Abstract
The binding activity to heparan sulphate is crucial for the mitogenic activity of fibroblast growth factor 4 (FGF4) in developing mammalian embryos. There are two conserved cysteine residues in FGF family proteins, Cys-84 and Cys-151 in mouse FGF4, and these constitute all of the cysteine residues in FGF4. However, the relationships among the heparin binding activity, growth promoting activity, and the two conserved cysteine residues in FGF4 are still unclear. Consequently, we generated in Escherichia coli three kinds of point-mutated mouse FGF4, namely C84S, C151S, and C84S;C151S, by converting the cysteine residues to serine residues. In heparin column chromatography, the heparin binding activities of these mutants were attenuated. In particular, the activity of the double-mutated C84S;C151S was weakened considerably. The growth promoting activities of these mutants correlated well with their heparin binding activities. We also demonstrated that an octapeptide, the Leu-76 to Tyr-83 region, which contained four basic amino acid residues and flanked Cys-84 in mouse FGF4, enhanced the heparin binding activity when fused to glutathione-S-transferase as a recombinant protein. Overall, our findings imply that the two conserved cysteine residues in FGF4 are both involved in the heparin binding activity and mitogenicity likely by affecting the configuration of heparin binding sites in FGF4.
Introduction
The human and mouse fibroblast growth factors (FGFs) are a family comprising 22 members of structurally related polypeptides. FGF1 and FGF2 were originally isolated from the brain and pituitary based on their potent mitogenicities on fibroblasts as a marker [Citation1]. In mice, FGF4 secreted from the inner cell mass, founder cells for the epiblast and the embryo proper, of blastocysts stimulates cell proliferation of the polar trophectoderm [Citation2]. Immediately after implantation, FGF4 secreted from the epiblast promotes consecutively the proliferation of extra-embryonic ectoderm, founder cells of the placenta, derived from the polar trophectoderm [Citation2–4]. Therefore, FGF4 is a crucial growth factor, especially for the development of the placenta in mouse embryos, and Fgf4-null mice showed a peri-implantation lethal phenotype [Citation3]. FGF4 also regulates organogenesis, including the development of the gastrointestinal tract, kidneys and teeth in fetuses [Citation5–7].
Notably, FGF4 promoted the establishment of trophoblast stem cells from mouse blastocysts and the maintenance of trophoblast stem cells in an undifferentiated state in vitro [Citation2]. FGF4 also promoted the generation of human intestinal organoids as three-dimensional spheroids of human epithelium through directed differentiation of human embryonic stem cells and induced pluripotent stem cells [Citation5,Citation8,Citation9]. Hence, FGF4 becomes more important not only in developmental biology but also in stem cell biology and regenerative medicine.
Heparin is an experimental model for heparan sulphate, constituting proteoglycans at the cell surface [Citation10]. The binding activity of FGFs, such as FGF1, FGF2 and FGF4, to heparin is substantially different and affects FGF signalling [Citation11–13]. Indeed, heparan sulphate is crucial as a tissue-specific regulator of FGF4 and FGF receptor recognition [Citation14,Citation15]. On the other hand, there are two conserved cysteine residues in the primary structure of FGFs. Almost all members of the FGFs contain more than three cysteine residues, such as three and four cysteine residues in human FGF1 (GeneBank accession No. AAH32697) and human FGF2 (No. EAX05222), respectively. Extensive analyses have been conducted to clarify the roles of these cysteine residues in disulphide bond formation and the mitogenic and heparin binding activities in FGFs [Citation16–21]. Two conserved cysteine residues, Cys-84 and Cys-151 in the primary structure of mouse FGF4, are the only cysteine residues in FGF4. However, the relationships among the heparin binding activity, growth promoting activity, and the two conserved cysteine residues in FGF4 is still unknown. Because FGF4 is a good model for analyzing the role of cysteine residues in FGFs, we generated in Escherichia coli all types of point-mutated mouse FGF4 by converting cysteine to serine residues.
Materials and methods
Construction of Bacterial Expression Vectors for Recombinant Proteins
We previously constructed a bacterial expression vector pET28/HismFGF4L encoding a hexahistidine-tagged and aminoterminally truncated mouse FGF4 (Leu-51 to Leu-202, predicted Mr of 19.1 kDa [Citation22]), herein referred to as FGF4L. FGF4L is fully bioactive compared with mature mouse FGF4 (Pro-31 to Leu-202) [Citation22]. In order to generate point-mutated mouse FGF4 derivatives by converting cysteine to serine residues, bacterial expression vectors were constructed using pET-28a(+) (Novagen, Madison, WI, USA) for the production of single-cysteine-mutated FGF4L(C84S), single-cysteine-mutated FGF4L(C151S), and double-cysteine-mutated FGF4L(C84S;C151S). These point mutations were introduced with the use of mutated PCR primers with KOD-plus DNA polymerase (Toyobo, Osaka, Japan) and pET28/HismFGF4L as a PCR template in combination with overlap extension PCR [Citation23].
Glutathione-S-transferase (GST) fused with an octapeptide Leu-76 to Tyr-83 (L76-Y83, Leu-76-Lys-77-Arg-78-Leu-79-Arg-80-Arg-81-Leu-82-Tyr-83 in mouse FGF4, underlines indicating basic amino acid residues) at both the N- and C-termini was constructed as follows. PCR primers encoding the octapeptide (forward primer, 5′-CGCGCGGCAGCCATATGCTGAAACGTCTGCGTCGTCTGTATATGTCCCCTATACTAGGTTAT-3′; reverse primer, 5′-GACGGAGCTCGAATTCTTAATACAGACGACGCAGACGTTTCAGATCCGATTTTGGAGGATGGTC-3′) and a GST expression vector (pGEX-6P-3, GE Healthcare Bio-Sciences, Piscataway, NJ, USA) as a PCR template were used for amplification of the DNA fragment with KOD-plus DNA polymerase. The resultant recombinant, hexahistidine-tagged GST was referred to here as GST(L76-Y83).
These amplified DNAs were inserted into the NdeI/EcoRI sites of pET-28a(+). Then, the nucleotide sequences of all of the recombinant DNAs generated in the present study were confirmed.
Expression of the Recombinant Proteins in E. coli and Evaluation of Their Heparin Binding Activity Using Heparin Column Chromatography
Culture of E. coli was performed as described previously [Citation24]. In brief, the expression of recombinant proteins was induced by adding isopropyl-1-thio-β-D-galactopyranoside (1 mmol/L, Nacalai Tesque, Kyoto, Japan) to cultures of an E. coli strain Rosetta(DE3)pLysS (Novagen) bearing the recombinant plasmids. The cells were lysed by three successive cycles of freezing and thawing, disrupted by sonication and cleared by centrifugation, and the bacterial supernatant was harvested.
By utilizing heparin column chromatography [Citation22], the heparin binding activity of the recombinant proteins was evaluated; the proteins eluted from the heparin column were analyzed by western blotting and by comparing the elution concentrations of NaCl and the positions in the fractions at 2 mL/tube. The concentrations of FGF4 derivatives in the collected fractions were quantified with purified HismFGF4L (FGF4L in this study) [Citation22] as a standard by western blotting.
Western Blotting
Western blotting analysis was performed as described previously [Citation25,Citation26]. Goat anti-human FGF4 (#sc-1361, 1:10,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and rabbit anti-GST (#G7781, 1:20,000, Sigma, St. Louis, MO, USA) were the primary antibodies, and horseradish peroxidase-conjugated, mouse anti-goat IgG (#sc-2354, 1:5,000, Santa Cruz Biotechnology) and horseradish peroxidase-conjugated, goat anti-rabbit IgG (#AP132P, 1:5,000, Chemicon, Temecula, CA, USA) were used as secondary antibodies. The enzyme reaction was detected using ECL prime Western Blotting Reagent (GE Healthcare Bio-Sciences) and a LAS-4000 image analyzer (Fujifilm, Tokyo, Japan).
Cell Growth Assay
Mouse embryonic fibroblast Balb/c 3T3 cells were used for the cell growth assay, as reported previously [Citation22]. In brief, the cells (2000 cells in 200 μL of medium/well) were precultured in a 96-well plate with 10% fetal calf serum overnight. Subsequently, the cells were cultured with medium supplemented with 0.4% calf serum for a 1-day culture period. Then, the cells were cultured further with medium (100 μL) containing various concentrations of FGF4 derivatives in the presence of 0.4% calf serum and heparin (1 μg/mL, #H9399, Sigma) for 3 days. Then, 10 μL of WST-8 reagent (Dojindo Laboratories, Kumamoto, Japan) was added to each well and incubated for an additional 3 h. The absorbance was measured using a microplate reader at 450 nm. The statistical significance of the difference among sample means was determined using one-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test.
Results and discussion
Effect of Point Mutations From Cysteine to Serine Residues on Heparin Binding in FGF4
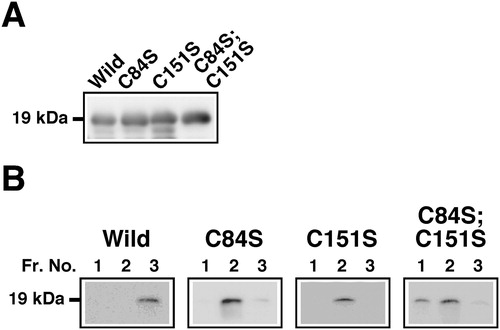
The expression levels of FGF4 derivatives were similar to each other (). Na+ binds competitively with heparin-binding sites, which contain basic amino acid residues, in FGF4 to heparin; thus the heparin binding activity among FGF4 derivatives could be compared using the elution profiles from heparin column chromatography. In order to improve the separation performance of column chromatography, a small amount of FGF4 derivatives was applied and followed by detection with western blotting. As shown in , almost all of the FGF4 derivatives retained on the column were eluted intensively at 0.92 mol/L NaCl. FGF4L, which is regarded as a wild-type FGF4, was eluted efficiently by stepwise increasing concentrations of NaCl at fraction No. 3 with 0.92 mol/L eluate. However, the double-cysteine-mutated FGF4L(C84S;C151S) was eluted quickly from fraction No. 1. Single-cysteine-mutated FGF4L(C84S) and FGF4L(C151S) were eluted from fraction No. 2. These results indicated that the heparin binding activity was considerably weaker in the double-cysteine-mutated FGF4L. Single-cysteine-mutated types of FGF4L showed intermediate heparin binding activity.
Figure 1. Heparin binding activity of point-mutated FGF4 derivatives with conversions of cysteine to serine residues. (a) Expression of FGF4 derivatives (Mr 19 kDa) in E. coli. Supernatants of E. coli cell lysates were analyzed by western blotting using an anti-FGF4 antibody. FGF4L was regarded as a wild-type (Wild). Single-cysteine-mutated FGF4L(C84S), C84S; single-cysteine-mutated FGF4L(C151S), C151S; double-cysteine-mutated FGF4L(C84S;C151S), C84S;C151S. (b) Elution profiles of FGF4 derivatives using 0.92 mol/L NaCl with heparin column chromatography. Supernatants of E. coli cell lysates were applied to heparin column chromatography. FGF4 derivatives retained on the column were eluted with increasing concentrations of NaCl and fractionated at 2 mL/tube. FGF4 derivatives in aliquots of proteins in the respective eluates using 0.92 mol/L NaCl were detected by western blotting. Fraction No. 1 in eluates using 0.92 mol/L NaCl corresponded to sequential fraction No. 31 from chromatography. Fr. No., fraction number.
Effect of Fusion of the Octapeptide Leu-76 to Tyr-83 (L76-Y83) Region Flanking Cys-84 in Mouse FGF4 on Heparin Binding Activity of Recombinant GST Protein
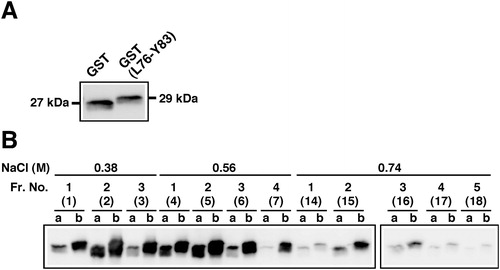
An amino-terminal region flanking Cys-84 in mouse FGF4 (GenBank accession No. NM_010202) and its corresponding regions in other mammals, such as human (No. NM_002007), cattle [Citation27] and pig [Citation28], contain a group of four basic amino acid residues. We predicted that this region is crucial for heparin binding activity in FGF4. In fact, because Lys-81 in human FGF4 (Lys-77 in mouse) was identified as a heparin binding lysine residue, the Lys-81-containing region was suggested as the heparin binding site [Citation29]. In order to analyze the heparin binding activity of the octapeptide containing Lys-77 in mouse FGF4, we generated GST(L76-Y83). As shown in , similar amounts of a hexahistidine-tagged GST (GST) and GST(L76-Y83) were applied for heparin column chromatography. GST(L76-Y83) retained on the column was eluted by concentrations of NaCl mainly between 0.38 and 0.74 mol/L. In these binding and elution conditions, more than 3-fold more GST(L76-Y83) was eluted from the column than for GST. Thus, fusion of the octapeptide L76-Y83 to GST resulted in an enhancement of heparin binding activity, whereas the activity of GST(L76-Y83) was much weaker than those of both wild-type and mutated FGF4L when considering the elution concentrations of NaCl. Our findings also support, at least in part, the possibility that the Lys-81-containing region plays a role in heparin binding activity in human FGF4, as reported previously [Citation29].
Figure 2. Heparin binding activity of GST fused with an octapeptide Leu-76 to Tyr-83 (L76-Y83) flanking Cys-84 in mouse FGF4. (a) Expression of recombinant GST proteins in E. coli. Recombinant GST proteins fused with (GST(L76-Y83), Mr 29 kDa) or without (GST, Mr 27 kDa) the L76-Y83 peptide at both termini were expressed in E. coli. Supernatants of cell lysates before applying to heparin column chromatography were analyzed by western blotting using an anti-GST antibody. (b) Elution profiles of recombinant GST proteins using heparin column chromatography. GST(L76-Y83) retained on the column was eluted with increasing concentrations of NaCl. GST proteins in aliquots of proteins in the respective fractions were detected by western blotting. The numbers in parentheses indicate the sequential fraction numbers from column chromatography. a, GST; b, GST(L76-Y83).
Effect of Point Mutations From Cysteine to Serine Residues on the Growth-promoting Activity of FGF4
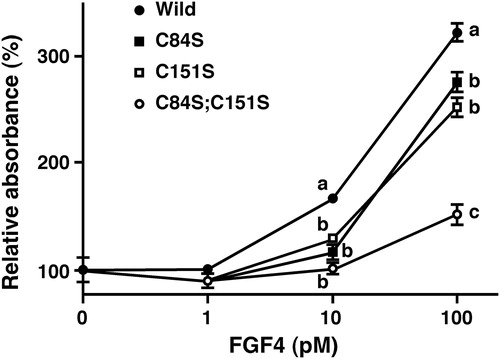
As shown in , FGF4L caused a dose-dependent stimulation of cell growth in Balb/c 3T3 cells, a representative mouse fibroblast cell line; 100 pmol/L FGF4L enhanced the absorbance of WST-8 3.2-fold compared with the absence of FGF4L. However, the mitogenic activity of the double-cysteine-mutated FGF4L(C84S;C151S) was weakened, thus WST-8 absorbance increased only 1.5-fold at 100 pmol/L. Single-cysteine-mutated FGF4L(C84S) and FGF4L(C151S) exhibited intermediate levels of mitogenic activity (2.5- to 2.7-fold at 100 pmol/L). Collectively, these results suggested that heparin binding activity and mitogenic activity correlated well in the FGF4 derivatives generated in this study.
Figure 3. Effect of point-mutated FGF4 derivatives on cell growth of mouse embryonic fibroblast Balb/c 3T3 cells. Balb/c 3T3 cells were precultured in a 96-well plate with 0.4% calf serum for a 1-day culture period. Then, the cells were cultured with medium containing FGF4 derivatives for 3 days in the presence of 0.4% calf serum. Then, WST-8 reagent was added to each well and incubated for an additional 3 h. Absorbance was measured using a microplate reader at 450 nm. Because the results obtained from trials matched well, data represent the sum of two independent trials and are expressed as mean ± SE (n = 16 wells). Absorbance values with different alphabets in the same concentrations of FGF4 differ significantly (P < 0.01).
An X-ray structure determination indicated that Cys-88, a conserved cysteine residue on the amino-terminal side of human FGF4, is a free thiol [Citation30]. Because Cys-88 is located at a solvent-inaccessible position, the role of Cys-88, such as the recognition of FGF receptors or the provision of a significant buried hydrophobic area that might contribute to the stability of FGF4, remain unclear [Citation31]. In our study, the C84S mutation in mouse FGF4 led to the attenuation of both heparin binding activity and mitogenicity. We consider that this mutation likely affected the configuration of surrounding regions, such as the Leu-76 to Tyr-83 region, and resulted in changes in heparin binding activity. In addition, the binding activity of FGF4 to heparin affects FGF signalling [Citation11,Citation13]. Accordingly, changes in the heparin binding activity likely resulted in changes in their growth promoting activity. In human FGF4, peptide regions containing Lys-142, Lys-144, or Lys-158, are also considered to act as heparin binding sites [Citation29]. These lysine residues flank C155, another conserved cysteine residue in human FGF4. Therefore, the C151S mutation in mouse FGF4 might affect both heparin binding and mitogenic activities, as shown in single-mutated C151S, and even more so, in double-mutated C84S;C151S, likely through modulation of the configuration of neighbouring heparin binding sites.
Conversion of cysteine to serine residues enhanced the mitogenic activity and led to less heparin dependency than with wild-type FGF1 [Citation19]. On the other hand, the cysteine-to-serine mutation resulted in a decrease in the thermostability in FGF1 [Citation32]. In keratinocyte growth factor (KGF), a member of FGF family proteins, cysteine-free KGF did not bind to the heparin column, and it exhibited less mitogenic activity compared with wild-type KGF [Citation20]. We considered that the roles of cysteine residues in FGF4 are similar to those in KGF, rather than in FGF1. The roles of cysteine residues in the stability of FGF4 remain to be clarified.
Conclusions
To the best of our knowledge, this is the first delineation of a direct relationship among heparin binding activity, mitogenicity, and two conserved cysteine residues in FGF4. Crystal structure and stability analyses of point-mutated FGF4 derivatives will provide direct evidence regarding a relationship among the conformation, the stability, and the two conserved cysteine residues in FGF4.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI No. 24580413 and No. 16K07993) and the Akita Prefectural University President’s Research Project to M. K.
References
- Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149:121–130.
- Tanaka S, Kunath T, Hadjantonakis A, et al. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075.
- Feldman B, Poueymirou W, Papaioannou VE, et al. Requirement of FGF-4 for postimplantation mouse development. Science. 1995;267:246–249.
- Niswander L, Martin GR. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992;114:755–768.
- Danopoulos S, Schlieve CR, Grikscheit TC, et al. Fibroblast growth factors in the gastrointestinal tract: twists and turns. Dev Dyn. 2017;246:344–352.
- Cancilla B, Ford-Perriss MD, Bertram JF. Expression and localization of fibroblast growth factors and fibroblast growth factor receptors in the developing rat kidney. Kidney Int. 1999;56:2025–2039.
- Kratochwil K, Galceran J, Tontsch S, et al. FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(-/-) mice. Genes Dev. 2002;16:3173–3185.
- Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109.
- Leslie JL, Huang S, Opp JS, et al. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect Immun. 2015;83:138–145.
- Xie M, Li JP. Heparan sulfate proteoglycan – a common receptor for diverse cytokines. Cell Signal. 2019;54:115–121.
- Guimond S, Maccarana M, Olwin BB, et al. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1. FGF-2, and FGF-4. J Biol Chem. 1993;268:23906–23914.
- Ishihara M, Shaklee PN, Yang Z, et al. Structural features in heparin which modulate specific biological activities mediated by basic fibroblast growth factor. Glycobiology. 1994;4:451–458.
- Ishihara M. Structural requirements in heparin for binding and activation of FGF-1 and FGF-4 are different from that for FGF-2. Glycobiology. 1994;4:817–824.
- Allen BL, Filla MS, Rapraeger AC. Role of heparan sulfate as a tissue-specific regulator of FGF-4 and FGF receptor recognition. J Cell Biol. 2001;155:845–858.
- Shimokawa K, Kimura-Yoshida C, Nagai N, et al. Cell surface heparan sulfate chains regulate local reception of FGF signaling in the mouse embryo. Dev Cell. 2011;21:257–272.
- Arakawa T, Hsu YR, Schiffer SG, et al. Characterization of a cysteine-free analog of recombinant human basic fibroblast growth factor. Biochem Biophys Res Commun. 1989;161:335–341.
- Caccia P, Nitti G, Cletini O, et al. Stabilization of recombinant human basic fibroblast growth factor by chemical modifications of cysteine residues. Eur J Biochem. 1992;204:649–655.
- Linemeyer DL, Menke JG, Kelly LJ, et al. Disulfide bonds are neither required, present, nor compatible with full activity of human recombinant acidic fibroblast growth factor. Growth Factors. 1990;3:287–298.
- Ortega S, Schaeffer MT, Soderman D, et al. Conversion of cysteine to serine residues alters the activity, stability, and heparin dependence of acidic fibroblast growth factor. J Biol Chem. 1991;266:5842–5846.
- Bare LA, Brown M, Goyal S, et al. Effect of cysteine substitutions on the mitogenic activity and stability of recombinant human keratinocyte growth factor. Biochem Biophys Res Commun. 1994;205:872–879.
- Seno M, Sasada R, Iwane M, et al. Stabilizing basic fibroblast growth factor using protein engineering. Biochem Biophys Res Commun. 1988;151:701–708.
- Sugawara S, Ito T, Sato S, et al. Production of an aminoterminally truncated, stable type of bioactive mouse fibroblast growth factor 4 in Escherichia coli. J Biosci Bioeng. 2014;117:525–530.
- Nelson MD, Fitch DH. Overlap extension PCR: an efficient method for transgene construction. Methods Mol Biol. 2011;772:459–470.
- Hosoi Y, Ando Y, Arakawa M, et al. Production of mouse fibroblast growth factor 4 in E. coli and its application for isolation and maintenance of mouse trophoblast stem cells in vitro. J Reprod Dev. 2011;57:650–654.
- Iha M, Watanabe M, Kihara Y, et al. Effect of ectopic expression of homeoprotein EGAM1C on the cell morphology, growth, and differentiation in a mouse embryonic stem cell line, MG1.19 cells. Reproduction. 2012;143:477–489.
- Mori Y, Sakuraoka M, Suzuki T, et al. Exogenous TPRX1 homeoprotein modulates the gene expression of lineage-specific transcription factors in human embryonal carcinoma cells. Biotechnol Biotec Eq. 2018;32:646–652.
- Sato S, Takahashi T, Nishinomiya H, et al. Common nucleotide sequence of structural gene encoding fibroblast growth factor 4 in eight cattle derived from three breeds. Anim Sci J. 2012;83:260–262.
- Sugawara S, Ito T, Suzuki H, et al. Common amino acid sequences deduced from coding exons of the porcine FGF4 gene in two breeds and production of the encoded protein in Escherichia coli. Biosci Biotechnol Biochem. 2013;77:173–177.
- Li Y, Sun C, Yates EA, et al. Heparin binding preference and structures in the fibroblast growth factor family parallel their evolutionary diversification. Open Biol. 2016; [cited 2019 Jan 05]6.
- Bellosta P, Iwahori A, Plotnikov AN, et al. Identification of receptor and heparin binding sites in fibroblast growth factor 4 by structure-based mutagenesis. Mol Cell Biol. 2001;21:5946–5957.
- Lee J, Blaber M. Structural basis of conserved cysteine in the fibroblast growth factor family: evidence for a vestigial half-cystine. J Mol Biol. 2009;393:128–139.
- Culajay JF, Blaber SI, Khurana A, et al. Thermodynamic characterization of mutants of human fibroblast growth factor 1 with an increased physiological half-life. Biochemistry. 2000;39:7153–7158.
