobial biofilms [Citation3]. In healthy individuals, the growth of oral biofilm is limited by innate host immune defence on mucosal interface and a rapid epithelial turnover [Citation4]. However, immunosuppression caused by disease allows the progression of mucosal candidal biofilms, which results in the clinical condition called oral candidiasis (thrush) [Citation5]. It has been reported that the biofilms in oral cavity are composed of a compact network of fungal cells and the resident bacteria are in close affiliation with Candida [Citation6]. Thus, the manner in which C. albicans interacts with the commensal bacteria is destined as an important factor in understanding C. albicans colonization and expansion on oral niches.
Several species of streptococci have been discovered in the oral cavity. Streptococcus sanguinis and Streptococcus mitis are facultative anaerobic, gram positive, non-motile and non-spore forming member of the viridans group [Citation7]. Since these streptococci are early colonizers of oral surfaces, they can contribute massively in fungal colonization and propagation by providing an additional adherence surface for C. albicans. Synergistic, antagonistic and/or mutualistic interactions between microorganisms contribute to the formation of polymicrobial biofilm communities on teeth, oral tissues and prosthetic materials [Citation8].
The perception of how mixed species form biofilms and how they influence human health is still very limited, even though it has been documented that microorganisms prevail in nature as polymicrobial colonies [Citation9]. Interconnections between oral bacteria and C. albicans may not only participate in colonization of C. albicans on oral mucosa, implants, dentures etc. but also during the progression of invasive infections [Citation10]. It is uncertain however, to what extent C. albicans–bacterial interactions prevent or promote disease.
The aim of this research was to assess the influence of two streptococcal species on the growth attributes of C. albicans on 24-h in vitro salivary biofilms consisting of C. albicans only or on dual/mixed species biofilms consisting of S. mitis and S. sanguinis. In addition to analyzing biofilms on biomass and cellular metabolic activity level, expression of ALS1, ALS2 and ALS3 genes was also compared in each group.
Materials and methods
Microbial strains and growth condition
In our study, S. mitis (ATCC 49456), S. sanguinis (BAA 1455) and Candida albicans (ATCC 14053) strains were used for the formation of respective in vitro salivary biofilms.
Candidal and bacterial cells maintained in 20% glycerol stocks were thawed and suspended in Tryptic soy broth (Sigma-Aldrich). A standard inoculum was adjusted to 1 × 106 cells/mL (OD550 nm = 0.144) for candida and for streptococci, in 10 mL Tryptic soy broth using Shimadzu, UV-1700 spectrophotometer [Citation11].
Collection and preparation of saliva for biofilm
Unstimulated saliva was obtained from a 30 year old, medically healthy donor with no persistent oral condition, and collected into ice-cold sterile falcon tubes. One donor was asked to contribute throughout the whole experiment to minimize any variations that may arise because of different subjects. The collected saliva was centrifuged for 30 min at 17,000g and filter-sterilized using sterile 0.2 µm cellulose acetate syringe filter (Sartorius) and stored at −20 °C prior to use [Citation12].
Formation and growth of biofilm
Using the prepared cell inoculums, single, dual and mixed species biofilms were developed on commercially available flat-bottom, polystyrene 96-well microtiter plates (Thermo Scientific Nunc). The surface of wells was coated with 50 μL of clarified saliva and incubated for 90 min at 37 °C. Excess saliva was aspirated. For single species biofilm, 200 µL of C. albicans cell suspension was poured in saliva coated wells. Equal volumes of each species were used to give a total volume of 200 µL for dual species biofilms – C. albicans with S. mitis and C. albicans with S. sanguinis. For mixed species biofilms, the ratio of C. albicans:S. mitis:S.sanguinis species were 1:1:1, to give a total volume of 200 µL. S. mitis and S. sanguinis alone were used as control. The plates were incubated for 24 h at 37 °C, medium was aspirated and the biofilms were washed twice with PBS to remove non-adherent cells.
Quantification of biofilm biomass using crystal violet staining
After washing, the biofilms were fixed with 10% formaldehyde for 10 min and stained with 100 μL of 0.1% w/v crystal violet (CV) solution for 15 min without any agitation. The biofilms were gently washed twice with PBS and destained with 95% ethanol. Next, 75 μL of the solution was transferred into new wells of new 96-well microtiter plates and their absorbance was measured at 595 nm using a microtiter plate reader (FC-Bios µQuant).
Quantification of biofilm cellular metabolic activity using XTT reduction assay
The XTT assay is based on the reduction of the XTT dye (tetrazolium salt) to a water-soluble formazan. The absorbance of the cell supernatant is proportional to the number of metabolically active microbial cells. After washing twice with PBS, a total of 100 μL XTT-menadione (10:1) solution (Sigma-Aldrich) was dispensed into each well. The plates were covered in aluminium foil and incubated in the dark for 2 h at 37 °C. Following this, 75 μL of the solution was transferred into new wells of new 96-well microtiter plates and the amount of colorimetric change (a depiction of the metabolic activity of biofilm cells) was measured at 490 nm using a microtiter plate reader (FC-Bios µQuant).
Scanning electron microscopy (SEM) ultrastructural analysis of coaggregated microorganisms
Since Streptococci appear the same under scanning electron microscopy (SEM), three types of biofilms were made: single species C. albicans, single species Streptococci and one mixed biofilm with all three species. Glass cover slips were placed in sterile wells of six-well plates. After 24 h, sample was fixed overnight with 4% glutaraldehyde solution and washed with 0.1 mol·L−1 sodium cacodylate buffer (pH 7.4). The samples were secondarily fixed overnight with 2% osmium tetroxide diluted in buffer solution. After washing, dehydration was done with an ascending series of ethanol concentrations (10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95% and 100%) for 15 min each succeeded with dehydration in ethanol–acetone mixture at ratios of 3:1, 1:1, 1:3 and pure acetone for 15 min each. Critical point drying of the samples was done in liquid CO2 for 2 h under 95 bar pressure (Balzers CPD 030; Bal-Tec AG, Balzers, Liechtenstein). Conclusively, an ion sputter coater (JOEL JFC1100; JEOL, Tokyo, Japan) was used to gold-coat samples under low pressure and observed with a scanning electron microscope (Quanta FEG 250).
The morphology index (Mi) originally described by Merson-Davies and Odds [Citation13] was calculated in the study to characterize the various pleomorphic forms of C. albicans in four groups, i.e. single species, two dual species and mixed species biofilm. The morphology index of randomly selected cells (n = 25 for each group) was calculated for the observed cell type based on the given formula: Mi = ls/d2. Where “l” is the length of the cell, “d” is the maximum diameter of the cell and “s” is the diameter at the septal junction in “µm”. Cell dimensions in all samples were measured with the Olympus DP80 microscope digital image analysis system. Microscopic fields were examined under magnification of 100× and visualized with a digital video camera.
Genomic analysis
RNA extraction
Expression of ALS1, ALS2 and ALS3 genes was analyzed in salivary biofilms of Candida with S. mitis and S. sanguinis. Primers that were used for analysis are shown in . For RNA extraction, easy-REDTM total RNA extraction kit was used. A 24 h single (C. albicans), two dual and one mixed species biofilms were developed on glass coverslips placed in a flat bottom six-well plate as described in a similar procedure above. Microbial cells were scrapped off from glass coverslip biofilms and transferred to 1.5 mL tubes, respectively. A pre-lysis buffer of 250 µL volume was added. The tube was incubated for 3 min at 95 °C. Following that, 750 µL of easy-RED™ solution was added, vortexed and incubated for 5 min at room temperature (RT). Then, 200 µL chloroform was added, vortexed and incubated for 5 min. After centrifugation, aqueous phase was pipetted and transferred to a clean tube and then 1 mL of cold isopropanol was added and left at −20 °C for 15 min. Samples were washed with 70% ethanol. RNA was dissolved using 50 µL RNAse free water and its quantity and purity were checked by spectrophotometer Nanodrop 2000 (Thermo Scientific).
Table 1. Forward (F) and reverse (R) primers used for qPCR analysis [Citation31].
cDNA synthesis
Complementary DNA (cDNA) was synthesized using 1-step RT-PCR kit (SuprimeScript RT-PCR kit and Premix 2×) according to instructions provided by manufacturer in Thermocycler (Eppendorf Mastercycler gradient). Reverse transcription reactions for synthesizing cDNA were done using 5 µL total RNA template (200 ng.µL-1), 1 μL 50 μg mL−1 random primers, 1 μL 10 mM dNTPs, 5 μL M-MLV reaction buffer (×5), 1 μL 25 U μL−1 RNase inhibitor and 1 μL 200 U μL−1Reaction mix (SuprimeScript RT-PCR kit). A final reaction volume of 25 μL was made by adding RNAse free water. The final reaction mix was then incubated for 5 min at 70 °C, followed by incubation for 60 min at 37 °C. The resultant cDNA was stored in freezer at –20 °C preceding qPCR.
Quantification of dominant gene using qPCR
Primers that were used in qPCR analysis are displayed in and were designed from full-length gene sequences obtained from the nucleotide platform in PubMed using Primer3 software [Citation14, Citation15].
Quantitative PCR was performed using 96-well qPCR plates as triplicates in an Applied Biosystems 7500 Fast Realtime PCR instrument. Each 20 μL reaction comprised of 2 μL cDNA, 1 μL of each primer (10 mmol/L), 10 μL (×2) Titan HotTaqEvaGreen® qPCR mix SYBR-Green PCR Master Mix and 6 μl RNAse free water. Thermal cycler protocol consisted of initial denaturation step for 2 min at 95 °C, followed by 40 cycles of denaturation for 15 s at 95 °C, then primer annealing for 30 s at 58 °C and primer extension for 30 s at 72 °C. A final extension for 2 min at 72 °C was conducted, succeeded by chilling at 4 °C. To verify the amplified product, a dissociation step at 60 °C was added and a melting curve was generated. After finishing qPCR, a threshold cycle was set in accordance with amplification curves of evaluated ALS genes. The control and experimental groups were compared on the basis of cycle number at which the reference and the target genes attained the threshold cycle (Ct). Relative gene expression analysis was achieved according to the double delta Ct analysis (ΔΔCt) method [Citation16].
Statistical analysis
All results were computed and expressed as mean values with standard deviation (±SD) from three biological and technical variants performed in triplicate (n = 9). Statistical analyses were performed using SPSS software version 22.0. A one-way analysis of variance (ANOVA) test was used to compare the significant differences between control (single species) two dual species (C. albicans with S. mitis) (C. albicans with S. sanguinis) and mixed species (C. albicans with both S. sanguinis and S. mitis) sample. An independent t-test was used to compare significant difference between two dual species groups. The morphology index (Mi) of various pleomorphic forms of C. albicans was analyzed by descriptive statistics (number of cells measured, average, SD, ANOVA test). A p < 0.05 was considered statistically significant.
Results and discussion
Biofilm biomass and cellular metabolic activity
Crystal violet analysis showed a significant increase in biofilm biomass of two dual species and mixed species in comparison to single species biofilm (p < 0.05). C. albicans formed denser biofilms in combination with S. sanguinis and S. mitis respectively. shows the absorbance values of different biofilms. Data are presented as mean ± SD of three independent experiments performed in triplicate. Although the two dual species did not show significant difference in biomass (p > 0.05), CV analysis showed that C. albicans formed denser biomass with S. sanguinis when compared to S. mitis. The analysis showed that both streptococci mixed with C. albicans formed an even denser biofilm when combined with both species in a mixed species biofilm (p < 0.05). The XTT (tetrazolium salt) assay accurately detected the observed differences in the extent of biofilm formation between the control biofilm, the two dual species biofilm and the mixed species biofilm (). Based on the XTT assay, the mixed species biofilm showed highest metabolic activity as compared to single and dual species (p < 0.05). The two dual species showed significantly higher cellular metabolic activity as compared to single species (p < 0.05). However, the difference in XTT values between two dual species was not significant (p > 0.05).
Figure 1. Absorbance values of crystal violet solutions obtained from C. albicans biofilm formation in single, dual and mixed species biofilms. Note: Data are presented as mean ± SD of three independent experiments performed in triplicate. C.a, Candida albicans; S.m, Streptococcus mitis; S.s, Streptococcus sanguinis. (*) Significant when compared to single species (One-way ANOVA; p < 0.05). (a) No significant difference between groups (p > 0.05).
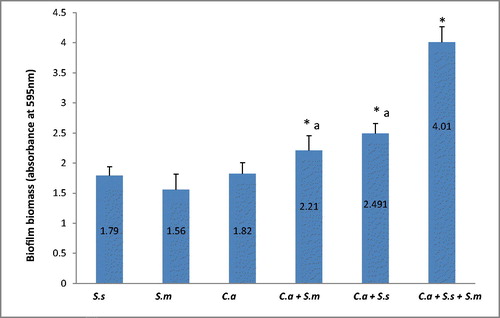
Figure 2. Absorbance values of XTT reduction assay obtained from C. albicans biofilm formation in single, dual and mixed species biofilms. Note: Data were represented as mean ± SD of three independent experiments performed in triplicate. C.a, Candida albicans; S.m, Streptococcus mitis; S.s, Streptococcus sanguinis. (*) Significant when compared to single species (one-way ANOVA; p < 0.05). (a) No significant difference between groups (p > 0.05).
Host adhesion and formation of biofilm are key areas for microbial virulence and antifungal property. But the science behind the formation of polymicrobial communities has always been under debate. It is well documented that an increase in the number of organisms in the same growth medium would cause them to compete for nutrients [Citation10] resulting in “survival of the fittest” strategy. However, the results of CV and XTT in this study demonstrated that both the biomass and cellular metabolic activity of candida biofilm escalateded under the influence of streptococci. The results indicate that not only do bacteria coexist with candida, but it is anticipated that they influence the local environment of C. albicans by altering parameters and creating a synergistic environment which may contribute in enhancing C. albicans hyphal transition, biofilm structure and subsequently its virulence [Citation17]. Previous studies have proved that streptococci bind directly to salivary proteins that are abundant in the pellicle covering teeth and mucosal surfaces, and this binding facilitates their initial colonization in the oral cavity [Citation18]. The results of this study show that there is high co-aggregation affinity between C. albicans and the two types of streptococci. Undoubtedly, it has been observed before that oral streptococcal bacteria interact with C. albicans [Citation19]. Diaz et al. showed in his study that the ability of oral streptococci to form biofilm on abiotic surfaces is substantially enhanced in the presence of C. albicans. Moreover, it is anticipated that these bacteria in dual and mixed species biofilms influence the local environment of C. albicans by altering parameters such as nutrient supply or carbon dioxide levels, which may contribute in enhancing C. albicans hyphal transition and subsequently virulence [Citation20].
Ultrastructure under SEM
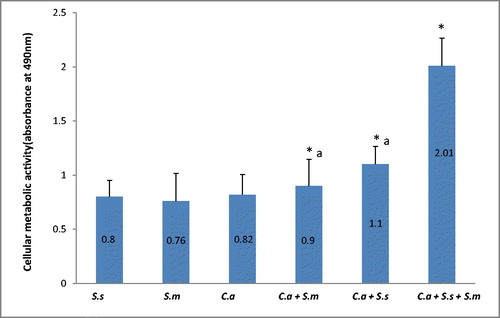
A photomicrograph of C. albicans species alone is represented in . The figure shows candidal blastoconidia as smooth-surfaced, ovoid and spherical cells. A ring of bud scars was visibly located at one pole (tip) of the blastoconidial cell and buds were seen emerging from the opposite pole of the cell. Fragile blastoconidial septum was also seen. The average length-to-width ratios of candida in the monospecies sample (n = 25) was 1.14. The ultrastructure of C. albicans in mixed species biofilm is represented in . In high contrast to the previous image, cells of candida were now visible as true hyphae. These true hyphal forms displayed homogenously elongated structure with lack of constrictions at the solid septum. Streptococcal diplococci cells were found as clusters, close to candidal hyphae (). The average length-to-width ratio of candida in the two dual species samples, i.e. with S. sanguinis and S. mitis, was 4.62 and 4.15, respectively (n = 25). The average length to width ratio of candida in mixed species sample (n = 25) was about 6.45. There was a significant difference in the morphology index of C. albicans (p < 0.05) () in the single versus the mixed species samples. An electron micrograph of streptococci in a single species biofilm is shown in as reference. Both streptococci revealed similar morphology so only one image is provided. Round to ovoid, smooth surfaced streptococci were visible, growing in chains of variable lengths. Growing cells were shown with distinctive plane of division. Fragile cell septum was visible between two cells.
Figure 3. Scanning electron micrograph of C. albicans in a 24-h single species biofilm. Ovoid blastoconidia are visible. Note the ring of scars (downward arrow) located on one pole. A bud (arrow head) emerging from opposite side of pole. Fragile blastoconidial septum (triangle) is also seen.
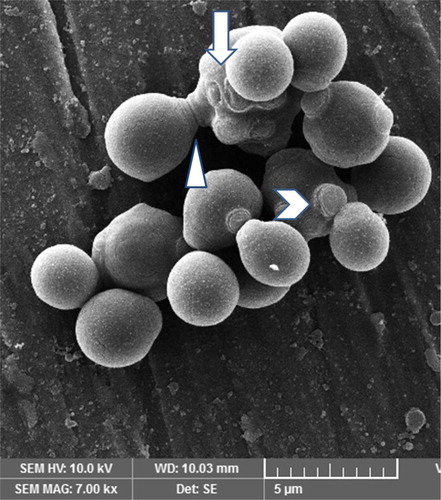
Figure 4. Scanning electron micrograph of C. albicans in a 24-h mixed species biofilm. True hyphal form of fungus is visible with no constriction at the septal junction (arrow).
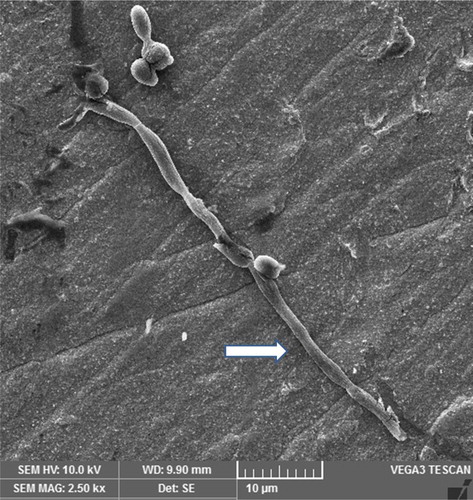
Figure 5. Scanning electron micrograph of C. albicans in a 24-h mixed species biofilm. True hyphal form of candida is visible (simple arrow). New blastoconidia can be seen budding off of hyphal cell (arrowhead). Clusters of streptococci can be seen near candida hyphae (stripped arrow).
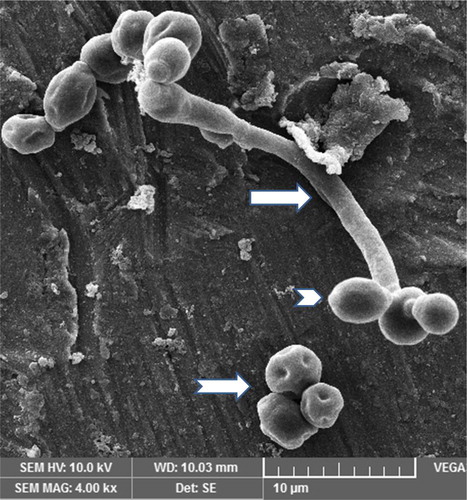
Figure 6. Scanning electron micrograph of streptococci in a 24-h single species biofilm. Image shows chains of streptococcal bacteria with plane of division (right directed arrow) and intercellular septa (downward directed arrow).
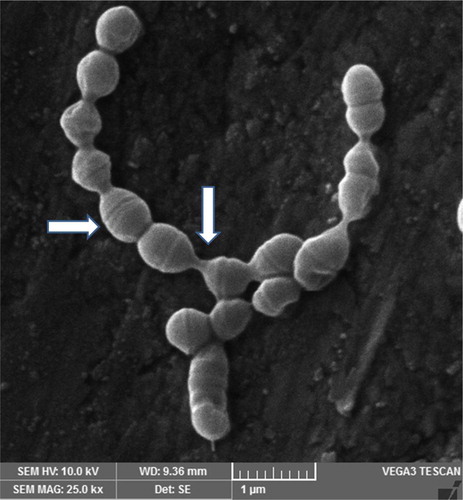
Table 2. Morphology index (Mi) values for pleomorphic cells of C. albicans in 24 h single species, two dual species and mixed species biofilms.
The morphological analysis gave an insight into the changes occurring in the structure of C. albicans under the influence of streptococci. In a 24-h biofilm, C. albicans formed true hyphae under the influence of streptococci, whereas in a bud stage it was observed in single species biofilm. The hyphal form of candida is critical for fungal adhesion and invasion of host surface [Citation21]. Hyphal formation is well known to trigger essential degrading enzymes required to attain full virulence[Citation22]. In symptomatic oral mucosal infections, e.g. thrush, denture stomatitis and oropharyngeal candidiasis, hyphal formation is clinically imperative as it is a critical marker of pathogenesis [Citation23]. Candidal hyphae formation is co-regulated with secretion of adhesins, secretory proteases and cytoplacmis proteins that aid in fungal biofilm stability over host surface and its invasion and subsequent dissemination of fungal pathogen [Citation24]. It has been observed in previous studies that when infecting humans and animals, C. albicans hyphae predominate at the primary site of infiltration of epithelial cell layers and tissues, whereas yeast cells are generally found either on the epithelial cell surface or emerging from penetrating hyphae that are infiltrating tissues [Citation25]. Hence, it could easily be stated that co-aggregation of C. albicans with streptococci in salivary biofilm poses a greater threat to local host tissues due to formation of hyphae when compared to fungus alone.
Expression of ALS1, ALS2 and ALS3 genes
The ability of C. albicans to form dual and mixed species biofilms with S. mitis and S. sanguinis was confirmed by genetic analysis of ALS1, ALS 2 and ALS 3 genes which encode large cell-surface glycoproteins implicated in the process of adhesion to host and other organisms. The expression was normalised to the standard ACT1 gene and results displayed in . Gene expression of ALS1 was significantly high in dual species and mixed species as compared to single species (p < 0.05). However, there was no significant difference in expression of ALS1 between dual and mixed species biofilm (p > 0.05). ALS2 however, did not show significant expression in either dual or mixed species biofilm (p > 0.05). Gene expression of ALS3, however, was significantly higher in the mixed species in comparison to the single or the dual species biofilm (p < 0.05). ALS3 gene expression was coherently high in dual species biofilm as compared to single species biofilm (p < 0.05), making it the most expressed gene amongst the three tested.
Figure 7. Relative gene expression of ALS1, ALS2 and ALS3 in C. albicans single, dual and mixed-species biofilms. C. albicans target genes were normalised using the C. albicans housekeeping gene (ACT1). Note: Analysis of quantitative RT-PCR was made by the ΔΔCt method. Single-species biofilms were used as reference samples for expression of C. albicans genes. C.a, Candida albicans; S.m, Streptococcus mitis; S.s, Streptococcus sanguinis. (*) Significant when compared to single species (C.a) (One-way ANOVA; p < 0.05).
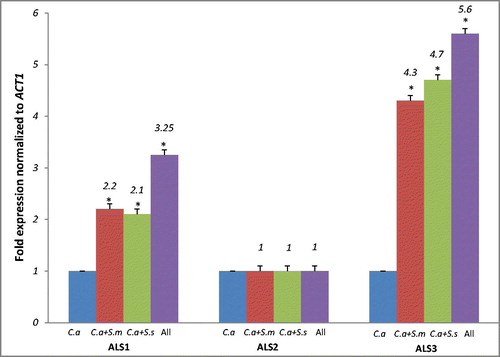
The results signify that ALS3 mainly and ALS1 are significantly expressed when C. albicans forms co-aggregation with commensal S. mitis and S. sanguinis of oral cavity. These putative genes are concerned with substratum adhesion [Citation26]. ALS1 is associated with biofilm maturation, whereas ALS3 is a cell surface, hyphae-specific protein and is known to be a receptor for the streptococcal adhesions SspB and SspA [Citation27]. Previous studies suggest that Als1p, encoded by the ALS1 gene, might adhere to host surfaces, rather than to other C. albicans cells or other microbial cells [Citation28]. High expression of the ALS1 gene in the dual and the mixed species biofilm indicates its role in biofilm maturation rather than cell–cell adhesion. On the other hand, ALS3 mutant C. albicans showed significant down-regulation in endothelial cell, buccal epithelial cell attachment and cell-cell adherence [Citation29]. Increased expression of ALS1 and ALS3 in dual and mixed species biofilms signify that C. albicans adhesion and propagation within surfaces may be enhanced under the influence of bacteria. Nailis et al. [Citation30] reported high expression of ALS1 and ALS3 genes in a 48-h biofilm. The high expression of the ALS1 and ALS3 genes in a 24-h biofilm, as indicated in this study, underlines high adherence capacity of C. albicans in initial stages of biofilm formation and maturation. This trait would lead to a more stable and resilient biofilm, increasing the pathogenicity of candida in local environments.
Conclusions
This study provided evidence that S. mitis and S. sanguinis enhanced the biomass, cellular metabolic activity in C. albicans biofilm, caused morphological changes in candida and stimulated expression of adherence genes. This is believed to be directly proportional to elevated virulence and pathogenic capability. These results can pave the way for researchers conducting clinical trials for treatment of recurrent fungal conditions like candidasis, denture stomatitis and implant failure cases.
Ethics statement
It is to confirm that this research has been conducted in full accordance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from the individuals who participated in this study.
Acknowledgements
“Balai Ungku Aziz Research Lab” in University of Malaya is highly acknowledged for providing microbial strains, kits and primers required for current research.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Diaz PI, Xie Z, Sobue T, et al. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect Immun. 2012;80:620–632.
- Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017.
- Zijnge V, van Leeuwen MBM, Degener JE, et al. Oral biofilm architecture on natural teeth. PLoS One. 2010;5:e9321.
- Dongari-Bagtzoglou A, Fidel PL. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J Dent Res. 2005;84:966–977.
- de Repentigny L, Lewandowski D, Jolicoeur P. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev. 2004;17:729–759.
- Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, et al. Characterization of mucosal Candida albicans biofilms. PLoS One. 2009;4:e7967.
- Silverman RJ, Nobbs AH, Vickerman MM, et al. Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii SspB adhesin promotes development of mixed-species communities. Infect Immun. 2010;78:4644–4652.
- Marcinkiewicz J, Strus M, Pasich E. Antibiotic resistance: a “dark side” of biofilmassociated chronic infections. Pol Arch Med Wewn. 2013;123:309–313.
- Peters BM, Jabra-Rizk MA, Scheper MA, et al. Microbial interactions and differential protein expression in Staphylococcus aureus–Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol. 2010;59:493–503.
- Hibbing ME, Fuqua C, Parsek MR, et al. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010;8:15.
- Nordin MAF, Razak FA, Himratul-Aznita WH. Assessment of antifungal activity of bakuchiol on oral-associated Candida spp. Evid-Based Compl Alt. Med. 2018;2015:7–8.
- Nordin MAF, Himratul-Aznita WH, Razak FA. Antifungal susceptibility and growth inhibitory response of oral Candida species to Brucea javanica Linn. extract. BMC Complement Altern Med. 2013;13:342.
- Merson-Davies LA, Odds FC. A morphology index for characterization of cell shape in Candida albicans. J Gen Microbiol. 1989;135:3143–3152.
- Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23:1289–1291.
- Untergasser A, Cutcutache I, Koressaar T, et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115.
- Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622.
- Diaz PI, Strausbaugh LD, Dongari-Bagtzoglou A. Fungal-bacterial interactions and their relevance to oral health: linking the clinic and the bench. Front Cell Infect Microbiol. 2014;4:101.
- Cavalcanti IM, Del Bel Cury AA, Jenkinson HF, et al. Interactions between Streptococcus oralis, Actinomyces oris, and Candida albicans in the development of multispecies oral microbial biofilms on salivary pellicle. Mol Oral Microbiol. 2017;32:60–73.
- Bowen WH, Burne RA, Wu H, et al. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26:229–242.
- Pathirana RU. Physiological adaptations in Candida albicans [dissertation]. Lincoln (NE): The University of Nebraska at Lincoln; 2016.
- da Silva Dantas A, Lee KK, Raziunaite I, et al. Cell biology of Candida albicans-host interactions. Curr Opin Microbiol. 2016;34:111–118.
- Nobile CJ, Andes DR, Nett JE, et al. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Path. 2006; 2:63.
- Desai JV. Candida albicans Hyphae: from growth initiation to invasion. J Fungi (Basel). 2018;4:10.
- Kumamoto CA, Vinces MD. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol. 2005;7:1546–1554.
- Blagojevic M. Epithelial cell death induced by Candidalysin, a cytolytic peptide toxin of Candida albicans [dissertation]. London (UK): King's College London; 2018.
- Zhu W, Filler SG. Interactions of Candida albicans with epithelial cells. Cell Microbiol. 2010;12:273–282.
- Swidergall M, Filler SG. Oropharyngeal candidiasis: fungal invasion and epithelial cell responses. PLoS Pathog. 2017;13:e1006056.
- Araújo D, Henriques M, Silva S. Portrait of Candida species biofilm regulatory network genes. Trends Microbiol. 2017;25:62–75.
- Richardson J, Ho J, Naglik J. Candida – epithelial interactions. J Fungi. 2018; 4:22.
- Nailis H, Kucharíková S, Řičicová M, et al. Real-time PCR expression profiling of genes encoding potential virulence factors in Candida albicans biofilms: identification of model-dependent and-independent gene expression. BMC Microbiol. 2010;10:114.
- Green CB, Cheng G, Chandra J, et al. RT-PCR detection of Candida albicans ALS gene expression in the reconstituted human epithelium (RHE) model of oral candidiasis and in model biofilms. Microbiology. 2004;150:267–275.
