Abstract
Rice blast is one of the most serious rice diseases. It is caused by Magnaporthe oryzae resulting in rice shortages. Biological control has become a new measure in the control of plant diseases. In this study, we isolated antagonistic bacteria from the traditional Chinese medicinal plant Baizhi (Angelica dahurica) against M. oryzae. Biochemical, physiological and 16S rDNA sequence analysis proved that it was Bacillus amyloliquefaciens and we named the strain Rdx5. The experiment proved that Rdx5 can produce cellulase, protease, indole-3-acetic acid and 1-amino-cyclopropane-1-carboxylate deaminase. The sterilized culture filtrate of Rdx5 had strong antifungal activity after treatment at 100 °C for 30 min. In addition, its antifungal activity did not change in strong acid, neutral and weak alkaline environment. However, under strong alkaline conditions, the antifungal activity decreased significantly. In vitro and in vivo experiments showed that Rdx5 could prevent and treat rice blast, since the prevention group performed better than the treatment group. B. amyloliquefaciens Rdx5 might provide an alternative resource for the biocontrol of rice blast.
Introduction
Rice feeds half the world’s population, while rice blast is a destructive disease that results in significant crop loss [Citation1,Citation2]. Therefore, it is particularly important and meaningful to carry out comprehensive measures for effective control of rice blast. This is currently achieved through planting resistant rice varieties or spraying chemical pesticides in agricultural production [Citation3]. However, due to the complexity in the aspect of genetic background of rice blast, physiological races can evolve easily to adapt to resistant rice varieties. Although a chemical fungicide has many advantages like low cost and high effectiveness, its overuse not only pollutes the environment but also enhances drug resistance of rice blast [Citation4]. With the merits of simplicity, high efficiency and no environment impact, biological control is more superior to chemical fungicides [Citation5]. In recent years, it has been widely used for biological control of microorganisms including Bacillus spp., Actinomycetes, Pseudomonas spp. and other antifungal bacteria that produce a series of antibiotics and enzymes [Citation6]. As a non-pathogenic bacterium, Bacillus spp. exists universally in nature and can produce a variety of antifungal substances. Since it was reported that Bacillus subtilis can produce antifungal substances, Bacillus spp. have attracted wide attention [Citation7]. Bacillus amyloliquefaciens has some potential applications in the biological control of plant diseases. For example, B. amyloliquefaciens strain RC-2 controlled disease on mulberry leaves through inhibiting against Colletotrichum dematium caused by Mulberry anthracnose [Citation8]. B. amyloliquefaciens as antagonists inhibited the growth and production of mycelia and sclerotia for control of Sclerotinia sclerotiorum [Citation9]. Understanding the mechanisms of action of the antagonistic endophytic B. amyloliquefaciens Bg-C31 is helpful in the control of plant diseases [Citation10]. Thus, B. amyloliquefaciens not only has a broad spectrum for plant pathogenic bacteria, but also has unlimited potential for use in biological control.
In this study, we isolated and screened an antifungal bacterial strain Rdx5 with strong inhibitory effect against Magnaporthe oryzae. It was proved to be B. amyloliquefaciens based on its morphology, biochemical and physiological properties and 16S rDNA sequence analysis. Furthermore, a series of preliminary tests were designed to validate its potential value as a biological control agent.
Materials and methods
Determination of in vitro antifungal activity against test phytopathogens
The target phytopathogenic fungal species, M. oryzae (physiological race of Guy11), was kindly provided by the Key Laboratory of Major Crop Diseases, Rice Research Institute, Sichuan Agricultural University (Chengdu, China) and maintained on complete medium (10.0 g, glucose; 2.0 g, peptone; 1.0 g, yeast extract; 1.0 g, acid hydrolyzed casein; 0.1% trace elements; 0.1% vitamin; 5% nitrogen source; 15.0 g agar; distilled water to1000 mL; 1 mol/L NaOH; adjusted to pH to 6.5).
Isolation of the antagonistic bacteria and the antifungal activity
During the screening related to the ingredients active against rice blast from a traditional Chinese herbal medicines library [Citation11], we accidentally discovered that several bacterial strains had inhibitory effects on M. oryzae. Then the microbial colonies were selected, inoculated in Potato Dextrose Agar (potato, 200.0 g; glucose, 20.0 g; agar, 20.0 g; distilled water, 1000 mL), and maintained under controlled conditions at 28 °C for 24 h. Then, a single colony was screened from several bacterial strains that had inhibitory effects on M. oryzae on Potato Dextrose Agar and was maintained in an aqueous solution containing 50% glycerol at −70 °C. Landy medium (glucose, 20.0 g; L-glutamate, 5.0 g; MgSO4, 0.5 g; KCl, 0.5 g; KH2PO4, 0.52g; FeSO4,0.15 mg; MnSO4, 5 mg; CuSO4, 0.16 mg; distilled water, 1000 mL) was used as a liquid medium. After incubation for 1–2 days at 37 °C, strain Rdx5, appeared on the plates and was isolated as single colonies on PSA (potato, 200 g; sucrose, 20 g; agar, 15 g; distilled water, 1000 mL) [Citation12]. To test the antifungal activity of strain Rdx5, the strain was transferred to 1 mL of Landy liquid medium in a 500 mL tube and incubated in shake flasks at 180 rpm at 37 °C for 2 days. Then, 10 μL fermented liquid and sterilized culture filtrate of Rdx5 were put on the PDA for confrontation training with M. oryzae at 28 °C. Finally, the confrontation training results were observed overnight. The inhibitory activity of the fermented liquid and sterilized culture filtrate of Rdx5 against M. oryzae growth was recorded as the percentage reduction of mycelia growth in comparison with that of the control plates.
Evaluation of antifungal activity
To test the inhibition of M. oryzae, we inoculated strain Rdx5 in Landy liquid medium and incubated it at 37 °C, 180 rpm in a rotary shaker. The fermentation broth was taken out at 48 h after inoculation and centrifuged at 8500 rpm at room temperature for 15 min to remove bacterial cells. The supernatant was filtered through a 0.22-μm membrane filter and then the different volume of the culture filtrate (30, 100, 300, 1000 and 3000 μL) was mixed with PDA to different concentrations (0.67, 2.2, 6.67, 22 and 66.7 μL/mL). Then, the mixture was poured into Petri dishes. Fungus cake of Guy11 was transferred to the centre of the medium and was incubated at 28 °C in the incubator. The M. oryzae colony diameter was measured after 7 days. The relative inhibition rate was calculated as follows:
Relative inhibition (%) = [(R1 − R2)/R1] × 100where R1 is the M. oryzae colony diameter in the control and R2 is the M. oryzae colony diameter in the dual culture plates with sterilized culture filtrate of Rdx5 mixed with PDA.
Identification of strain Rdx5
Biochemical and physiological procedures were employed to identify strain Rdx5. These methods included cell staining, reactions with catalase and oxidase, fermentation assay [Citation13] and 16S rDNA sequence analysis. The primers used for polymerase chain reaction (PCR) amplification of the bacterial 16S rDNA were 8-27Fw (5′-AGAGTTTGATCCTGGCTCAG-3′ and 1522-1541Re (5′-AAGGAGGTGATCCAGCCGCA-3; by TSINGKE (Chengdu, China) [Citation14]. The 50-μL reaction mixture of PCR was placed in a small 50 μL mixture of PCR containing 2 μL of forward primer, 2 μL of reverse primer, 10 μL of 5 × TransStart Fastplu fly Buffer, 1 μL of TransStart Fastplu Fly DNA Polymerase, 2 μL of the extracted DNA, 5 μL of 2.5 mM High pure dNTPs and 28 μL of sterile water. The PCR program was an initial denaturation for 5 min at 95 °C, followed by 25 cycles of denaturation (0.5 min at 95 °C), annealing (0.5 min at 55 °C) and extension (10 min at 72 °C), plus a final extension for 10 min at 72 °C. The PCR products were purified through a PCR purification kit and identified by horizontal electrophoresis on 1% agarose gel. The fragments of amplified 16S rDNA were transformed into Escherichia coli. The results were sequenced by TSINGKE (Chengdu, China) and compared with similar 16S rDNA sequences retrieved from the DNA databases by using the BLAST search program in the National Center for Biotechnology Information (NCBI). A neighbour-joining phylogenetic tree of Rdx5 based on 16S rDNA sequence analysis was constructed using MEGA5.
Correlation between cell growth and antifungal activity
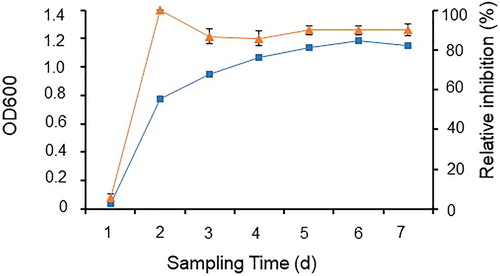
In this series of experiments, 500 mL of Landy medium in a 1-L flask was inoculated with 1% of strain Rdx5 for overnight culture at 37 °C 180 rpm for 72 h. During incubation, 1 mL of the culture broth was sampled at intervals to measure its OD600 and test its antifungal activity against M. oryzae [Citation15].
Effects of pH and temperature on the stability of antifungal metabolites
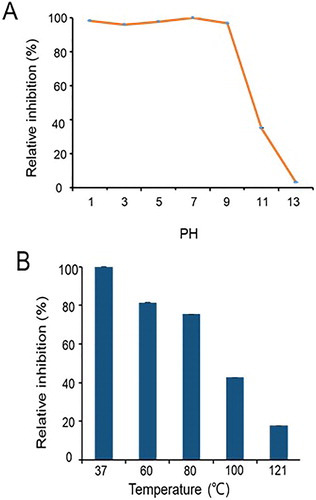
In the test for pH stability, samples of the filtered and sterilized culture broth of strain Rdx5 were adjusted to various pH in the range from 1.0 to 14.0 and maintained at 4 °C for 24 h. Antifungal activity was assayed after the samples had been readjusted to pH 7.0. A similar procedure was adopted to assess thermal stability of the antifungal metabolites produced by strain Rdx5. Separate samples of the sterilized culture filtrate were treated at temperatures of 37, 60, 80, 100 and 121 °C for 30 min and then, antifungal activity was assayed after the samples had been cooled to room temperature.
The antifungal activity of the samples against M. oryzae after the treatments was determined using the procedure described above. The relative remaining activity was measured by comparing the samples treated with pH and temperatures with the samples held at pH 7.0 and room temperature, respectively. The experiment was repeated three times and the data were collected for analysis.
Determination of enzymes and metabolites produced by B. amyloliquefaciens Rdx5
The experimental methods to test the produced enzymes and metabolites included cellulase, protease, hydrocyanic acid, peroxidase, chitinase and gelatinase production. All experiments were performed at 37 °C for 2 days [Citation16–18].
In vitro vs. in vivo inoculation for biological control against blast fungus
In vitro experiments on leaves
The rice susceptible variety Lijiangxintuan was planted into the field after 3% hydrogen peroxide disinfection. Healthy, 7-cm long and disease-free rice leaves were placed in Petri dishes containing 25 mL 6-benzylaminopurine adjusted to pH 7.0 by diluted HCl. The concentration of M. oryzae (Guy11) spore suspension 1x105 cfu/mL (0.1% Tween 20) was obtained from incubation on CM plate at 25 °C for 12–15 days [Citation19].
The experiment was divided into prevention, treatment and control groups [Citation20]. In the prevention group, 10 μL of the fermented liquid and sterilized culture filtrate of Rdx5 were dropped into the prepared hole and the leaves were incubated at 25 °C in the dark for 24 h. Then 10 μL of the spore suspension of M. oryzae was dropped into the prevention group and treatment group, respectively, at the same time. The method was the same as with the prevention group. In the treatment group, 10 μL of the spore suspension of M. oryzae were dropped in the prepared hole at 25 °C in the dark for 24 h subsequently, all were incubated in the light at room temperature [Citation21]. In the control group, sterile water (negative control) and 100 mg/mL of carbendazim (positive control) was used instead of the sterilized fermented liquid and sterilized culture filtrate and the other steps were the same. After 5–7 days, the lesion length was measured.
In vivo experiments on rice
Fifty rice plants of the susceptible variety Lijiangxintuan were grown healthily in the field for 15–20 days. The in vivo experiments on rice were the same as the in vitro experiments on leaves. A few steps were different: dropping of 10 μL spore suspension of M. oryzae was replaced with spraying of 10 mL for inoculation and the fermented liquid and sterilized culture filtrate of Rdx5 was increased from 10 μL to 100 mL. In addition, the rice plants were covered with a plastic membrane that keeps the relative humidity to create conditions for the rice blast to occur. Overnight, the film was removed to expose the rice to ensure normal growth. After 5–7 days, the field incidence was recorded.
Data analysis
The experiments were repeated at least in three times. Statistical analysis of the data was done using SPSS 20.0 software. Values p < 0.05 and p < 0.01 were considered to indicate statistical significance. All data are expressed as mean values with standard deviation (±SD).
Results and discussion
Isolation of antifungal strains from Baizhi
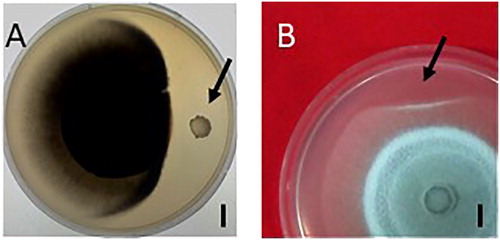
The use of antagonistic microorganisms during plant growth to control plant diseases is an active area of research [Citation22,Citation23]. Practice has proved that the application of antagonistic bacteria in biological control of plant diseases is an economical and effective method, while the traditional chemical control method will not only cause certain resistance to plant pathogens, but also damage the environment. Single bacteria can produce systemic resistance through different mechanisms [Citation24]. Isolation of single bacteria in the laboratory can be used to evaluate their performance in rice disease management. In our study, we screened out strain Rdx5 as having strong antagonistic activity against M. oryzae (). The sterilized culture filtrate of this antifungal strain also had the same inhibititory effect (). Its strong antifungal activity and lack of toxicity to plants provide strong evidence in support of its potential use in the biological control of plant diseases.
Evaluation of antifungal activity
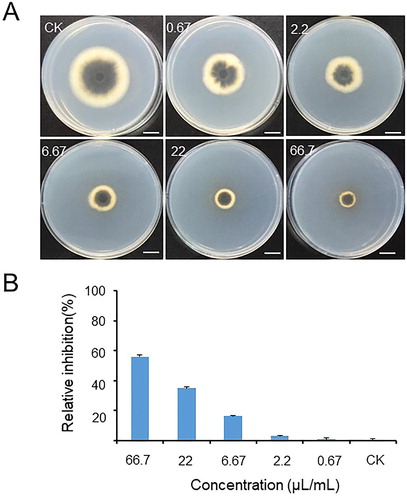
The sterilized culture filtrate of Rdx5 had significant inhibitory effect on the growth of M. oryzae (). The inhibitory activity was directly proportional to the concentration of the sterilized culture filtrate. When the concentration was 66.70 μL/mL, the relative inhibitory rate was 55.9%. These results indicated that strain Rdx5 had strong antagonistic effect against M. oryzae and a great research value.
Figure 2. Effect of different concentrations of the sterilized culture filtrate of Rdx5 on the inhibition of M. oryzae growth. Note: Bar = 1 cm. The inhibitory effect of different concentrations of Rdx5 sterilized culture filtrate on the growth of M. oryzae (A), and the relative inhibitory rate of different concentrations of Rdx5 sterilized culture filtrate on the growth of M. oryzae (B).
Identification of strain Rdx5
Morphological, physiological and metabolic characteristics of strain Rdx5 are summarized in [Citation25–27]. It is a gram-positive, motile, spore forming, rod-like and oval-shaped bacterium (900–4000 nm) (). The sequence analysis (1653 bp) of 16S rDNA (see the Supplemental Data) demonstrated that strain Rdx5 had 99.9% similarity with B. amyloliquefaciens BCRC 11 (NR 116022.1) from NCBI (http://www.ezbiocloud.net). A phylogenetic tree displayed the relationship between strain Rdx5 and other strains, as shown in . Due to the potential application of Rdx5 as a biocontrol agent, we deposited it in the Chinese Typical Culture Collection Center (CCTCCNO: M2017406).
Figure 3. Morphological characteristics of Rdx5. Colony morphology (A); Gram staining (B); Scanning electron micrograph (C); Flagella staining (D); Spore staining (E); Amylolysis (F). Note: Magnification 400× (B); 10,000× (C and D). Bar = 1cm.
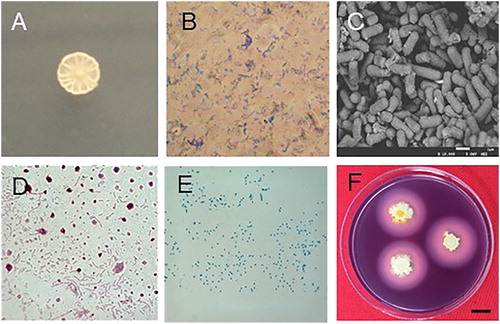
Figure 4. Neighbour-joining phylogenetic tree of Rdx5 based on 16S rDNA sequence analysis. Bar = 1 cm.
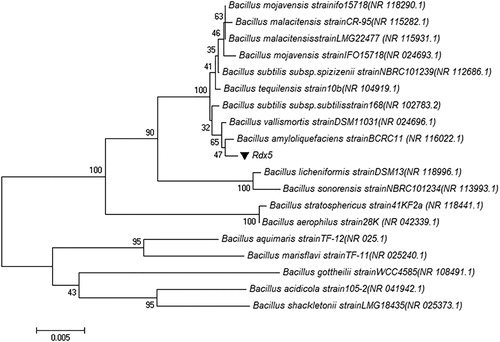
Table 1. Comparison of physiological and biochemical results of strain Rdx5 and the standard strain of B. amyloliquefaciens.
The stability of the antifungal activity
The results shown in demonstrate that the antifungal activity of the culture filtrate of strain Rdx5 against M. oryzae was significant under pH range 1–9. However, with the increase of alkaline, this activity remarkably decreased. In addition, the antifungal activity of the culture filtrate of strain Rdx5 against M. oryzae exhibited thermal stability when the culture filtrate was exposed to 37, 60 and 80 °C. When the culture filtrate was exposed to 100 °C, the inhibition rate decreased significantly to 50% (). By evaluating the influence of pH and temperature on the stability of antifungal metabolites, the experiment showed that the antifungal metabolites were stable at high temperature, strong acid and weak base. This lays a good foundation for biological control [Citation18].
Growth curve of strain Rdx5 and its production of antifungal metabolites
Bacteria are cultivated in limited environments such as laboratories. After 1 day of culture, Rdx5 entered into the logarithmic growth period, reached its stationary phase and showed the strongest antifungal activity after 2 days. It is reported that the synthesis of antibiotics is usually at the end of the exponential stage of bacterial growth and reaches its maximum concentration after cell growth stops, which does not fully conform to the production kinetics of strain Rdx5. As shown in , strain Rdx5 grew relatively quickly. It reached stationary phase 1 day after inoculation and there was almost no lag phase. With the increase of cultivation time, the concentration of Rdx5 increased and the growth trend showed an S curve. After 1 day, Rdx5 entered the logarithmic growth period when the cells reproduced the fastest. Moreover, the antifungal activity of the filtrate sampled at different time intervals was significantly correlated with the growth of the strain over the two-day growth period. The strongest antifungal activity against M. oryzae was obtained 2 days after incubation, and then the activity declined over the next 1 day. It seems that the antibiotic produced by strain Rdx5 was positively correlated with cell growth. Further research is needed to study the dynamics of antibiotics produced by strain Rdx5 and to optimize the media design and product recovery procedures. It may therefore be concluded that the optimal harvest time for the antifungal metabolites of strain Rdx5 is 2 days after inoculation, under the culture conditions used in this study.
Enzymes and secondary metabolites production by Rdx5
When B. amyloliquefaciens Rdx5 acts as a biological control agent, the resulting metabolites can promote the disease resistance of plants and induction systems, as well as the synthesis of a variety of organic acids, enzymes and physiologically active substances [Citation28,Citation29]. Our results showed that Rdx5 can produce a series of enzymes, laying the basis for studying the mechanism of action of M. oryzae (). It was reported that most antagonistic bacteria can secrete a series of cell-wall-degrading enzymes, resulting in degradation of cell wall and abnormal mycelium morphology [Citation30]. Experiments showed that a series of extracellular enzymes produced by Rdx5 include cellulase and protease. Rdx5 also secreted IAA and ACC deaminase, both of which can promote plant growth by reducing the effect of ethylene [Citation31]. The production of active secondary metabolites and fungal cell-wall-degrading enzymes is a prominent character of many biological control agents. Rdx5 could produce protease and cellulase, as indicated by clear zones surrounding the colonies on the test media. Although chitinase is an important enzyme degrading cell walls, and HCN could also suppress the disease development, we were unable to find their production by Rdx5 (). Some secondary metabolites might promote plant growth, which improved host plant resistance against pathogens. Rdx5 could produce plant hormone IAA and ACC deaminase, promoting plant growth by reducing the harmful effects. As known, plant-growth promoting microorganisms can indirectly improve the disease resistance of plants [Citation5]. Perhaps B. amyloliquefaciens Rdx5 protects rice against M. oryzae also by promoting rice growth. Therefore, further studies are conducted to identify compounds that are resistant to M. oryzae in Rdx5.
Figure 7. Production of enzymes and secondary metabolites by Rdx5. Chitinase test (A); Peroxidase test (B); HCN test (C); Protease test (D); Gelatinase assay (E); Cellulase assay (F). Note: Bar = 1 cm.
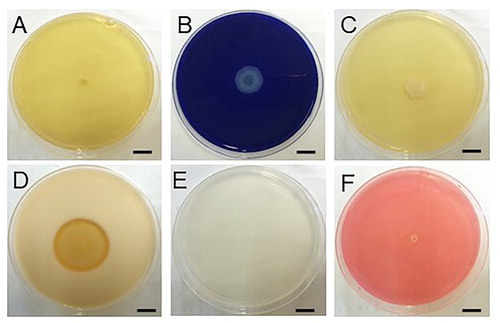
Table 2. Products secreted by Rdx5
Evaluation of biocontrol efficacy
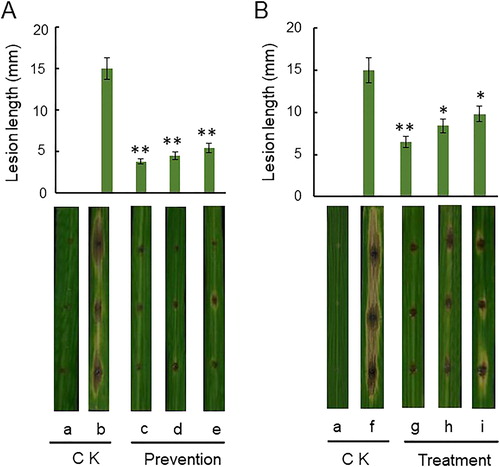
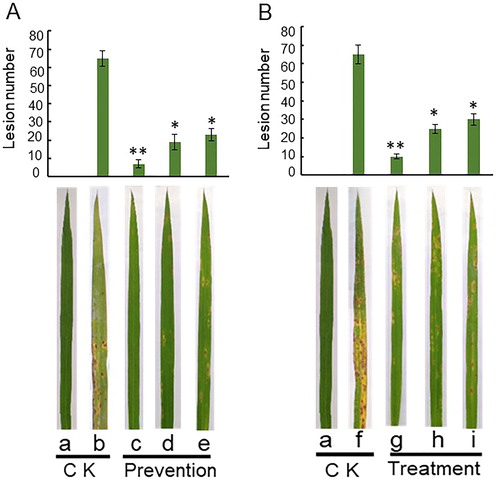
As shown in and Citation9, Rdx5 significantly inhibits the growth of rice blast as shown by in vitro and field experiments. In , the lesion diameter of the leaves in the prevention group (d and e) was smaller than that in the control group (b) (p < 0.01). In , the number of lesions in the treatment group (h) and (i) was smaller than in the control group (f) (p < 0.05). In the in vitro leaf experiments, the prevention group and the treatment group had similar results that the bacteria fermentation broth and sterilized culture filtrate of Rdx5 on blast disease and strain Rdx5 has significant preventive and therapeutic effects. By comparing the prevention group and treatment group (), this indicates that strain Rdx5 had a significant preventive effect, which was higher than the therapeutic effect. At the same time, the fungicide carbendazim group (c and g) was more effective than the control group (b and f) in the prevention and treatment of rice blast (p < 0.01) and the preventive effect was better than that of the therapeutic effect. In addition, the bacterial fermentation broth and the sterilized culture filtrate of Rdx5 had a similar effect on blast disease to that of the fungicide carbendazim. These similar effects provide reliable basis for further study of strain Rdx5 as a new biocontrol agent to control rice blast in agricultural production.
Figure 8. Biocontrol efficiency of Rdx5 against M. oryzae evaluated based on the size of lesions on rice leaves. Upper panel: Preventive effect (A) and therapeutic effect (B); mean values from three experiments on nine leaves. The leaves were incubated 5 to 7 days. *p < 0.05 level, **p < 0.01 level. Lower panel: Representative images. Control group, without any treatment (a). CK, sterile water was inoculated to slightly punctured sites of leaves. Preventive effect: Sterile water was inoculated to slightly punctured sites of leaves and then spore suspension of M. oryzae (b), 100 mg/mL of carbendazim (c), bacterial suspension of Rdx5 (d) or sterilized culture filtrate of Rdx5 (e) were applied to slightly punctured sites of leaves and then spore suspension of M. oryzae was inoculated. Therapeutic effect: Spore suspension of M. oryzae was inoculated to slightly punctured sites of leaves and then sterile water (f); 100 mg/mL of carbendazim (g), bacterial suspension of Rdx5 (h) or sterilized culture filtrate of Rdx5 (i) were applied to the same site.
As shown in , the lesion diameter of the leaves in the prevention group (d and e) was smaller than that in the control group (b) (p < 0.05). In , the number of lesions in the treatment group (h and i) was smaller than that in the control group (f) (p < 0.05). In the in vitro leaf experiments, the prevention group and the treatment group had similar results to the bacterial fermentation broth and sterilized culture filtrate of Rdx5 on blast disease, and strain Rdx5 had significant preventive and therapeutic effects. By comparing the prevention group and the treatment group (), this indicates that strain Rdx5 had a significant preventive effect, which was higher than the therapeutic effect. At the same time, the fungicide carbendazim group (c and g) was more effective than the control group (b and f) in the prevention and treatment of rice blast (p < 0.01) and the preventive effect was better than that of the therapeutic effect. In addition, the bacterial fermentation broth and the sterilized culture filtrate of Rdx5 had a similar effect on blast disease to that of the fungicide carbendazim. It may therefore be suggested that Rdx5 could be used as a biological control agent against rice blast. We found that the preventive effect was better than the therapeutic effect. The results showed that strain Rdx5 had a significant preventive effect, which was stronger than the therapeutic effect. In addition, in both the prevention group and the treatment group, the bacterial fermentation broth and the sterilized culture filtrate of strain Rdx5 had a similar effect on blast disease to that of the fungicide carbendazim. In other words, Strain Rdx5 showed a similar effect on blast disease to that of the fungicide carbendazim. Therefore, it can be considered that strain Rdx5 in the prevention group can effectively contribute to the resistance to plant disease by producing antifungal metabolites and growth promoters. In short, B. amyloliquefaciens Rdx5 had a strong and inhibitory effect on the fungus causing rice blast and could be further explored as a potentially efficient biological control agent against rice blast in agricultural applications. Although the mechanism of B. amyloliquefaciens Rdx5 to control blast disease is still unclear, we will further study this biological control mechanism [Citation32,Citation33].
Figure 9. Biocontrol efficiency of Rdx5 against M. oryzae on rice evaluated based on the number of lesions on leaves. Upper panel: Preventive effect (A) and therapeutic effect (B). Mean values from three experiments on fifty rice plants. The rice plants were incubated 5 to 7 days. Lower panel: Representative images. Control group, without any treatment (a). CK, sterile water was inoculated to the leaves. *p < 0.05level, **p < 0.01. Preventive effect: Sterile water was inoculated to the leaves and then spore suspension of M. oryzae (b), 100 mg/mL of carbendazim (c), bacterial suspension of Rdx5 (d) or sterilized culture filtrate of Rdx5 (e) were applied to slightly punctured sites of leaves and then spore suspension of M. oryzae was inoculated. Therapeutic effect: Spore suspension of M. oryzae was inoculated to the leaves and then sterile water (f), 100 mg/mL of carbendazim (g), bacterial suspension of Rdx5 (h) or sterilized culture filtrate of Rdx5 (i) were applied to the same site.
Biological control is an important part of integrated disease management, so it is important to find the anti-disease mechanism of the antifungal compounds and to evaluate the control effect in field conditions [Citation34,Citation35].
Conclusions
In this study, we isolated antagonistic bacteria from the traditional Chinese medicinal plant Baizhi (Angelica dahurica) active against M. oryzae. Biochemical, physiological and 16S rDNA sequence analysis proved that the isolated strain Rdx5 was B. amyloliquefaciens. The sterilized culture filtrate of Rdx5 had strong antifungal activity after treatment at high temperature, strong acid, neutral and weak alkaline environment. More experiments proved that Rdx5 can produce cellulase, protease, indole-3-acetic acid and 1-amino-cyclopropane-1-carboxylate deaminase. Strain Rdx5 could prevent and treat rice blast in vitro and in vivo. B. amyloliquefaciens Rdx5 might provide an alternative resource for the biocontrol of rice blast. This study provided useful information towards the development of new low cost, low toxicity and low residue biocontrol and microbial agents.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The work was supported by the National Natural Science Foundation of China under Grant number 31271802.
References
- Li W, Zhu Z, Chern M, et al. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell. 2017;170:114–126.
- Rossman AY, Howard RJ, Valent B. Pyricularia grisea. The correct name for the rice blast disease fungus. Mycologia. 1990;82:509–512.
- Ishii H. Impact of fungicide resistance in plant pathogens on crop disease control and agricultural environment. JARQ. 2006;40:205–211.
- Barbosa MS, Barbosa SN, Nagamoto NS, et al. Lack of correlation between micro fungi species and chemical control method of atta treated with toxic baits. Cienc Rural. 2018;48:e20170353. http://10.1590/0103-8478cr20170353
- Raupach GS, Kloepper JW. Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology. 1998;88:1158–1164.
- Shan H, Zhao M, Chen D, et al. Biocontrol of rice blast by the phenaminomethylacetic acid producer of Bacillus methylotrophicus strain BC79. Crop Prot. 2013;44:29–37.
- Kloepper JW, Ryu CM, Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94:1259–1266.
- Yoshida S, Hiradate S, Tsukamoto T, et al. Antimicrobial activity of culture filtrate of Bacillus amyloliquefaciens RC-2 isolated from mulberry leaves. Phytopathology. 2001;91:181–187.
- Mansourt A, Niday A, Patrice S. Biological control of Sclerotinia sclerotiorum (lib.) de bary with Trichoderma harzianum and Bacillus amyloliquefaciens. Crop Prot. 2008;27:1354–1359.
- Hu HQ, Li XS, He H. Characterization of an antimicrobial material from a newly isolated Bacillus amyloliquefaciens from mangrove for biocontrol of capsicum bacterial wilt. Biol Control. 2010;54:359–365.
- Seneviratne CJ, Wong RWK, Samaranayake LP. Potent anti-microbial activity of traditional Chinese medicine herbs against Candida species. Mycoses. 2010;51:30–34.
- Zhao Z, Wang Q, Wang K, et al. Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal components. Biores Technol. 2010;101:292–297.
- Vaneechoutte M, Devriese LA, Dijkshoorn L, et al. Acinetobacter baumannii-infected vascular catheters collected from horses in an equine clinic. J Clin Microbiol. 2000;38:4280–4281.
- Nick G, De LP, Eardly BD, et al. Sinorhizobium arboris sp. nov. and Sinorhizobium kostiense sp. nov., isolated from leguminous trees in Sudan and Kenya. Int J Syst Bacteriol. 1999;49:1359–1368.
- Liu CH, Chen X, Liu TT, et al. Study of the antifungal activity of Acinetobacter baumannii LCH001 in vitro and identification of its antifungal components. Appl Microbiol Biotechnol. 2007;76:459–466.
- Agrawal T, Kotasthane AS. Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chhattisgarh in Central India. SpringerPlus. 2012;1:1–10.
- Gopalakrishnan S, Pande S, Sharma M, et al. Evaluation of actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea. Crop Prot. 2011;30:1070–1078.
- Bose A, Chawdhary V, Keharia H, et al. Production and characterization of a solvent-tolerant protease from a novel marine isolate Bacillus tequilensis P15. Ann Microbiol. 2014;64:343–354.
- Jia Y, Valent B, Lee FN. Determination of host responses to Magnaporthe grisea on detached rice leaves using a spot inoculation method. Plant Dis. 2003;87:129–133.
- Zhang Y, Li S, Jiang D, et al. Antifungal activities of metabolites produced by a termite-associated Streptomyces canus BYB02. J Agric Food Chem. 2013;61:1521–1524.
- Chan HP, Shirsekar G, Bellizzi M, et al. The E3 ligase APIP10 connects the effector AvrPiz-t to the NLR receptor Piz-t in rice. Plos Pathog. 2016;12:e1005529.
- Chowdhury SP, Hartmann A, Gao XW. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42 – a review. Front Microbiol. 2015;6:780.
- Leibinger W, Breuker B, Hahn M, et al. Control of postharvest pathogens and colonization of the apple surface by antagonistic microorganisms in the field. Phytopathology. 1997;87:1103–1110.
- Loon LCV, Bakker PAHM. Induced systemic resistance as a mechanism of disease suppression by rhizobacteria. In: Siddiqui ZA, editors. PGPR: Biocontrol and Biofertilization. Dordrecht (Netherlands): Springer; 2005. p. 39–66.
- Zhu Y, Shuna LI, Yuan H, et al. Isolation and identification of the antagonistic strain DM-54 of Bacillus amyloliquefacien against Verticillium dahliae, and optimization of antifungal protein producing conditions. Front Agric China. 2009;3:16–23.
- Singh S, Moholkar VS, Goyal A. Isolation, identification, and characterization of a cellulolytic Bacillus amyloliquefaciens strain SS35 from rhinoceros dung. ISRN Microbiol. 2013;2013:1–7.
- Wu WJ, Ahn BY. Isolation and identification of Bacillus amyloliquefaciens BY01 with high productivity of menaquinone for cheonggukjang production. J Korean Soc Appl Biol Chem. 2011;54:783–789.
- Zakira N, Adamh P, Fauziay H, et al. Identification of rice blast disease-suppressing bacterial strains from the rhizosphere of rice grown in Pakistan. Crop Prot. 2009;28:1052–1060.
- Wang S-L, Shih I-L, Liang T-W, et al. Purification and characterization of two antifungal chitinases extracellularly produced by Bacillus amyloliquefaciens V656 in a shrimp and crab shell powder medium. J Agric Food Chem. 2002;50:2241–2248.
- Shrestha A, Sultana R, Chae JC, et al. Bacillus thuringiensis C25 which is rich in cell wall degrading enzymes efficiently controls lettuce drop caused by Sclerotinia minor. Eur J Plant Pathol. 2015;142:577–589.
- Idriss EE, Makarewicz O, Farouk A, et al. Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiology. 2002;148:2097–2109.
- Someya N, Nakajima M, Hibi T, et al. Induced resistance to rice blast by antagonistic bacterium, Serratia marcescens strain B2. J Gen Plant Pathol. 2002;68:177–182.
- Kazempour MN. Biological control of Rhizoctonia solani, the causal agent of rice sheath blight by antagonistics bacteria in greenhouse and field conditions. Plant Pathol J. 2004;3:88–96.
- Kim YS, Kotnala B, Kim YH, et al. Biological characteristics of Paenibacillus polymyxa GBR-1 involved inroot rot of stored Korean ginseng. J Ginseng Res. 2016;40:453–461.
- Sellem I, Triki MA, Elleuch L, et al. The use of newly isolated Streptomyces strain TN258 as potential biocontrol agent of potato tubers leak caused by Pythium ultimum. J Basic Microbiol. 2017;57:393–342.