Abstract
This study investigated the effect of insulin-like growth factor 1 (IGF-1) on rat gastric smooth muscle cell apoptosis under high glucose conditions, and explored the involvement of the PI3K-Akt-PKC-Ca2+ pathway. Rat gastric smooth muscle cells were cultured in vitro under normal and high glucose conditions and treated with IGF-1. Related protein expression, PKC activity, changes of intracellular Ca2+ concentration and cell apoptosis were detected at 24 and 48 h by western blotting, enzyme-linked immunosorbent assay, confocal laser-scanning microscopy and flow cytometry, respectively. Compared with the non-treated group, PKCβ1, p-PKCβ1, PI3K and p-Akt expression in IGF-1-treated cells in normal and high glucose conditions at 24 and 48 h were increased; PKCα expression was increased in the 24-h high glucose + IGF-1 group, and decreased in the 48-h normal glucose + IGF-1 group; p-PKCα expression was decreased in the 24-h normal glucose + IGF-1 group, and increased in the 24-h and 48-h high glucose + IGF-1 group. PKC activity was increased in the 24-h normal glucose + IGF-1, 24-h and 48-h high glucose + IGF-1 groups compared with the non-treated group. After 24 and 48 h of IGF-1 treatment, the Ca2+ concentration was significantly increased in the normal glucose group, and decreased in the high glucose group compared with the non-treated group. The apoptosis rate in the 48-high glucose + IGF-1 group was significantly lower than that in the 48-h normal glucose + IGF-1 and 24-h high glucose + IGF-1 groups. Under high glucose conditions, IGF-1 can inhibit apoptosis in rat gastric smooth muscle cells through activating the PI3K-Akt-PKC pathway, and decreasing intracellular Ca2+ concentration.
Introduction
Diabetic gastroparesis (DGP) is one of the common complications of diabetes, which is characterized by delayed gastric emptying. Delayed gastric emptying can lead to large fluctuations in blood glucose levels and impaired drug absorption, thereby accelerating the occurrence of diabetes-related acute and chronic complications [Citation1, Citation2]. Therefore, it is very important to investigate the relevant mechanisms of DGP and explore effective interventions for the treatment of DGP. Long-term hyperglycemia is considered the main cause of GDP [Citation3]. Although gastric smooth muscle motility is regulated by nerve and body fluids, the contribution of the functional status of gastric smooth muscle cells in regulating smooth muscle motility cannot be neglected [Citation4–6]. The gastric smooth muscle contraction can accelerate gastric emptying, and the effective gastric smooth muscle cell numbers can maintain the gastric smooth muscle contraction. Apoptosis is the programmed death of a cell which can alter cell numbers. Wang et al. [Citation7] demonstrated that high glucose can induce apoptosis in nucleus pulposus cells. Feng et al. [Citation8] reported that high glucose can cause increased apoptotic rate in H9C2 cells. Zhang et al. [Citation9] found that apoptosis was involved in the development of DGP. This evidence indicated that high glucose can induce apoptosis.
The phosphoinositide 3-kinase (PI3K)-Akt pathway is an essential intracellular signaling pathway in cell cycle. Studies have found that the PI3K-Akt pathway can control cell proliferation, growth, apoptosis and metabolism [Citation10, Citation11]. It has been confirmed that the PI3K-Akt pathway plays an important role in the development of diabetic nephropathy, diabetic cardiomyopathy and DGP [Citation12–14]. Protein kinase C (PKC) family is classified into three subfamilies, including classical, novel and atypical PKCs. Classical PKCs include PKCα, PKCβI, PKCβII, and PKCγ [Citation15]. Classical PKCs, also known as second messenger calcium (Ca2+)-dependent PKC, are a downstream signal of the PI3K-Akt pathway. Classical PKCs have a typical structure of Ca2+-binding domain (C2 domain), which requires both diacylglycerol (DAG) and Ca2+ for activation. PKCs are also involved in the regulation of Ca2+ channel opening, affect the influx of extracellular Ca2+, and have a major influence on the changes in intracellular Ca2+ concentration. Ca2+ is one of the most widespread second messengers in eukaryotic cells. Ca2+ can activate many different protein kinases and participate in various biological processes such as cell growth, differentiation, movement and apoptosis.
Insulin-like growth factor 1 (IGF-1) is a 70-amino acid peptide that plays an important role in the regulation of cell proliferation, differentiation, metabolism and regulation via endocrine, autocrine and paracrine modes [Citation16–18]. IGF-1 can activate PKC through the PI3K-Akt pathway, and the activation of PKC can affect the changes in the intracellular Ca2+ concentration. Intracellular Ca2+ can act as apoptotic signal to initiate apoptosis. PKC has different effects on Ca2+ in different cell types and cells under different conditions. This may be due to the activation of different PKC subtypes. Two PKC subtypes, PKCα and PKCβ, are very closely related to Ca2+. Ma and Sansom [Citation19] found that under normal glucose conditions, PKCα is activated and regulates the extracellular Ca2+ influx, whereas PKCβ is activated and regulates the intracellular Ca2+ concentration under high glucose conditions.
The gastrointestinal tract is one of the most sensitive target tissues for IGF-1 [Citation20]. However, the effects of IGF-1 on apoptosis through the PI3K-Akt-PKC-Ca2+ pathway during the development of DGP have not been elucidated. In the present study, we cultured gastric smooth muscle cells in vitro under high glucose conditions, and observed the effects of exogenous IGF-1 on the expression of PI3K, p-Akt, PKCα, p-PKCα, PKCβ1 and p-PKCβ1, changes in Ca2+ concentration in cells, and apoptosis of gastric smooth muscle cells at different time points. We also explored the mechanism of action of IGF-1 in gastric smooth muscle cell apoptosis under high glucose conditions. The study aimed to further clarify the possible therapeutic mechanism of IGF-1 for GDP, provide a scientific theoretical basis and experimental basis for exploring new treatments for GDP.
Materials and methods
Ethics of experimentation statement
All animal experimental procedures were carried out in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China and were approved by the Ethics Committee of Yanbian University College of Medicine (Yanji, China).
Animals and reagents
Twenty adult Sprague-Dawley male rats (weighing 200 ± 20 g) were provided by the Experimental Animal Center of Yanbian University. Some specific reagents used in this study included PI3K (ab127617), p-Akt (ab38449), PKCα (ab32376), p-PKCα (ab76016), PKCβ1 (ab195039) and p-PKCβ1 (ab75657) antibodies (all from Abcam, USA), internal reference β-actin (A5316, SIGMA, USA); horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (SIGMA, USA), rat PKC ELISA kit (CSB-E12801r), FITC-Annexin V and PI apoptosis detection kit (BD company).
Primary culture of rat gastric smooth muscle cells and grouping
The rats were decapitated and sacrificed after 24 h of fasting, and soaked in 75% alcohol for 30 minutes. The abdominal cavity of rats was opened on a clean bench; the stomach and the intestine connected to the stomach were removed, placed in phosphate-buffered saline (PBS) and washed with PBS to remove remaining food residues. The junction of the stomach and intestine was cut into small pieces with scissors, and then incubated in 10 mL of 0.25% trypsin at 4 °C overnight. The digested tissues were taken and filtered using a 200-mesh filter, and centrifuged at 251.55×g for 5 min, the supernatant was discarded. The cell pellets were washed with PBS buffer, and centrifuged again. After adding low glucose-Dulbecco's modified Eagle's Medium (L-DMEM) containing 10% fetal bovine serum (FBS), the cultures were placed in a 37 °C incubator. Cells were divided into normal glucose (5 mmol/L) group, high glucose (35 mmol/L) group, normal glucose + IGF-1 group and high glucose + IGF-1 group. After 24 and 48 h of culture, related protein expression, PKC activity, changes in intracellular Ca2+ concentration in rat gastric smooth muscle cells and cell apoptosis were detected by western blot analysis, enzyme-linked immunosorbent assay (ELISA), confocal laser-scanning microscopy and flow cytometry, respectively.
Detection of related protein expression by Western blot analysis
Total proteins were exacted from cells, and the protein concentration of the samples was determined by a full-wavelength spectrophotometer. Proteins were boiled for 2 min, 40 µg of proteins were loaded per well of a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel. After separation by electrophoresis, the proteins in the gel were transferred onto a PVDF membrane by semi-dry transfer. The membranes were blocked with 5% skimmed milk powder in TBS-T blocking solution (25 mmol/L Tris, 150 mmol/L NaCl, 1% Tween 20, pH 7.5). After washing, the membranes were incubated with PI3K (1:1000), p-Akt (1:500), PKCα (1:1000), p-PKCα (1:1000), PKCβ1 (1:1000), p-PKCβ (1:1000) antibodies and β-actin (1:500) at 4 °C overnight. After washing, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:1000) at room temperature for 1 h. Finally, the membranes were washed and exposed with a gel-imaging analysis system. Images were analyzed and relative expression levels of proteins were calculated relative to β-actin, which served as the internal reference.
Detection of PKC activity in rat gastric smooth muscle cells by ELISA
All prepared reagent solutions (wash solution, biotin-conjugated antibody working solution and horseradish peroxidase-conjugated avidin working solution) were equilibrated to room temperature (18–25 °C) for at least 30 minutes before use. Standard and sample wells were set; 100 μL of the standards and samples were added into each well, respectively; the solutions in all wells were mixed well by shaking the plate gently; then the plate was sealed with a plate cover and incubated at 30 °C for 2 h. After discarding the liquids and drying the plate without washing, 100 μL of biotin-conjugated antibody working solutions were added to each well; then the plate was sealed with a plate cover and incubated at 30 °C for 1 h. After discarding the liquids from each well and drying the plate, the plate was washed three times (soaking for 2 minutes each time) with 250 µL/well wash buffer. After drying the plate, 100 μL of horseradish peroxidase-conjugated avidin working solution per well was added; then the plate was covered with a plate cover, and incubated at 30 °C for 1 h. After discarding the liquids and drying the plate, the plate was washed five times (soaking for 2 minutes each time) with 200 µL/well wash buffer; then the plate was dried and 90 μL substrate solution was added into each well. The plate was incubated at 37 °C for 15–30 minutes in the dark. Finally, 50 μL of stop solution was added to the wells to terminate the reaction; 5 minutes after termination of the reaction, the optical density (OD value) of each well was measured at a wavelength of 450 nm using a microplate reader.
Detection of changes in intracellular Ca2+ concentration in rat gastric smooth muscle cells by confocal laser-scanning microscopy
When the cultured cells reached 80% confluence, the cells in the normal glucose + IGF-1 and high glucose + IGF-1 groups were treated with IGF-1. Fluo-4 AM was diluted to 0.5–5 μmol/L in PBS. After removing the culture medium, the cells were washed three times with PBS or Hanks' balanced salt solution (HBSS), then fluo-4 AM working solution was added in an amount sufficient to cover the cells. After incubation at 20–37 °C for 10–60 min, the cells were washed three times with PBS or HBSS; then the cells were reincubated with fluo-4 AM working solution for another 20–30 min to ensure that fluo-4 AM was converted into its functional form fluo-4 in the cells. The fluorescence of fluo-4 was detected using confocal laser-scanning microscopy to determine the changes in intracellular Ca2+ concentration.
Detection of cell apoptosis by flow cytometry
Annexin V can be used as a sensitive indicator to detect early apoptosis of cells through detection of the exposure of phosphatidylserine on the outside of the cell membrane. Propidium iodide (PI) can pass through the cell membrane in late apoptotic cells and necrotic cells, and stain the nucleus red. Cells were washed twice with PBS and centrifuged at 447.2×g for 5 min; 1 × 105 cells were collected, and suspended in 500 μL binding buffer. After adding 5 μL of Annexin V-EGFP and mixing, 5 μL of PI were added, the samples were mixed and incubated at room temperature in darkness for 5–15 min. Cell apoptosis was observed by flow cytometry 24 and 48 h after the cells were treated with IGF-1. In the flow cytometry plots, cells were divided into four quadrants, with the x-axis indicating Annexin V-FITC-stained cells and the y-axis indicating PI-stained cells. The upper right quadrant represented late apoptotic cells; the upper left quadrant represented necrotic cells; the lower left quadrant represented normal cells and the lower right one represented early apoptotic cells. Apoptotic rates were the ratio of apoptotic cells divided by the total number of cells.
Statistical analysis
Statistical analyses were performed with GraphPad Prism5 software (GraphPad Software, San Diego, CA). Measurement data are expressed as mean values with standard error of the means (±SEM). Differences between groups were compared using t-test and one-way analysis of variance (ANOVA). P < 0.05 was considered to indicate a significant difference.
Results and discussion
Effect of IGF-1 on rat gastric smooth muscle cell apoptosis
DGP is a complex pathophysiological process. At present, research on the pathogenesis of DGP mainly focuses on neuropathy caused by hyperglycemia, disturbed gut hormone secretion, stress and some microangiopathies. DGP is characterized by decreased gastric motility-induced delayed gastric emptying. Gastric motility disorders are closely related to changes in smooth muscle contraction. Any factors that affect the normal contraction and relaxation of gastric smooth muscles can cause gastric motility disorders. It has been confirmed that apoptosis may be involved in the development of DGP by changing the effective cell numbers. The structure of IGF-1 is highly similar to that of insulin [Citation21, Citation22]. Studies revealed that the IGF-1/IGF-1R signaling pathway can promote the cell proliferation in peripheral tissues and inhibit apoptosis through PI3K or mitogen-activated protein kinase (MAPK) signaling pathway [Citation23, Citation24]. However, the effect of IGF-1 on the apoptosis of rat gastric smooth muscle cells under high glucose condition remains unclear. Therefore, in the present study, we cultured rat gastric smooth muscle cells in vitro under different glucose conditions, and 24 and 48 h after the cells were treated with IGF-1. The analysis of apoptosis by flow cytometry showed that there was no significant difference in the apoptosis rates between the normal glucose and high glucose groups at 24 h, but there was a decreasing trend in the high glucose group. Compared with the 24-h high glucose + IGF-1 group and 48-h normal glucose + IGF-1 group, the rates of apoptosis in the 48-h high glucose + IGF-1 group were significantly decreased (4.997 ± 0.243 vs. 2.090 ± 0.121, p<0.001; 4.973 ± 0.274 vs. 2.090 ± 0.121, p<0.001, respectively; ). There was no significant difference in the rates of apoptosis between the 48-h normal glucose and 24-h normal glucose groups. Therefore, the results indicated that IGF-1 had an inhibitory effect on the apoptosis of rat gastric smooth muscle cells under high glucose conditions, the inhibitory effect being more obvious with the prolonged treatment time. We subsequently investigated the potential mechanism underlying the regulatory effect of IGF-1 on gastric smooth muscle cell apoptosis.
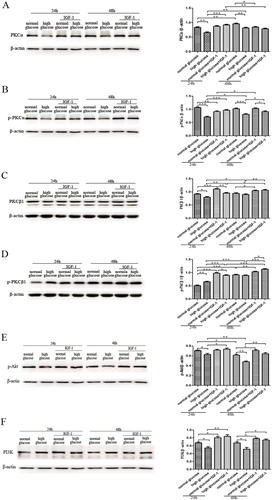
Effect of IGF-1 on the expression of PKCα, p-PKCαPKCβ1, p-PKCβ1, p-Akt and PI3K
Studies have found that the PI3K-Akt pathway is activated in many diabetes complications and is a downstream signal of IGF-1. Zhang et al. [Citation9] found that apoptosis is involved in the occurrence of DGP. During this process, PI3K-Akt expression gradually decreased with the disease progression, indicating that the PI3K-Akt pathway is involved in the development of DGP and has a regulatory effect on apoptosis. As PKC is a downstream signal factor of PI3K-Akt pathway, PKC activity is controlled by the PI3K-Akt pathway [Citation25]. Lahaye et al. [Citation26] reported that under high glucose conditions, activated PKC can reduce Na+/Ca2+ pump activity and increase intracellular Ca2+ concentration in cardiac myocytes. However, inhibition of PKC activity by PKC inhibitors can enhance the Na+/Ca2+ pump activity, reduce intracellular Ca2+ accumulation, suggesting that PKC can regulate Na+/Ca2+ pump activity [Citation27]. Ozdemir et al. [Citation28] found that the activation of PKC inhibited the activity of sarcoplasmic reticulum calcium pump (SERCA), reduced SERCA Ca2+ transport efficiency and caused continued increase in the intracellular Ca2+ concentration, resulting in impaired myocardial contraction and relaxation. After the cells were treated with PKC inhibitors, the intracellular Ca2+ transport was significantly improved. Chaudhari et al. [Citation29] found that store-operated Ca2+ entry (SOCE) is involved in the development of diabetic nephropathy, diabetic cardiomyopathy and diabetic eye diseases, and PKC plays an important role in regulating the process of SOCE. These examples suggest that the activation of PKC is associated with various diabetes complications, and PCK achieves its effect mainly by affecting the intracellular Ca2+ concentration. The changes in Ca2+ concentration have a great influence on cell apoptosis. KoraMagazi et al. [Citation30] found that rhein increased the Ca2+ concentration in human hepatic cell line (HL-7702), leading to apoptosis. Seo et al. [Citation31] reported that curcumin inhibited the SERCA activity in ovarian cancer cells, which can cause an increase in intracellular Ca2+ concentration and apoptosis. Another study provided evidence that chetomin can induce apoptosis in human triple-negative breast cancer cells by promoting calcium overload and mitochondrial dysfunction [Citation32].
To investigate the changes in the PI3K-Akt-PKC pathway and Ca2+ concentration in rat gastric smooth muscle cells cultured under high glucose conditions, we used western blot analysis to detected the protein expression of PKCα, PKCβ1, p-PKCα, p-PKCβ1, PI3K and p-Akt in cells. The results showed that after 24 and 48 h of culture, the expression of PKCα, PKCβ1 and p-PKCα in the high glucose group was lower than that in the normal glucose group (p<0.01) (). The expression of PI3K and p-Akt in the high glucose group was also lower than that in the normal glucose group, and p-Akt expression in the high glucose group gradually decreased with time (p<0.05) (). However, p-PKCβ1 expression was significantly higher in the 24-h high glucose group than in the 24-h normal glucose group (p<0.01), and there was no significant difference between the 48-h high glucose and the 48-h normal glucose groups ().
Figure 2. Effect of IGF-1 on protein expression of PKCα (A), p-PKCα (B), PKCβ1 (C), p-PKCβ1 (D), p-Akt (E) and PI3K (F) in rat gastric smooth muscle cells after 24 and 48 h of culture. Western blot analysis. ***p < 0.001; **p < 0.01; *p < 0.05.
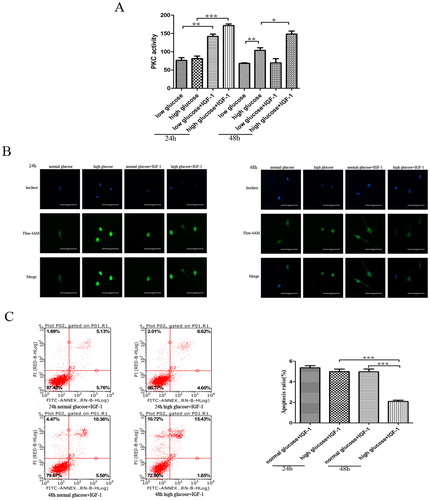
We subsequently used ELISA to detect the PKC activity. The results showed that there was no statistically significant difference in PKC activity in the 24-h high glucose group and 24-h normal glucose group, but there was an increasing trend in the 24-h high glucose group. The PKC activity in the 48-h high glucose group was significantly higher than that in the 48-h normal glucose group (p<0.01) ().
We then used confocal laser-scanning microscopy to detect Ca2+ concentration in rat gastric smooth muscle cells, we found that after 24 and 48 h of culture, the intracellular Ca2+ concentration was significantly higher in the high glucose group than that in the normal glucose group (). These results indicated that under high glucose conditions, the Ca2+ concentration in rat gastric smooth muscle cells was significantly increased, resulting in disturbance of Ca2+ homeostasis. The expression of p-PKCα and p-PKCβ were significantly different in the normal and high glucose groups at different time points. This may be because under normal glucose conditions, the PI3K-Akt pathway mainly activates PKCα to ensure the intracellular Ca2+ homeostasis. But under high glucose conditions, the PI3K-Akt pathway is inhibited, resulting in decreased PKCα activity, and increased PKCβ1 activity, which cause an increase in intracellular Ca2+ concentration. Our findings suggested that the PI3K-Akt-PKCα pathway was down-regulated, while PKCβ1 activity was increased and the intracellular Ca2+ concentration was increased under high glucose conditions.
In the present study, we further investigated whether the inhibitory effects of IGF-1 on rat gastric smooth muscle cell apoptosis were associated with the PI3K-Akt-PKC pathway and Ca2+ concentration. At different time points, 24 and 48 h, after the rat gastric smooth muscle cells were cultured under different glucose conditions and treated with IGF-1, western blot analysis showed that, compared with untreated cells, the expression of PKCβ1, p-PKCβ1, PI3K and p-Akt in IGF-1-treated cells of normal and high glucose groups were both increased at 24 and 48 h (p<0.05) (). PKCα expression in IGF-1-treated cells of normal and high glucose groups were both increased at 24 h (p<0.001), and were both decreased at 48 h compared with untreated cells (p<0.05) (). The expression of p-PKCα was lower in the 24-h normal glucose + IGF-1 group than that in the non-treated group (p<0.01), and there was no significant difference in p-PKCα expression in the 48-h normal glucose group before and after IGF-1 invention. Compared with untreated cells, p-PKCα expression was increased in IGF-1-treated cells in both 24- and 48-h high glucose group (p<0.05) (). With time, the effect of IGF-1 on the expression of these proteins in the high glucose group was significantly stronger than that in the normal glucose group.
Effect of IGF-1 on PKC activity in rat gastric smooth muscle cells
After the cells were treated with IGF-1, the PKC activity detected by ELISA showed that PKC activity in IGF-1-treated cells of the normal and high glucose groups was significantly increased as compared to that in non-treated cells at 24 h (p<0.01), and the increase in PKC activity was more pronounced in the high glucose group. There was no significant difference in PKC activity in the 48-h normal glucose group before and after IGF-1 intervention, but the PKC activity was significantly increased in the 48-h high glucose group after IGF-1 intervention (p<0.05) ().
Effect of IGF-1 on Ca2+ concentration in rat gastric smooth muscle cells
The confocal laser-scanning microscopy showed that after 24 and 48 h of IGF-1 treatment, Ca2+ concentration in the IGF-1-treated gastric smooth muscle cells of the normal glucose group was significantly increased compared to that in the non-treated cells, while Ca2+ concentration in the IGF-1-treated cells of the high glucose group was significantly decreased (). Therefore, the results indicated that IGF-1 can cause disturbance of intracellular Ca2+ homeostasis under normal glucose conditions, and improve the intracellular Ca2+ homeostasis under high glucose conditions. Under high glucose conditions, IGF-1 can reduce the intracellular Ca2+ concentration and improve the intracellular calcium homeostasis by activating the PI3K-Akt-PKC pathway, thereby reducing rat gastric smooth muscle cell apoptosis; while under the normal glucose conditions, although IGF-1 can activate the PI3K-Akt-PKC pathway. It is interesting that IGF-1 can increase the intracellular Ca2+concentration, cause the occurrence of disturbance of intracellular calcium homeostasis and increase the rates of cell apoptosis. It is considered that IGF-1 may be a new stimulus that activates other signaling pathways under normal glucose conditions.
Conclusions
Our results suggested that the PI3K-Akt-PKCα pathway was down-regulated, while PKCβ1 activity was increased and intracellular Ca2+ concentration was increased under high glucose conditions. IGF-1 can cause disturbance of the intracellular Ca2+ homeostasis under normal glucose conditions, and improve the intracellular Ca2+ homeostasis under high glucose conditions. IGF-1 had an inhibitory effect on the apoptosis of gastric smooth muscle cells under high glucose conditions perhaps through a mechanism that involved a decrease in the intracellular Ca2+ concentration and activation of the PI3K-Akt-PKC pathway.
Disclosure statement
The authors have no conflicts of interest to declare.
Funding
This study was financially supported by the National Natural Science Foundation of China under grants number 81360070 and 81560142.
References
- Marathe CS, Rayner CK, Jones KL, et al. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. 2013;36:1396–1405.
- Ma J, Rayner CK, Jones KL, et al. Diabetic gastroparesis: diagnosis and management. Drugs. 2009;69:971–986.
- Lee AA, Hasler WL. Diabetes and the stomach. Curr Treat Options Gastroenterol. 2017;15:441–459.
- Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–356.
- Phillips LK, Deane AM, Jones KL, et al. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. 2015;11:112–128.
- Ramzan Z, Duffy F, Gomez J, et al. Continuous glucose monitoring in gastroparesis. Dig Dis Sci. 2011;56:2646–2655.
- Wang W, Li P, Xu J, et al. Resveratrol attenuates high glucose-induced nucleus pulposus cell apoptosis and senescence through activating the ROS-mediated PI3K/Akt pathway. Biosci Rep. 2018;38:BSR20171454.
- Feng CC, Pandey S, Lin CY, et al. Cardiac apoptosis induced under high glucose condition involves activation of IGF2R signaling in H9c2 cardiomyoblasts and streptozotocin-induced diabetic rat hearts. Biomed Pharmacother. 2018;97:880–885.
- Zhang MH, Jiang JZ, Cai YL, et al. Significance of dynamic changes in gastric smooth muscle cell apoptosis, PI3K-AKT-mTOR and AMPK-mTOR signaling in a rat model of diabetic gastroparesis. Mol Med Rep. 2017;16:1530–1536.
- Lu D, Qian J, Li W, et al. β-hydroxyisovaleryl-shikonin induces human cervical cancer cell apoptosis via PI3K/AKT/mTOR signaling. Oncol Lett. 2015;10:3434–3442.
- Cui H, Wu S, Shang Y, et al. Pleurotus nebrodensis polysaccharide(PN50G) evokes A549 cell apoptosis by the ROS/AMPK/PI3K/AKT/mTOR pathway to suppress tumor growth. Food Funct. 2016;7:1616–1627.
- Ma Y, Chen F, Yang S, et al. Silencing of TRB3 ameliorates diabetic tubule interstitial nephropathy via PI3K/AKT signaling in rats. Med Sci Monit. 2017;23:2816–2824.
- Wu Z, Huang A, Yan J, et al. Resveratrol ameliorates cardiac dysfunction by inhibiting apoptosis via the PI3K/Akt/FoxO3a pathway in a rat model of diabetic cardiomyopathy. J Cardiovasc Pharmacol. 2017;70:184–193.
- An X, Long C, Deng X, et al. Higenamine inhibits apoptosis and maintains survival of gastric smooth muscle cells in diabetic gastroparesis rat model via activating the β2-AR/PI3K/AKT pathway. Biomed Pharmacother. 2017;95:1710–1717.
- Menne J, Shushakova N, Bartels J, et al. Dual inhibition of classical protein kinase C-α and protein kinase C-β isoforms protects against experimental murine diabetic nephropathy. Diabetes. 2013;62:1167–1174.
- Lupachyk S, Watcho P, Stavniichuk R, et al. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes. 2013;62:944–952.
- Humbel RE. Insulin-like growth factors I and II. Eur J Biochem. 1990;190:445–462.
- Cohick WS, Clemmons DR. The insulin-like growth factors. Annu Rev Physiol. 1993;55:131–153.
- Ma R, Sansom SC. Epidermal growth factor activates store-operated calcium channels in human glomerular mesangial cells. J Am Soc Nephrol. 2001;12:47–53.
- Carroll PV. Treatment with growth hormone and insulin-like growth factor-I in critical illness. Best Pract Res Clin Endocrinol Metab. 2001;15:435–451.
- Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology. 2002;63:317–332.
- Elijah IE, Branski LK, Finnerty CC, et al. The GH/IGF-1 system in critical illness. Best Pract Res Clin Endocrinol Metab.. 2011;25:759–767.
- Vanamala J, Reddivari L, Radhakrishnan S, et al. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer. 2010;10:238.
- Sukhanov S, Higashi Y, Shai SY, et al. Differential requirement for nitric oxide in IGF-1-induced anti-apoptotic, anti-oxidant and anti-atherosclerotic effects. FEBS Lett. 2011;585:3065–3072.
- Le Good JA, Ziegler WH, Parekh DB, et al. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045.
- Lahaye P, Tazi KA, Rona JP, et al. Effects of protein kinase C modulators on Na+/K+ adenosine triphosphatase activity and phosphorylation in aortae from rats with cirrhosis. Hepatology. 1998;28:663–669.
- Wickley PJ, Shiga T, Murray PA, et al. Propofol modulates Na+-Ca2+ exchange activity via activation of protein kinase C in diabetic cardiomyocytes. Anesthesiology. 2007;106:302–311.
- Ozdemir S, Ugur M, Gürdal H, et al. Treatment with AT(1) receptor blocker restores diabetes-induced alterations in intracellular Ca(2+) transients and contractile function of rat myocardium. Arch Biochem Biophys. 2005;435:166–174.
- Chaudhari S, Ma R. Store-operated calcium entry and diabetic complications. Exp Biol Med (Maywood). 2016;241:343–352.
- KoraMagazi A, Wang D, Yousef B, et al. Rhein triggers apoptosis via induction of endoplasmic reticulum stress, caspase-4 and intracellular calcium in primary human hepatic HL-7702 cells. Biochem Biophys Res Commun. 2016;473:230–236.
- Seo JA, Kim B, Dhanasekaran DN, et al. Curcumin induces apoptosis by inhibiting sarco/endoplasmic reticulum Ca2+ ATPase activity in ovarian cancer cells. Cancer Lett. 2016;371:30–37.
- Dewangan J, Srivastava S, Mishra S, et al. Chetomin induces apoptosis in human triple-negative breast cancer cells by promoting calcium overload and mitochondrial dysfunction. Biochem Biophys Res Commun. 2018;495:1915–1921.