Abstract
Survivin is a bifunctional protein which regulates cell division and inhibits apoptosis. Survivin is a member of the inhibitor of apoptosis protein (IAP) family. Expression of survivin has been shown to be responsible for apoptosis and resistance to ionizing radiation. The aim of the present study was to investigate the association between −31 G/C promoter polymorphism, survivin protein and glial tumour grading, and to compare survivin versus Ki-67 as a marker. In this study, DNA was isolated from paraffin-embedded sections of 29 patients diagnosed with glial tumours. Survivin gene promoter −31 G/C polymorphism was investigated using PCR-RFLP. For the analysis, 10 µm sections were stained with survivin protein and Ki-67 antibody. Immunohistochemical staining was performed. Survivin showed a positive correlation with Ki-67 (r = 0.604; p = 0.001). The tumour grades correlated with survivin; however, the relationship was not statistically significant (r = 0.345; p > 0.05). We found a significant correlation between tumour grades and Ki-67 (r = 0.663; p < 0.01), suggesting that Ki-67 is a more sensitive marker compared to survivin.
Keywords:
Introduction
Primary brain tumours account for 2% of all primary tumours with a global annual incidence of 5–6 per 100,000 individuals, resulting in mortality before 70 years of age in 7% of affected patients [Citation1]. Glial tumours are responsible for approximately 45% of all intracranial tumours [Citation1,Citation2].
Gliomas consist of a group known as neoplasms of the central nervous system including various tumours which involve the brain and spinal cord. Tumours originating from different types of cells that constitute the brain are termed brain tumours. These tumours are often limited to the brain and rarely metastasize to other parts of the body. Glioblastoma is the most common brain tumour in adults with an invariably fatal course. Glioblastomas are mostly seen in the age group of 50–70 years and account for less than 10% of childhood tumours. Despite extensive research aimed at identifying major risk factors, no apparent cause is determined in the majority of primary brain tumours. In the patients with such primary tumours, there is no relevant family history or inherited mutation likely to be involved in the development of a brain tumour. To date, no apparent relationship has been established with an environmental risk factor, except for exposure to ionizing radiation [Citation3,Citation4].
As a member of the inhibitor of apoptosis (IAP) family, survivin is involved in the inhibition of apoptosis as well as the regulation of mitosis. Inhibitor of apoptosis proteins typically contain 1 to 3 repetitive domains of baculovirus IAP proteins that are involved in cell death [Citation5,Citation6]. The promoter region of survivin includes an element which suppresses transcription during the G1 phase that is transactivated during mitosis, suppressed through G1, and highly expressed during the G2/M phase, indicating the cell cycle regulator role of survivin. The anti-apoptotic effect of survivin is thought to occur during cell proliferation. While the interaction between survivin and caspases remains a subject of debate, the fact that survivin is phosphorylated at threonine 34 indicates that it may interact with caspase 3, 7 and 9 in vitro. Chandele et al. [Citation7] have reported evidence that the phase-specific anti-apoptotic effect of survivin is associated with inhibition of caspase 9 activity. Caspase 9 is a key caspase which activates mitochondria-dependent apoptosis [Citation7,Citation8]. Our aim in this study was to compare the clinical significance of Ki-67 versus survivin in glial tumours.
Materials and methods
Patients
The present study included 29 paraffin-embedded blocks of tissue obtained from 29 patients diagnosed with glial tumours between 1 January 2012 and 31 January 2016 at Goztepe Training and Research Hospital of Istanbul Medeniyet University Medical Faculty. The clinical phase of this study was conducted at the Neurosurgery Department and the experimental stages were performed with the collaboration of the Pathology and Biophysics Departments. A retrospective analysis was performed.
Ethics of experimentation
The study design was approved by the ethics committee of Istanbul Medeniyet University. All patients were given information about the study and informed consent forms were obtained. Tumour samples were obtained from consenting patients.
Identification of -31G/C polymorphism
Sections of 4–10 µm thickness were obtained from paraffin-embedded tissues, paraffin was removed with xylene, and tissues were then dehydrated through alcohol series. DNA isolation from paraffin-embedded tissues was performed using PureLink Genomic DNA Mini Kit (USA) in line with the manufacturer’s instructions. A sample volume of 2 µL DNA was used per PCR reaction. For each reaction, 5′-GTTCTTTGAAAGCAGTCGAG-3′ (forward) and 5′-CCAGTTCTTGAATGTAGAG-3′ (reverse) primers were used for the relevant gene region. The pre-specified PCR conditions were 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 57 °C for 90 seconds, and elongation at 72 °C for 90 s, and the procedure was completed with a final elongation phase at 72 °C for 5 min, yielding PCR products of 341 bp (Techne TC-3000). The amplicon was subjected to restriction with the restriction enzyme EcoO1091 (New England) at 37 °C for 2 h. Restriction products were stained with ethidium bromide and observed under ultraviolet light in 2% agarose gel. The restriction resulted in two bands for the G-allele (236 and 105 bp), while the C-allele was not restricted. PCR and enzyme restriction protocols were applied as previously specified in [Citation9]. At the end of the study, 10% of the samples were selected randomly and PCR was repeated with these samples, yielding consistent genotype findings with previous results.
Immunohistochemical investigation
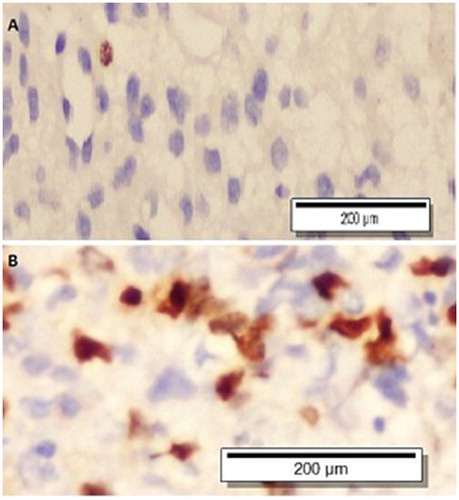
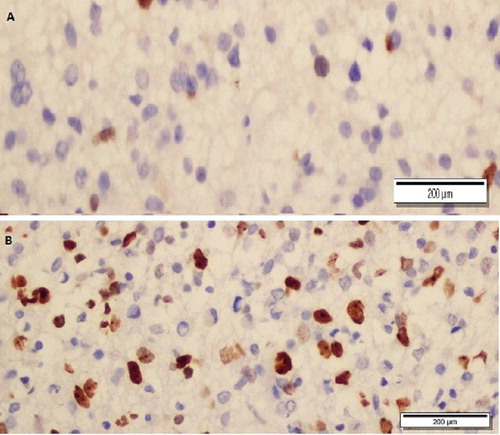
The same pathologist evaluated the nuclear staining of survivin- and Ki-67 stained preparations by counting 1000 cells at 200-fold magnification in the densest region. The presence of survivin was determined as a percentage and was also identified semi-quantitatively by comparing the nuclear staining against the control as follows: nuclear survivin staining at the same intensity as the control 3+, moderate staining 2+, weak staining 1+ ( and ).
Statistical analysis
Statistical analysis was performed using the NCSS (Number Cruncher Statistical System) 2007 Statistical Software (Utah, USA) program. Kruskal–Wallis test and Mann–Whitney U test were used to compare descriptive statistics (mean, standard deviation, median, frequency and ratio) as well as a non-normal distribution of variance between the groups. Spearman’s correlation analysis was utilized to determine inter-variable correlations. Fisher’s Exact test and Fisher–Freeman–Halton test were used for the comparison of qualitative data. Results were evaluated with a confidence interval of 95% at a significance level of p < 0.05.
Results and discussion
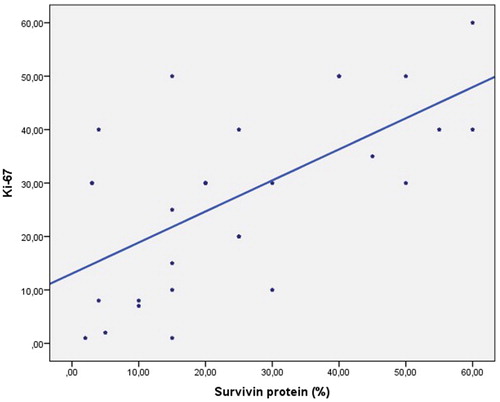
There were no statistically significant gender-related differences in terms of tumour grades (p > 0.05). There was no statistically significant correlation between age and Ki-67 (r = −0.016; p > 0.05). No statistically significant correlation was seen between age and survivin protein levels (r = 0.061; p > 0.05). There were also no statistically significant difference between the gender distributions with regard to tumour sizes (p > 0.05) and no statistically significant difference was seen between the gender distributions in terms of survivin genotypes () (p > 0.05). There was a moderate correlation between tumour grades and survivin protein; however, this association was not statistically significant (r = 0.345; p > 0.05). A statistically significant relationship was shown between tumour grades and Ki-67 level in 66.3% of the samples (r = 0.663; p < 0.01) (). There was no correlation between survivin protein and Ki-67 expression (). The Ki-67 and survivin protein levels did not demonstrate any statistically significant difference between genders (p > 0.05). Furthermore, the survivin genotypes did not show any statistically significant difference across genders (p > 0.05). There was no correlation between the survivin genotype and the immunohistochemical expression of the survivin protein ().
Table 1. Evaluations by gender.
Table 2. Relationship of tumour grades with survivin protein and Ki-67 grade.
The survivin gene polymorphism and allele distribution percentages are presented in . The survivin protein levels varied from 2% to 60%, with a mean value of 24.51 ± 18.22%, while the Ki-67 staining levels ranged from 1% to 60%, with a mean value of 27.31 ± 16.82%. Based on the intensity of Ki-67 staining, we observed the following distribution of Ki-67 protein levels in patients: 13.8% were evaluated as 1+, 6.9% as 1+/2+, 24.1% as 2+, 3.4% as 2+/1+, 10.3% as 2+/3+, 37.9% as 3+ and 3.4% as 3+/2+.
Table 3. Distribution of survivin genotypes.
Mitosis and proliferative activity are reliable methods to determine the biological behaviour of tumours; therefore, studies continue to focus on relevant biological biomarkers for different tumour types. Currently, gene methylations are considered biomarkers in glial tumours [Citation10]. In our study, we evaluated survivin and Ki-67 as potential indicators and performed comparative analysis to determine whether one was superior to the other in terms of distinguishing glial tumour grades.
Some limitations of the present study include the fact that a limited number of patients were included and that oncological treatment and follow-up of the patients continued at external centres, as there is no oncology clinic at our hospital. Since the study was made with patients’ brain tissue, a control group was not used. Furthermore, only inadequate information could be obtained in terms of prognosis and survival of the patients due to limited medical registries of the hospital. Therefore, we could not evaluate the levels of survivin and Ki-67 as potential indicators for prognosis and survival.
The Ki-67 antigen is a nuclear protein complex detected with the MIB-1 antibody. The Ki-67/MIB-1 index is commonly used for the diagnosis of several malignant tumours, including gliomas, and a higher percentage of cells positive for Ki-67 is associated with poor survival [Citation11]. Ki-67/MIB-1 has been shown to be a reliable prognostic and diagnostic biomarker indicating proliferative activity in glial tumours. In astrocytomas, proliferative activity shows correlation with tumour grade and prognosis [Citation12,Citation13]. Increased levels of survivin in cancer cells have been associated with increased proliferative index and unfavourable prognosis [Citation14].
While survivin immunoreactivity is seen in both the cytoplasm and the nucleus, nuclear staining appears to be more prominent and readily detectable [Citation15]. It remains a subject of debate whether nuclear survivin or cytoplasmic survivin is associated with prognosis, and several studies have reported different findings in this regard.
Localization of survivin in the nuclear compartment correlates with cell division and prognosis [Citation15]. Increased expression of cytoplasmic survivin determined by immunohistochemistry shows correlation with advanced tumour grade in gliomas [Citation16]. In high-grade gliomas, simultaneous survivin expression in the nucleus and cytoplasm is associated with poor prognosis, providing a more reliable prognostic indicator compared to MIB-1, p53 expression and EGFR expression [Citation16,Citation17].
Xie et al. [Citation18] compared cytoplasmic and nuclear survivin expression in glioblastomas and demonstrated that cytoplasmic survivin is associated with rapid progression and aggressive phenotype. In a study by Uematsu et al. [Citation19], which included 29 cases, all gliomas were shown to express survivin, and the percentage of survivin-immunoreactive cells (survivin index) demonstrated a strong inverse correlation with survival among these patients. Especially in low-grade gliomas, the survivin index was found to correlate with survival. While the survivin index and MIB-1 index were shown to be similar in anaplastic astrocytomas and malignant gliomas, the survivin index was demonstrated to be a more sensitive marker than the MIB-1 index to predict prognosis in low-grade gliomas. The aforementioned study found no significant difference between anaplastic astrocytomas and low-grade astrocytomas with regard to Ki-67 staining and suggested that survivin index is a better marker than Ki-67 in all grades of glioma [Citation19]. Skjulsvik et al. [Citation13] demonstrated a substantially higher Ki-67 index in high-grade gliomas (grades III and IV) compared to that in low-grade ones (grade I-II), indicating that it is a useful marker to distinguish high-grade from low-grade gliomas [Citation13,Citation19,Citation20]. However, Ki-67 was found to be insufficient to distinguish grade I and II gliomas or grade III and IV gliomas due to the overlapping values [Citation13,Citation21]. Habberstad et al. [Citation12] conducted a study in 27 cases, revealing a substantial correlation between survivin and mitotic activity; however, such a relationship was not shown for survivin. The apparent correlation between survivin and other proliferative markers such as Ki-67 and mitosin has led to the interpretation that nuclear survivin positivity may be employed as a reliable proliferation marker in astrocytic tumours. Another relevant study demonstrated a relationship between survivin and mitotic activity, while there was no correlation with Ki-67, and similar to our study, a lower value was detected for survivin-stained nuclei compared to Ki-67 [Citation12].
Polymorphism studies conducted to determine genotype incidences in the population are performed with large numbers of individuals by comparing the patient population versus healthy individuals and allow understanding whether the polymorphism in question leads to a predisposition to disease. In the present study, we could not analyze survivin polymorphism versus the healthy population as it would not be statistically significant owing to the limited number of preparations we were able to retrieve from the hospital archive. In a meta-analysis by Wang et al. [Citation22] aiming to determine the association between polymorphisms in the survivin −31 G > C promoter region and cancer risk, polymorphism studies on cervical cancer, bladder cancer, gastric cancer, pancreatic cancer, urothelial cancer, lung cancer, colorectal cancer, oesophagal cancer, hepatocellular cancer, nasopharyngeal cancer and ovarian cancer were compared, and the frequency of the C allele was found to be higher in Asian populations compared to Caucasians. The substantially a lower proliferation activity of the −31 G allele compared to the -−31 C allele in the survivin (BIR5) promoter indicates that −31 G/C polymorphism influences survivin expression. These results suggest that the survivin −31 G > C polymorphism may be associated with increased cancer risk, especially in Asian populations [Citation22]. Functional studies on the survivin −31 G > C polymorphism show that the −31 C allele has substantially a higher transcriptional activity compared to −31 G. Increased survivin has also been demonstrated with −31 CC genotypes compared to GC genotypes [Citation23–26].
Conclusions
In our study, nuclear survivin was assessed and cells with positive staining for survivin were seen in all grades of gliomas. Comparison of survivin and Ki-67 immunohistochemical staining rates revealed a statistically significant positive correlation. The statistical findings of our study indicate that Ki-67 could be considered a more sensitive marker than survivin to demonstrate the grade when all grades of gliomas are taken into account. Because there are limited studies on the immuno-staining of survivin, its use as a marker in glial tumours remains a matter of debate and warrants evaluation in a larger case series across a range of tumour grades. As we found no studies investigating survivin −31 G/C polymorphism in glial tumours in the literature, to our knowledge, the present study is the first to be conducted in this field. In conclusion, Ki-67 still appears to be a good indicator of cell differentiation in glial tumours compared to the anti-apoptotic protein, survivin.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alifieris C, Trafalis DT. Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther. 2015;152:63–82.
- Gao Y, Li L, Song L. Expression of p16 and Survivin in gliomas and their correlation with cell proliferation. Oncol Lett. 2015;10:301–306.
- Nieto-Sampedro M, Valle-Argos B, Gómez-Nicola D, et al. Inhibitors of glioma growth that reveal the tumour to the Immune System. Clin Med Insights Oncol. 2011;5:265–314.
- Li J, Han Y, Zhou D, et al. Downregulation of survivin gene expression affects ionizing radiation resistance of human T98 glioma cells. Cell Mol Neurobiol. 2018;38:861–868.
- Li F, Ambrosini G, Chu EY, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584.
- Bae IS, Kim CH, Kim JM, et al. Correlation of survivin and B-cell lymphoma 2 expression with pathological malignancy and anti-apoptotic properties of glial cell tumours. Biomedical Reports. 2017;6:396–400.
- Chandele A, Prasad V, Jagtap JC, et al. Upregulation of survivin in G2/M cells and inhibition of caspase 9 activity enhances resistance in staurosporine-induced apoptosis. Neoplasia. 2004;6:29–40.
- Zhang F, Chu J, Wang F. Expression and clinical significance of cyclooxygenase 2 and survivin in human gliomas. Oncol Lett. 2017;14:1303–1308.
- Gazouli M, Tzanakis N, Rallis G, et al. Survivin -31G/C promoter polymorphism and sporadic colorectal cancer . Int J Colorectal Dis. 2009;24:145–150.
- Kim HR, Lee JJ, Lee J-I, et al. Malignant glioma with neuronal marker expression: a clinicopathological study of 18 cases. J Korean Neurosurg Soc. 2015;59:44–51.
- Prayson RA. The utility of MIB-1/Ki-67 immunostaining in the evaluation of central nervous system neoplasms. Adv Anat Pathol. 2005;12:144–148.
- Habberstad AH, Gulati S, Torp SH. Evaluation of the proliferation markers Ki-67/MIB-1, mitosin, survivin, pHH3, and DNA topoisomerase IIα in human anaplastic astrocytomas-an immunohistochemical study. Diagnostic Pathol. 2011;6:1–8.
- Skjulsvik AJ, Mørk JN, Torp MO, et al. Ki-67/MIB-1 immunostaining in a cohort of human gliomas. Int J Clin Exp Pathol. 2014;7:8905–8910.
- Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther.. 2006;5:1087–1098.
- Shirai K, Suzuki Y, Oka K, et al. Nuclear survivin expression predicts poorer prognosis in glioblastoma. J Neurooncol. 2009;91:353–358.
- Sasaki T, Lopes BM, Hankins GR, et al. Expression of survivin, an inhibitor of apoptosis protein, in tumors of the nervous system. Acta Neuropathol. 2002;104:105–109.
- Saito T, Arifin MT, Hama S, et al. Survivin subcellular localization in high-grade astrocytomas: simultaneous expression in both nucleus and cytoplasm is negative prognostic marker. J Neurooncol. 2007;82:193–198.
- Xie D, Zeng Y, Wang H, et al. Expression of cytoplasmic and nuclear survivin in primary and secondary human glioblastoma. Br J Cancer.. 2006;94:108–114.
- Uematsu M, Ohsawa I, Aokage T, et al. Prognostic significance of the immunohistochemical index of survivin in glioma: a comparative study with the MIB-1 index. J Neurooncol. 2005;72:231–238.
- Song Z, Pan Y, Ling G, et al. Escape of U251 glioma cells from temozolomide-induced senescence was modulated by CDK1/survivin signalling. Am J Transl Res. 2017;9:2163–2180.
- Conde M, Michen S, Wiedemuth R, et al. Chromosomal instability induced by increased BIRC5/Survivin levels affects tumorigenicity of glioma cells. BMC Cancer. 2017[Cited Dec 31 2018].17:889.
- Wang X, Huang L, Xu Y, et al. Association between survivin − 31G > C promoter polymorphism and cancer risk: a meta-analysis. Eur J Hum Genet.. 2012;20:790–795.
- Xu Y, Fang F, Ludewig G, et al. A mutation found in the promoter region of the human survivin gene is correlated to overexpression of survivin in cancer cells. DNA Cell Biol. 2004;23:527–537.
- Jang JS, Kim KM, Kang KH, et al. Polymorphisms in the survivin gene and the risk of lung cancer. Lung Cancer. 2008;60:31–39.
- Budak M, Yildiz M. Epigenetic modifications and potential treatment approaches in lung cancers. In: Costa Torres AF, editor. Lung cancer-strategies for diagnosis and treatment. IntechOpen; 2018; p. 115–135.
- Yalcin O, Budak M. Un-methylation of the survivin gene has no effect on immunohistochemical expression of survivin protein in lung cancer patients with squamous cell carcinoma. Bratislavske Lekarske Listy. 2017;118:160–163.