Abstract
The aim of this study was to investigate the phenotype and function of NKT cells and the effect of NKT cells on the functions of vascular endothelial cells. The study included 30 pregnant women with hypertension and 20 pregnant women with normal delivery. Peripheral blood and placental tissues were collected from all patients and control subjects. Lymphocytes were isolated from peripheral blood and tissues. NKT cell ratio, expression of surface receptors and expression of effector molecules were detected by flow cytometry. Culture supernatant of NKT cells was collected and co-cultured with human umbilical vein endothelial cells (HUVECs). Proliferation was determined by CCK-8 assay, and migration, by transwell assay. The results showed elevated ratios of NKT and NK cells in peripheral blood and placental tissues from women with pregnancy-induced hypertension (PIH) compared with women with normal pregnancies. The expression of NKp30, NKG2D and NKG2A receptors on the surface of NKT cells from women with PIH was altered: the ratio of CD4+ NKT cells was increased but the ratio of CD8+ NKT cells was decreased. The ratios of GranB+ NKT cells in peripheral blood, IFN-γ-positive T cells in placental tissues and IL-17-positive NKT cells in both peripheral blood and placental tissues were elevated in PIH patients. The present study demonstrated that the ratio and activity of NKT cells from peripheral blood and placental tissues of women with PIH were elevated. In addition, NKT cells from peripheral blood of PIH patients inhibited the proliferation and migration of vascular endothelial cells by secreting IL-17.
Introduction
Pregnancy-induced hypertension (PIH) is a clinically common disease in pregnant women, which is characterized by hypertension, proteinuria and edema, leading to convulsions, coma or even death [Citation1,Citation2]. Clinical statistics show that PIH is one of the main causes leading to maternal death [Citation3]. The pathogenesis of PIH can be divided into two stages: placental perfusion reduction stage and multi-organ perfusion disorder stage caused by dysfunction of endothelial cells [Citation4,Citation5]. The decrease of placental perfusion leads to the formation of placental ischaemia, which is mainly secondary to placental shallow implantation. However, the reason for placental perfusion reduction is not clear, and it is reported to be related to genetic and immune abnormalities [Citation6]. Endothelial dysfunction is one of the most common pathological changes in PIH patients, being secondary to pathological changes of the placenta [Citation7]. It is discovered that oxidative stress, immune inflammation and cytokine disorders are involved in this process [Citation8].
In recent years, researchers have gained understanding of the human pregnancy process from the perspective of immunity. The embryo tissues carrying allogenic antigens from the father are actually a semi-homograft for the mother’s body. After the antigens are identified by the maternal immune system, no rejection is produced, and a protective immune response is active until the foetus is delivered [Citation9]. Since foetal delivery depends on the maternal body's own immune balance, PIH itself may be caused by immune system imbalance of the body [Citation10]. Recent studies support this hypothesis. For example, placental interface immune balance is closely related to the balance of Th1 and Th2 with their cytokines [Citation11]. Imbalance of Th1 and Th2 is prevalent in PIH, and results in cytokine disorder and placental trophoblastic dysplasia that are related to endothelial dysfunction [Citation12].
Natural killer T (NKT) cells are a class of heterogeneous cells in natural immunity. On the one hand, NKT cells have properties of NK cells, and activate perforin and granzyme pathways to kill cells via binding of activated receptors with ligands [Citation13]. On the other hand, NKT cells also have T cell receptors (TCR) that are restrictedly recognized by CD1d, and the cells can secrete a large number of cytokines such as tumour necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-4 and IL-17 after activation [Citation14,Citation15], thereby participating in local inflammatory response and immunoregulation. NKT cells have many subtypes. According to whether T and NK cell markers are expressed simultaneously, CD3+CD56+ cells are defined as NKT cells. Depending on whether CD4 and CD8 are expressed, NKT cells are further divided into CD4+, CD8+ and CD4−CD8− subgroups. CD3+CD56+ NKT cells play important regulatory roles in the balance of Th1 and Th2 cell subtypes. CD4−CD8− NKT cells tend to promote Th2-type immune response, while CD4+CD8− and CD4−CD8+ NKT cells tend to promote Th1-type immune response [Citation16,Citation17]. The role of NKT cells in vascular injury in patients with PIH is still unclear. In the present study, we investigated the phenotype and function of NKT cells at tissue and cellular levels, as well as the effect of NKT cells on the functions of vascular endothelial cells.
Subjects and methods
Subjects
A total of 30 pregnant women with PIH who had caesarean section at our hospital between October 2015 and October 2017 were included in the present study (age range 28–38 years; mean age 32.7 years; median age 33 years). In addition, 20 pregnant women with normal delivery were included into control group (age range 25–32 years; mean age 25.5 years; median age 24 years). Placental tissues and peripheral blood (5 mL) were collected from all patients and control subjects. Among the 30 pregnant women with hypertension, 13 women had only PIH (blood pressure rose after 20 weeks of pregnancy, without proteinuria), 11 women had combined mild preeclampsia (mPE; systolic pressure ≥ 140 mmHg or diastolic pressure ≥ 90 mmHg; proteinuria ≥ 300 mg within 24 h), and 6 women had combined severe preeclampsia (sPE; systolic pressure ≥ 160 mmHg or diastolic pressure ≥ 100 mmHg; proteinuria ≥ 2 g within 24 h). All subjects in both groups had singleton pregnancies. None of the pregnant women in either group had heart, liver or kidney diseases, blood system diseases, diabetes, rheumatism or autoimmune diseases. In addition, none of the subjects in either group had history of infection or medication within four weeks before surgery. After the delivery of the foetus, placental tissues (1 cm × 1 cm × 1 cm) were cut from the placental maternal surface to which the umbilical cord was attached. Necrosis, bleeding and calcification were avoided during cutting. The tissues were rinsed with saline and ground in an ice bath to obtain single-cell suspensions.
Ethics statement
All procedures performed in the present study were approved by the Ethics Committee of Children & Women's Healthcare of Laiwu. Written informed consent was obtained from all patients or their families.
Cells
Human umbilical vein endothelial cells (HUVECs; Cell Bank, Chinese Academy of Sciences, Shanghai, China) were cultured in low-glucose DMEM (Dulbecco's modified Eagle medium) supplemented with 10% foetal bovine serum (FBS). When reaching 90% confluency, the cells were trypsinised and passaged. HUVECs in logarithmic growth were used for functional experiments. In high glucose-induced endothelial cell model, HUVECs were cultured in high-glucose DMEM medium supplemented with 10% FBS.
To isolate peripheral blood mononuclear cells (PBMCs), peripheral blood (2 mL) from healthy subjects was treated with Ficoll lymphocyte separation solution (GE Healthcare, Chicago, IL, USA) according to the manufacturer’s manual. Then, PBMCs were mixed with five volumes of phosphate-buffered saline (PBS), and centrifuged at 1200g for 6 min. After discarding the supernatant, PBMCs were resuspended with PBS and mixed thoroughly.
High-glucose DMEM (250 μL) containing 10% FBS was used to culture HUVECs for 48 h at 37 °C and under 5% CO2. Then, the culture supernatant was collected and mixed with low-glucose DMEM supplemented with 10% FBS at a ratio of 1:1. The resulted medium was used for co-culture with NK cells.
To purify NKT cells, PBMCs were treated with CD3+CD56+ NKT Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s manual. First, PBMCs were resuspended with 10 mL sterile PBS before centrifugation at 300g for 10 min. After discarding the supernatant, flow cytometry was used to count the number of cells. Then, the cells (106) were resuspended with 400 μL PBS, and biotinylated antibody (100 μL) against CD3+CD56+ NKT cells was added, followed by dark incubation at 4 °C for 15 min. Unconjugated biotinylated antibody was washed off by cold PBS, and 5 mL PBS was added before centrifugation at 300g at room temperature for 10 min. After removing the supernatant, the cells were resuspended in 400 μL PBS. Anti-biotin magnetic beads (100 μL) were subsequently added, and the mixture was incubated in darkness at 4 °C for 15 min. Afterwards, the mixture was mixed with 1 mL PBS and centrifuged at 300g at room temperature for 10 min. Then, the washed cells were resuspended with 500 μL PBS, and subjected to magnetic bead column sorting. After 15 min, the resulting transparent liquid was transferred to a new vial, and the collected cells were NKT cells, which were then cultured in RPMI-1640 medium supplemented with 10% FBS and 100 IU IL-2 at 37 °C and 5% CO2 for 48 h. Then, NKT cells were collected for further experiments.
In NKT and HUVECs co-culture experiments, purified NKT cells that were cultured with RPMI-1640 medium supplemented with 10% FBS and 100 IU IL-2 were used as a negative control (NC) group. NKT cells from PIH patients co-cultured with HUVECs in 24-well plates were used as the experimental group, and NKT cells from healthy subjects co-cultured with HUVECs in 24-well plates were used as the control group. Each well contained 500 μL of medium and the medium was replenished every 24 h. At 72 h, the cells were used for tests.
In cytokine stimulation experiments, recombinant protein IL-17 (R&D Systems, Minneapolis, MN, USA) was added into DMEM containing 10% FBS to a final concentration of 0.5 ng/mL, and was used to culture HUVECs for 48 h. Then, HUVECs were collected for further experiments.
Flow cytometry
According to the manufacturer’s manual, 1 × 106 cells were mixed with 5 μL of fluorescence-labelled antibodies () before incubation at room temperature in darkness for 10 min. NKT cell membrane and intracellular markers were detected by flow cytometry. The mononuclear lymphocyte population was identified by FSC/SSC, and CD3+CD56+ NKT cell population was chosen for further analysis. Normal human PBMCs or NKT cells were cultured with 100 U/mL IL-2 in vitro for 24 h before centrifugation at 1000g for 5 min. CD3+CD56+ NKT cells were collected and used for detection of the expression of NKG2D, NKP30, CD4, CD8, TCRvα24, IL-4, IL-17, perforin and granzyme B (GranB). Each test was repeated at least three times.
Table 1. Fluorescence-labelled antibodies.
Flow cytometry was also used to detect cell cycles. At 24 h after transfection, 1 × 106 HUVECs of each group were washed twice with precooled PBS. Cell Cycle Assay Kit (BD Biosciences, Franklin Lakes, NJ, USA) was used to determine cell cycle according to the manufacturer’s manual. The cells were incubated with 200 μL liquid A for 10 min, and 150 μL liquid B for another 10 min. Then, the cells were incubated with 120 μL liquid C in darkness for 10 min before flow cytometry. The result was analysed using ModFit software version 3.2 (Verity Software House, Topsham, ME, USA).
CCK-8 assay
HUVECs were seeded at a density of 2000/well in 96-well plates. At 0, 24, 48 and 72 h, 20 μL CCK-8 reagent (5 g/L; Beyotime, Shanghai, China) was added onto the cells. On the last day, 150 μL CCK-8 reaction solution was added and the cells were incubated at 37 °C for 2 h. Then, the absorbance of each well was measured at 490 nm for plotting cell proliferation curves. Each group was tested in five replicate wells and the values were averaged.
Transwell assay
Transwell chambers (Corning, Inc., Corning, NY, USA) were used to evaluate the migration ability of HUVECs. Transfected cells were collected by trypsin digestion and resuspended to a density of 5 × 105 cells/mL using RPMI-1640 medium containing 0.1% bovine serum albumin. The cell suspension (200 μL) was added into the migration chamber. In the lower chamber, 500 μL RPMI-1640 medium supplemented with 20% FBS was added. After 24 h, the cells were fixed with 4% methanol at room temperature for 10 min, and then washed with PBS. Afterwards, the cells in the upper chamber were wiped off by a cotton swab. After Giemsa staining, the number of cells that crossed the membrane was counted under a microscope (200x; 5 fields). Each group was tested in five replicate wells and the values were averaged.
Data analysis
The results were analysed using Graph Pad Prism 7.0 statistical software (GraphPad Software, La Jolla, CA, USA). The data are expressed as means with standard deviations (±SD). Data were tested for normality. Multi-group measurement data were analysed using one-way analysis of variance (ANOVA). In case of homogeneity of variance, least significant difference and Student–Newman–Keuls methods were used; in case of heterogeneity of variance, Tamhane’s T2 or Dunnett’s T3 method was used. Comparison between two groups was carried out using Student’s t-test. P < 0.05 indicated statistically significant differences.
Results and discussion
Ratios of NKT cells and NK cells are elevated in peripheral blood and placental tissues from pregnant women with PIH as compared to women with normal pregnancies
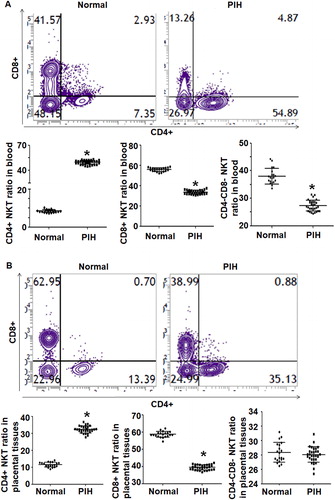
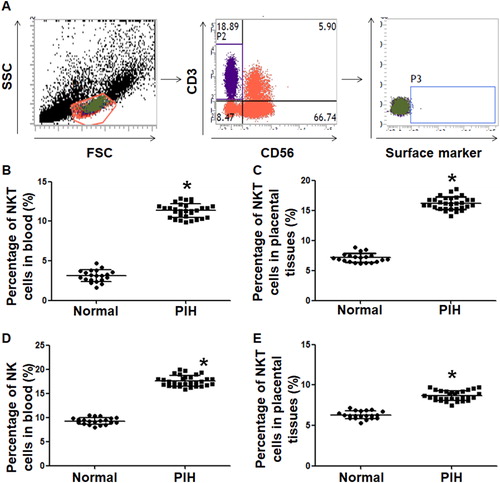
To examine the ratios of NKT and NK cells in peripheral blood and placental tissues, we used flow cytometry. The data showed that the percentage of NKT cells in peripheral blood from PIH patients (11.5 ± 0.21%) was significantly higher than that from the control group (2.9 ± 0.17%) (P < 0.05) (). Similarly, the percentage of NKT cells in placental tissues from PIH patients (16.3 ± 0.33%) was significantly higher than that from the control group (7.1 ± 0.3%) (P < 0.05) (). The ratio of CD3−CD56+ NK cells in peripheral blood from PIH patients (17.2 ± 0.29%) was significantly higher than that from the control group (9.4 ± 0.27%) (P < 0.05) (). In addition, the ratio of CD3−CD56+ NK cells in placental tissues from PIH patients (8.9 ± 0.16%) was significantly higher than that from the control group (6.2 ± 0.22%) (P < 0.05) (). These results suggest that the ratios of NKT cells and NK cells in peripheral blood and placental tissues from pregnant women with PIH are elevated compared to women with normal pregnancies.
Figure 1. Distribution of NKT and NK cells in peripheral blood and placental tissues from women with normal pregnancies and women with PIH. (a) Flow cytometry of NKT and NK cells. (b–e) Percentages of NKT and NK cells in peripheral blood and placental tissues from women with normal pregnancies and women with PIH. Note: * P < 0.05 compared to women with normal pregnancies.
PIH is a specific condition during pregnancy [Citation18]. PIH is one of the important causes of morbidity and mortality of perinatal mothers and children. The etiology and pathogenesis are still not fully elucidated and there are no effective preventive treatment measures at present [Citation19]. It is discovered that the immune system plays an important role in the occurrence and development of PIH. Immune cells play an important role in the pathogenesis of PIH by synthesizing and secreting cytokines [Citation20,Citation21]. In the present study, we demonstrated that the ratio and activity of NKT cells in peripheral blood and placental tissues from PIH patients was elevated. In vitro experiments show that NKT cells inhibit the proliferation and migration of vascular endothelial cells by secreting IL-17 (data not shown).
Expression of NKp30, NKG2D and NKG2A receptors on the surface of NKT cells from pregnant women with PIH is altered compared to women with normal pregnancies
To investigate the expression and distribution of activated receptors NKp30 and NKG2D and inhibitory receptors NKG2A and 158 b on NKT cell surface, these receptors were labelled with fluorescent antibodies and flow cytometry was carried out. The data showed that the percentage of NKT cells with positive expression of NKp30 in peripheral blood from PIH patients (64.7 ± 2.8%) was significantly higher than that from the control group (52.5 ± 1.8%) (P < 0.05). The percentage of NKT cells with positive expression of NKG2D in peripheral blood from PIH patients (77.8 ± 1.4%) was not different from that from the control group (73.8 ± 2.4%) (P > 0.05). In addition, the percentages of NKT cells with positive expression of NKp30 or NKG2D in placental tissues from PIH patients were significantly higher than those from the control group (P < 0.05). Regarding the inhibitory receptors, the percentage of NKT cells with positive expression of NKG2A in peripheral blood from PIH patients (31.8 ± 1.8%) was significantly lower than that from the control group (64.8 ± 1.6%) (P < 0.05), but the percentage of NKT cells with positive expression of 158 b in peripheral blood from PIH patients was not different from that from the control group (data not shown). Similarly, the percentage of NKT cells with positive expression of NKG2A in placental tissues from PIH patients (25.8 ± 2.6%) was significantly lower than that from the control group (57.2 ± 2.8%) (P < 0.05), but the percentage of NKT cells with positive expression of 158 b in placental tissues from PIH patients was not different from that from the control group (data not shown) (). The results indicate that the expression of NKp30, NKG2D and NKG2A receptors on the surface of NKT cells from pregnant women with PIH is altered as compared to women with normal pregnancies.
Figure 2. Expression of receptors on the surface of NKT cells in peripheral blood and placental tissues from women with normal pregnancies and women with PIH. Ratios of NKT cells expressing indicated receptors in peripheral blood (a) and placental tissues (b) women with normal pregnancies and women with PIH. Note: Flow cytometry was performed to detect surface receptors. * P < 0.05 compared to women with normal pregnancies.
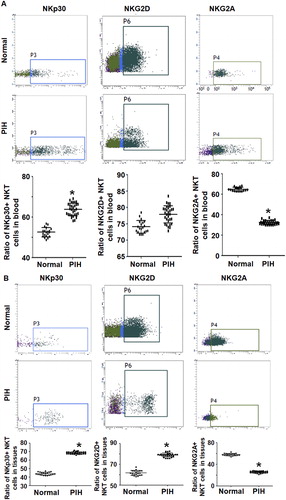
From an immunological point of view, pregnancy is similar to organ transplantation. The embryo with the father’s antigens is a transplant to the mother, so it is recognized by the mother’s immune system and produces an immune response [Citation22]. Normally, the mother produces a protective immune response to the foetus until the foetus is delivered. There are acute atheromatous changes in the uterine spiral arterioles of PIH patients, as well as deposition of IgM and complements on the walls of blood vessels [Citation23,Citation24]. This lesion is similar to that of typical vasculitis seen in graft rejection, which provides evidence for the relationship between hypertensive disorders in pregnancy and immune responses. NKT cells originate from pluripotent hematopoietic stem cells. They are an important part of the body’s natural immune system and a group of heterogeneous immune cells [Citation13]. Similar to NK cells, NKT cells can produce cytotoxic effects spontaneously without the activation of primary immune response, playing important roles in immune surveillance and tumour therapy [Citation25]. In addition, some subgroups of NKT cells express CD1d-restricted TCR on the surface, which can identify limited lipid antigens. After activation, they can secrete a large number of cytokines and play immunomodulatory roles [Citation26]. CD3+CD56+ NKT cells are a group of subsets that are more widely used in clinical researches. These NKT cells have been confirmed to be different subsets from iNKT. CD3+CD56+ NKT cells can be further divided into CD4+CD8−, CD4−CD8+ and CD4−CD8− subtypes, which secrete different cytokines and play different roles [Citation27,Citation28]. Studies have shown that NKT cells secrete many cytokines during pregnancy, regulate the balance of Th1/Th2 cells, and affect the process of pregnancy [Citation29]. However, the proportion and role of NKT cells in PIH are still unclear. The present study showed that the NKT ratio in peripheral blood from PIH patients was elevated. In addition, the expression of activated receptors NKp30 and NKG2D in placental tissues was up-regulated, and the expression of NKp30 was up-regulated only in peripheral blood. By contrast, the expression of inhibitory receptor NKG2A in peripheral blood and placental tissues was both down-regulated. No difference was observed for 158 b expression.
The ratio of CD4+ NKT cells is increased but the ratio of CD8+ NKT cells is decreased in peripheral blood and placental tissues from pregnant women with PIH compared to women with normal pregnancies
To examine the ratios of CD4+, CD8+ and CD4−CD8− subtypes of CD3+CD56+ NKT cells, flow cytometry was carried out. The data showed that the percentage of CD4+ NKT cells in peripheral blood from PIH patients (27.0 ± 0.41%) was significantly higher than that from the control group (8.4 ± 0.77%) (P < 0.05), the percentage of CD8+ NKT cells in peripheral blood from PIH patients (33.7 ± 1.5%) was significantly lower than that from the control group (56.7 ± 0.51%) (P < 0.05), and the percentage of CD4−CD8− NKT cells in peripheral blood from PIH patients (32.7 ± 1.9%) was significantly lower than that from control group (37.4 ± 1.20%) (P < 0.05) (). In addition, the percentage of CD4+ NKT cells in placental tissues from PIH patients (31.8 ± 0.71%) was significantly higher than that from the control group (11.3 ± 0.48%) (P < 0.05), the percentage of CD8+ NKT cells in placental tissues from PIH patients (39.1 ± 2.5%) was significantly lower than that from the control group (58.3 ± 2.7%) (P < 0.05), and the percentage of CD4−CD8− NKT cells in placental tissues from PIH patients (27.4 ± 0.55%) was not significantly different from that from the control group (28.3 ± 0.47%) (P > 0.05) (). The results suggest that the ratio of CD4+ NKT cells is increased but the ratio of CD8+ NKT cells is decreased in peripheral blood and placental tissues from pregnant women with PIH compared to women with normal pregnancies.
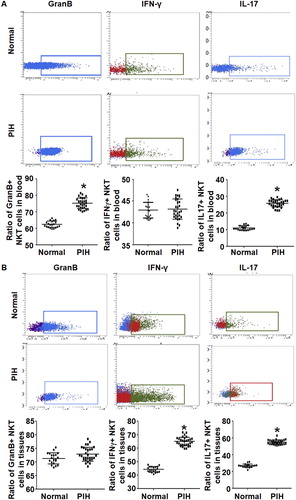
The ratios of GranB+ NKT cells in peripheral blood, IFN-γ-positive T cells in placental tissues and IL-17-positive NKT cells in both peripheral blood and placental tissues are elevated in PIH patients compared to women with normal pregnancies
To test the expression of effector cells and relevant cytokines in NKT cells, flow cytometry was employed. The data showed that the percentage of GranB+ NKT cells in peripheral blood from PIH patients (74.5 ± 1.8%) was significantly higher than that from the control group (62.7 ± 2.6%) (P < 0.05). The percentage of IFN-γ-positive NKT cells in peripheral blood from PIH patients (43.9 ± 1.6%) was not significantly different from that from the control group (42.8 ± 0.85%) (P > 0.05). The percentage of IL-17-positive NKT cells in peripheral blood from PIH patients (25.8 ± 1.8%) was significantly higher than that from the control group (11.7 ± 0.65%) (P < 0.05) (). Moreover, the percentage of GranB+ NKT cells in placental tissues from PIH patients (73.2 ± 2.8%) was not significantly different from that from the control group (71.6 ± 2.4%) (P > 0.05). The percentage of IFN-γ-positive NKT cells in placental tissues from PIH patients (65.9 ± 2.5%) was significantly higher than that from the control group (45.2 ± 1.7%) (P < 0.05). The percentage of IL-17-positive NKT cells in placental tissues from PIH patients (55.2 ± 2.0%) was significantly higher than that from control group (26.3 ± 3.1%) (P < 0.05) (). The results indicate that the ratios of GranB+ NKT cells in peripheral blood, IFN-γ-positive T cells in placental tissues, and IL-17-positive NKT cells in both peripheral blood and placental tissues are elevated in PIH patients compared with normal pregnant women.
Figure 4. Expression of effector cells and cytokines in NKT cells in peripheral blood and placental tissues from women with normal pregnancies and women with PIH. Ratios of NKT cells expressing indicated intracellular markers in peripheral blood (a) and placental tissues (b) from women with normal pregnancies and women with PIH. Note: Flow cytometry was performed to detect intracellular markers. * P < 0.05 compared to women with normal pregnancies.
NKT cells isolated from peripheral blood of PIH patients inhibit the proliferation and migration abilities of HUVECs
To study whether NKT cell activation affects the biological functions of vascular endothelial cells, NKT cells from peripheral blood of PIH patients were co-cultured with HUVECs for 48 h before CCK-8 and transwell assays. CCK-8 assay showed that the absorbance of HUVECs co-cultured with NKT cells was significantly lower than that in the control group at 24 h, 48 h and 72 h (P < 0.05 at all points) (). Transwell assay showed that the number of migrated HUVECs co-cultured with NKT cells (25.7 ± 1.8) was significantly lower than that of HUVECs in the control group (55.7 ± 2.8) (P < 0.05) (). These results suggest that NKT cells isolated from peripheral blood of PIH patients inhibit the proliferation and migration abilities of HUVECs.
Figure 5. Effect of NKT cells and IL-7 on the biological functions of HUVECs. Proliferation (a) and migration (b) of HUVECs co-cultured with NKT cells in the absence and presence of IL-17 antibody (n = 5). Control, NKT cells from healthy subjects co-cultured with HUVECs. * P < 0.05 compared with control; # P < 0.05 compared with co-culture. Proliferation (c) and migration (d) of HUVECs cultured in the absence and presence of IL-17 (n = 5). NC, negative control. * P < 0.05 compared with NC; # P < 0.05 compared with co-culture.Note: Proliferation was determined by CCK-8 assay and migration was detected by transwell assay.
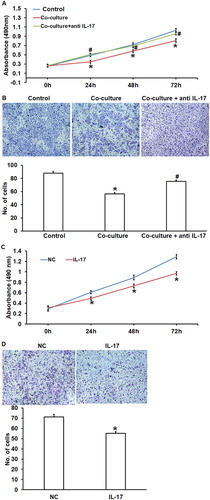
NKT cells from peripheral blood of PIH patients inhibit the proliferation and migration of HUVECs by secreting IL-17
To test whether NKT cells exerted their effect via IL-17, we added IL-17 antibody or IL-17 in the co-cultured NKT cells and HUVECs. The data showed that IL-17 antibody significantly enhanced the proliferation and migration abilities of HUVECs co-cultured with NKT cells (P < 0.05) (). In addition, culture with medium containing IL-17 inhibited the proliferation and migration abilities of HUVECs co-cultured with NKT cells (P < 0.05) (). These results indicate that NKT cells from peripheral blood of PIH patients inhibited the proliferation and migration of HUVECs by secreting IL-17.
Vascular injury is the most common complication of PIH, and its underlying mechanism is endothelial cell injury [Citation30]. As a cell barrier of the intravascular wall, vascular endothelial cells not only act as the physical barrier in the maintenance of vascular function, but also regulate vascular pressure, blood coagulation and inflammation by secreting cytokines and signal substances [Citation31,Citation32]. After vascular injury, endothelium will be repaired by in situ proliferation and distant migration [Citation33]. During pregnancy, NKT cells in placenta and peripheral blood can interact with vascular endothelial cells by secreting cytokines and direct contact [Citation34]. In the present study, we demonstrated that expression of IL-17 in NKT cells from peripheral blood of PIH patients was elevated. Therefore, we tested the effect of NKT cells on HUVECs. Our results showed that NKT cells from PIH patients inhibited the proliferation and migration of HUVECs, and IL-17 antibody reversed this biological effect. IL-17 is a cytokine secreted by various immune cells, and plays important roles in cell proliferation, metastasis and inflammation regulation [Citation35]. It is reported that IL-17 causes elevated blood pressure during pregnancy [Citation36] and induces inflammatory responses of endothelial cells [Citation37]. Our results showed that IL-17 recombinant protein inhibited the proliferation and migration of HUVEC cells, indicating that NKT cells can regulate the biological behaviour of vascular endothelial cells through IL-17.
Conclusions
The present study demonstrated that the ratio and activity of NKT cells were elevated in peripheral blood and placental tissues of PIH pregnant women. In addition, NKT cells from peripheral blood of PIH patients inhibited the proliferation and migration of vascular endothelial cells by secreting IL-17 and hence, affecting the repair of vascular injury.
Acknowledgements
The authors wish to thank their department and research team for their help and dedication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Birdir C, Droste L, Fox L, et al. Predictive value of sFlt-1, PlGF, sFlt-1/PlGF ratio and PAPP-A for late-onset preeclampsia and IUGR between 32 and 37weeks of pregnancy. Pregnancy Hypertension. 2018;12:124–128.
- Ren LQ, Sun XX, Guan Y. Effects of sevoflurane or propofol combined with remifentanil anesthesia on clinical efficacy and stress response in pregnant women with pregnancy-induced hypertension. Eur Rev Med Pharmacol Sci. 2018;22:1825–1829.
- Han N, Li Y, Dong Y. Therapeutic effect of long-term epidural block in rats with pregnancy induced hypertension. Biomed Res Int. 2018;2018:1. [cited 2018 Oct 22]. doi:10.1155/2018/1639623
- Swiatkowska-Stodulska R, Kmiec P, Stefanska K, et al. Renin-Angiotensin-aldosterone system in the pathogenesis of pregnancy-induced hypertension. Exp Clin Endocrinol Diab. 2018;126:362–366.
- Tripathy K, Chawla R, Mutha V, et al. Spontaneous suprachoroidal haemorrhage with exudative retinal detachment in pregnancy-induced hypertension. BMJ Case Rep. 2018;[cited 2018 Oct 22]2018. doi:10.1136/bcr-2017-223907
- Sentilhes L, Azria E, Schmitz T. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:2399–2400.
- Wang C, Liu X, Kong D, et al. Apelin as a novel drug for treating preeclampsia. Exp Ther Med. 2017;14:5917–5923.
- Singh SK, Bhatia K. Ultrasonographic optic nerve sheath diameter as a surrogate measure of raised intracranial pressure in severe pregnancy-induced hypertension patients. Anesth Essays Res. 2018;12:42–46.
- Jammalamadaga VS, Abraham P. Spectrum of factors triggering endothelial dysfunction in PIH. J Clin Diagn Res. 2016;10:Bc14–Bc17.
- Sharma R, Magoon R, Choudhary R, et al. Anaesthesia for emergency caesarean section in a morbidly obese achondroplastic patient with PIH: feasibility of neuraxial anaesthesia?. Indian J Anaesth. 2017;61:77–78.
- Wang J, Su L, Zhu T, et al. [Changes in the subsets of dendritic cells and T cells in peripheral blood of patients with preeclampsia]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.]. 2013;29:72–75. Chinese.
- Zhou Y, Huang P, Zhang P, et al. [Changes in the expression of three markers in T lymphocytes of peripheral blood and immunoregulatory mechanisms of burned mice with sepsis at early stage]. Zhonghua Shao Shang Za Zhi [Chin J Burns]. 2016;32:89–96. Chinese.
- Tan X, Ding Y, Zhu P, et al. Elevated hepatic CD1d levels coincide with invariant NKT cell defects in chronic hepatitis B virus infection. JI.. 2018;200:3530–3538.
- Schonrich G, Raftery MJ. CD1-restricted T cells during persistent virus infections: “sympathy for the devil”. Front Immunol. 2018;[cited 2018 Oct 22]9:545.
- Nishioka Y, Masuda S, Tomaru U, et al. CD1d-restricted type II NKT cells reactive with endogenous hydrophobic peptides. Front Immunol. 2018;[cited 2018 Oct 22]9:548. doi:10.3389/fimmu.2018.00548
- Gharagozloo M, Rezaei A, Kalantari H, et al. Decline in peripheral blood NKG2D + CD3 + CD56+ NKT cells in metastatic colorectal cancer patients. BLL.. 2018;119:6–11.
- Nowicka D, Grywalska E, Fitas E, et al. NK and NKT-like cells in patients with recurrent furunculosis. Arch Immunol Ther Exp (Warsz). 2018;66:315–319.
- Maru L, Verma M, Jinsiwale N. Homocysteine as predictive marker for pregnancy-induced hypertension-a comparative study of homocysteine levels in normal versus patients of PIH and its complications. J Obstet Gynecol India. 2016;66:167–171.
- Takahashi Y, Yamashita T, Morihara R, et al. Emergency caesarean section saved both an anti-MuSK antibody-positive myasthenia gravis mother with pregnancy-induced hypertension and her premature baby. Intern Med.. 2017;56:3361–3364.
- Maruta E, Wang J, Kotani T, et al. Association of serum asymmetric dimethylarginine, homocysteine, and l-arginine concentrations during early pregnancy with hypertensive disorders of pregnancy. Clin Chim Acta; Int J Clin Chem. 2017;475:70–77.
- Ding J, Kang Y, Fan Y, et al. Efficacy of resveratrol to supplement oral nifedipine treatment in pregnancy-induced preeclampsia. Endocr Connect. 2017;6:595–600.
- Laule CF, Wing CR, Odean EJ, et al. Effect of nicotine on placental ischemia-induced complement activation and hypertension in the rat. J Immunotoxicol. 2017;14:235–240.
- Jafri S, Ormiston ML. Immune regulation of systemic hypertension, pulmonary arterial hypertension, and preeclampsia: shared disease mechanisms and translational opportunities. Am J Physiol Regul Integr Comp Physiol. 2017;313:R693–r705.
- Persson G, Melsted WN, Nilsson LL, et al. HLA class Ib in pregnancy and pregnancy-related disorders. Immunogenetics. 2017;69:581–595.
- Hosseini S, Shokri F, Pour SA, et al. Diminished frequency of menstrual and peripheral blood NKT-like cells in patients with unexplained recurrent spontaneous abortion and infertile women. Reprod Sci. 2018; [cited 2018 Oct 22]:1933719118766261.
- Kasler HG, Lee IS, Lim HW, et al. Histone deacetylase 7 mediates tissue-specific autoimmunity via control of innate effector function in invariant natural killer T cells. eLife. 2018; [cited 2018 Oct 22]7.
- Thanapati S, Ganu MA, Tripathy AS. Differential inhibitory and activating NK cell receptor levels and NK/NKT-like cell functionality in chronic and recovered stages of chikungunya. PLoS One. 2017;12:e0188342. [cited 2018 Oct 22].
- Das R, Tripathy A. Increased expressions of NKp44, NKp46 on NK/NKT-like cells are associated with impaired cytolytic function in self-limiting hepatitis E infection. Med Microbiol Immunol. 2014;203:303–314.
- Zhang Y, Wang Y, Li MQ, et al. IL-25 promotes Th2 bias by upregulating IL-4 and IL-10 expression of decidual γδT cells in early pregnancy. Exp Ther Med. 2018;15:1855–1862.
- Feng Y, Wang N, Xu J, et al. Alpha-1-antitrypsin functions as a protective factor in preeclampsia through activating Smad2 and inhibitor of DNA binding 4. Oncotarget. 2017;8:113002–113012.
- Oliveira JSS, Santos GDS, Moraes JA, et al. Reactive oxygen species generation mediated by NADPH oxidase and PI3K/Akt pathways contribute to invasion of Streptococcus agalactiae in human endothelial cells. Mem Inst Oswaldo Cruz. 2018;113:e140421 [cited 2018 Oct 22].
- Wang J, Wang Y, Li Y, et al. Platelet-rich plasma protects HUVECs against oX-LDL-induced injury. Open Med (Wars). 2018;13:41–52.
- Singh N, Geethika M, Eswarappa SM, et al. Manganese-based nanozymes: multienzyme redox activity and effect on the nitric oxide produced by endothelial nitric oxide synthase. Chem Eur J.. 2018;24:83–93. [cited 2018 Oct 22].
- Hoya M, Nagamatsu T, Fujii T, et al. Impact of Th1/Th2 cytokine polarity induced by invariant NKT cells on the incidence of pregnancy loss in mice. Am J Reprod Immunol. (NY: 1989). 2018;[cited 2018 Oct 22]79: e12813.
- Jiang Y, Liu Y, Lu H, et al. Epigenetic activation during T helper 17 cell differentiation is mediated by Tripartite motif containing 28. Nat Commun. 2018; [cited 2018 Oct 22]9:1424.
- Cornelius DC, Wallace K, Kiprono L, et al. Endothelin-1 is not a mechanism of IL-17 induced hypertension during pregnancy. Med J Obstet Gynecol. 2013; [cited 2018 Oct 22]1:1006.
- Xing X, Yang J, Yang X, et al. IL-17A induces endothelial inflammation in systemic sclerosis via the ERK signaling pathway. PLoS One. 2013;8:e85032. [cited 2018 Oct 22].