Abstract
Atrial septal defect (ASD) is one of the most prevalent types of congenital heart disease (CHD). The pathogenic role of miRNAs in the development of ASD has not yet been fully elucidated. The aim of this study was to examine the miRNA profile of ASD patients, and to identify the role of miRNAs in the pathogenesis of ASD. We performed a miRNA comparison between the atrial septa of three normal fetuses and three ASD patients by microarray, followed by chromosome clustering and bioinformatic analysis to identify the dysregulated miRNA clusters between these two groups. Furthermore, qRT-PCR in the mouse developing heart was used to exclude differences resulting from the use of unpaired stage patient samples. After normalization, 70 dysregulated miRNAs were detected between the two groups. Advanced chromosome clustering and bioinformatic analysis showed that two upregulated miRNA clusters (miR-29 and miR-143/145) and three downregulated miRNA clusters (miR-17-92, miR-106b-25 and miR-503/424) were associated with ASD. Further qRT-PCR in the mouse developing heart found that the dysregulated expression levels of all the clusters, except the miR-143/145 cluster, were associated with the occurrence of ASD. This study reveals four dysregulated miRNA clusters, which will enable further elucidation of the pathogenic mechanism of ASD.
Introduction
Congenital heart disease (CHD), a structural abnormality of the fetal heart or the major vessels, is one of the most common congenital anomalies and the main non-infectious cause of death among children [Citation1]. The prevalence of CHD varies from 5.4 to 16.1 per 1000 births worldwide [Citation2]. The risk factors for CHD are complex and multifactorial, including genetic and environmental factors [Citation3]. CHD is clinically classified into more than 20 distinct categories, including atrial septal defect (ASD), ventricular septal defect (VSD), tetralogy of Fallot (TOF), patent ductus arteriosus (PDA), double outlet right ventricle, transposition of the great arteries (TGA), endocardial cushion defect, persistent truncus arteriosus and hypoplastic left ventricle [Citation4]. Of these, ASD is one of the most common types of CHD, with an estimated incidence of 100 per 100,000 live births [Citation5, Citation6]. ASD is a non-cyanotic CHD where there is abnormal blood flow between the left and right atria caused by the ASD [Citation5]. Although the embryology and physiology of ASD have been elucidated, its etiology and pathogenesis remain largely unknown.
MicroRNAs (miRNAs) are a class of small single-stranded noncoding RNA molecules, typically 18–22 nucleotides in length, which can negatively regulate gene expression at the post-transcriptional level by binding to targeted messenger RNA (mRNA) [Citation7,Citation8]. Although miRNAs only represent 1% of human genes, studies have estimated that miRNAs can potentially regulate 30% of human mRNA through complex regulatory networks [Citation9,Citation10]: they participate in diverse physiological and pathological processes, such as proliferation, differentiation, development, apoptosis and cell death [Citation7,Citation11–14]. miRNAs play an important role in embryogenesis and organogenesis [Citation15]: they are involved in embryonic heart development, myocardial cell growth and differentiation, and contribute to cardiovascular disease and cardiac remodeling [Citation16–18]. miRNAs also play an increasingly important role in the diagnosis and treatment of heart-related diseases [Citation19]. Dysregulation of miRNA expression has been implicated in many kinds of CHD [Citation20], including VSD, TOF, HLHS and BAV [Citation20,Citation21]. However, few studies have been carried out to reveal the regulatory functions of miRNAs in ASD [Citation22].
Microarray analysis of miRNAs for gene expression profiles is one of the most important methods used to screen and study the pathophysiology of specific disease-associated miRNAs. Therefore, to throw more light on the role of miRNA in the ASD regulatory network, we performed microRNA microarray analysis of the tissues of patients with ASD and ASD-free controls, and investigated the changes in global miRNA expression levels. Advanced bioinformatic analyses, including prediction of target genes, identification of interaction relationships between target genes and functional enrichment analysis were also employed to comprehensively understand the molecular mechanisms of translational modulation by miRNAs in ASD. Our findings provide valuable evidence to clarify the pathogenesis of ASD at the molecular level.
Subjects and methods
Participants
Three ASD patients and three normal control participants were enrolled in this study. Experimental tissue samples were obtained from ASD patients aged 6–12 months, who were diagnosed by echocardiography and verified to have no other deformities, and who underwent ASD repair surgery in Yan’an Affiliated Hospital of Kunming Medical University. The ASD samples were the rims of the holes in the atrial septa that were excised and cast off before a tissue patch was fixed in. The control tissues were the atrial septa of fetuses with gestational ages of 30–33 weeks, obtained from pregnant women who underwent voluntary abortion in Yan’an Affiliated Hospital of Kunming Medical University. Fetal echocardiography was used to exclude any kind of CHD.
Experimental animals
Wild-type C57BL/6J mice were selected for collection of mouse embryonic atrial septum tissue at defined stages from timed mating pairs. Pregnancy was detected by visual inspection of a distended abdomen and weight gain. Heart tubes were collected at E8.5 using a dissecting microscope, and atrial septa of the embryos were obtained from E10.5, E12.5, E13.5, E14.5, E15.5, E17.5, E18.5, E21.5 and adult mice. The E21.5 embryos were newborn mice, and adult mice were 6 months old.
Ethics of experimentation
All procedures in this study were performed in compliance with the Helsinki Declaration and national laws. The study was approved by the Ethics Committee of Yan'an Affiliated Hospital of Kunming Medical University and signed informed consent was collected from all patients and guardians.
All experimental protocols involving animals were approved by the Yan'an Affiliated Hospital of Kunming Medical University Animal Care and Use Committee.
RNA extraction, quality control and labelling
Total RNA was isolated using the mirVana™ miRNA Isolation Kit (AM1560, Ambion, Austin, TX, USA) according to the manufacturer’s instructions. The RNA was detected by a NanoDrop 2000 Spectrophotometer (Thermo Scientific, USA) and its purity was assessed by the ratio of the absorbances at 260 and 280 nm (A260/A280). A value between 1.8 and 2.1 was considered acceptable. The quality was also assessed by an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, US). The miRNA was labelled by the miRNA Complete Labeling and Hyb Kit (Agilent Technologies, Santa Clara, CA, USA) following the manufacturer’s instructions.
miRNA microarray hybridization
Labelled RNA samples were detected using Agilent’s human miRNA microarray, version 19.0. Each slide was hybridized with 100 ng Cy3-labeled RNA using the miRNA Complete Labeling and Hyb Kit (Agilent Technologies, Santa Clara, CA, USA) in a hybridization oven at 55 °C with 20 rpm for 20 hours according to the manufacturer’s instructions. After hybridization, the slides were washed in staining dishes (Thermo Shandon, Waltham, MA, USA) with Gene Expression Wash Buffer Kit (Agilent Technologies, Santa Clara, CA, USA).
Microarray scanning and image data acquisition
An Agilent Microarray Scanner (Agilent Technologies, Santa Clara, CA, USA) and Feature Extraction software 10.7 (Agilent Technologies, Santa Clara, CA, USA) with default settings were used to scan the slides. Raw data were normalized by Quantile algorithm using Gene Spring Software 11.0 (Agilent Technologies, Santa Clara, CA, USA). Then, differentially expressed miRNAs between the ASD and control groups were identified using an unpaired Student’s t-test with P values > 0.05 and fold change > 2.0 or < 0.5.
miRNA target gene prediction and bioinformatic analysis
The target genes of the differentially expressed miRNAs were predicted by miRTarBase Release 7.0 (http://miRTarBase.mbc.nctu.edu.tw/), which is a public platform providing known experimentally validated miRNA targets [Citation23]. GO analysis was applied to analyse the main functions of the targets of the differentially expressed miRNAs by Gene Ontology, the key functional classification of the NCBI [Citation24]. GO functional and pathway enrichment analyses were conducted for the target genes using the DAVID online tools [Citation25]. Statistical analysis of the GO terms and pathway analysis was done by the Fisher’s exact and χ2 tests. Significant results were defined as a P value < 0.05, where a smaller P-value indicated a more significant function or pathway.
Quantitative real-time polymerase chain reaction (qRT-PCR)
qRT-PCR was performed on the CFX96 Touch™ detection system (BIORAD, USA). The first strand of the cDNA was synthesized with adjusted concentrations of RNA, and the corresponding genes were amplified with EVA Green Supermix (BIORAD, USA). All the primers used for the qRT-PCR were obtained from GeneCopoeia (USA). The miRNA levels were calculated relative to U6 RNA as an internal control using the 2−ΔΔCt method.
Results and discussion
Dynamic miRNA profiles and differentially expressed miRNA chromosome clustering
In vertebrate embryogenesis, heart development is an important biological event. miRNAs show spatially and temporally restricted expression patterns that play essential roles in the maintenance of heart development [Citation26]. Previous studies have suggested that some cardiac-specific miRNAs, including miR-1, miR-133a, miR-181c, miR-92 and the miR-17-92 cluster, may be involved in the pathogenesis of VSD, which, like ASD, is a discontinuation in the septal wall of the heart [Citation21]. To better understand the molecular mechanisms of abnormal atrial septum formation, miRNAs were isolated and purified from three ASD patients and three controls. After quality assessment, the miRNA expression patterns were detected by the Agilent V19.0 miRNA array to identify miRNA expression differences between these two groups. After normalization of the raw data, we found 70 differentially expressed miRNAs in total (fold change (FC) > 2 or < 0.5, P-value < 0.05) between the ASD and control groups, which included 33 downregulated and 37 upregulated miRNAs ( and Supplemental Table S1). The heatmap and a hierarchical analysis of the 70 miRNAs are shown in . To identify the function of the differentially expressed miRNAs, we aligned the co-downregulated miRNAs to their chromosomal locations based on the chromosome coordinates of each miRNA (). Based on previous research, we speculated that miRNAs that are located close together may have the same biological function [Citation27,Citation28]. From all 70 differentially expressed miRNAs, we found nine miRNA clusters where the distance between the miRNAs was no greater than 5000 bp ( and ). There were two upregulated miRNA clusters, including five miRNAs, and seven downregulated miRNA clusters, including 19 miRNAs.
Figure 1. An overview of the miRNA data of the atrial septum tissue samples from ASDs patients and normal controls. (a) The differentially expressed miRNAs between ASD and control groups. (b) Heatmap and hierarchical clustering of differentially expressed miRNAs. (c) Chromosome clustering analysis of differentially expressed miRNAs.
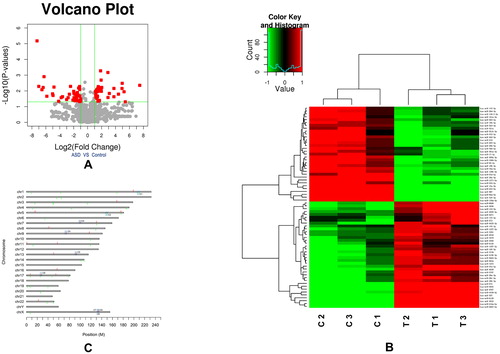
Table 1. Chromosome clusters of upregulated and downregulated miRNAs in ASDs patients vs. controls.
miRNA target gene prediction and bioinformatic analysis
To further characterize the functions of the miRNAs we identified, we used miRTarBase Release 7.0, in which the target genes of each included miRNA have been validated by reporter assay, qRT-PCR, western blot, microarray or pSILAC, to predict the target genes of each miRNA in the cluster. Then, to determine the function of the miRNA cluster, we determined any co-target genes in each cluster for further bioinformatic analysis, which showed that the miR-29, miR-143/145, miR-17-92, miR-106a-36, miR-106b-25 and miR-503/424 clusters have co-target genes (). Next, by employing the annotation tool of the DAVID bioinformatics database, we analyzed the GO terms of the target genes in these six clusters. We focused on the significant GO terms that were associated with heart development and found that in the upregulated group, some target genes of the miR-29 cluster and miR-143/145 cluster participated in cardiac morphogenesis (). In the downregulated group, we found that some target genes of the miR-17-92, miR-106b-25 and miR-503/424 clusters were also associated with this process, including cell proliferation, cell migration, cell adhesion and others, all of which play important roles in the embryonic heart [Citation29–31]. We also established the relationship between the miRNA clusters, target genes and GO terms (). Previous studies have shown similar interactions. miRNA-940 can influence the proliferation and migration of hCMPCs to cause TOF [Citation32], and miR-1 and miR-133 have been shown to play fundamental roles in ventricular cardiomyocyte proliferation, as their overexpression can lead to VSD [Citation33,Citation34].
Figure 2. Interaction network of dysregulated miRNA clusters, target genes and GO terms of upregulated (a) and downregulated (b) groups.
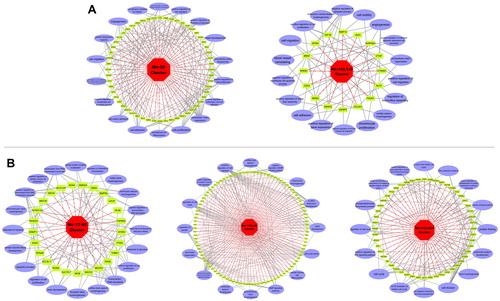
Table 2. Co-target genes of six differentially expressed miRNA clusters in patients with ASDs.
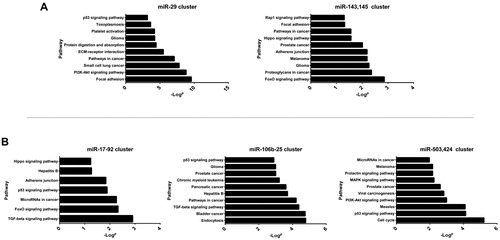
To further explore the pathways affecting the morphogenesis of the atrial septum during heart development, we performed pathway analysis based on KEGG databases using Fisher’s exact test. We focused on the pathways that might participate in cardiac morphogenesis (). The results indicated that in the upregulated group, both the miR-29 and miR-143/145 clusters were involved in focal adhesion (), a pathway that plays a central role in cell migration, cell proliferation and cell differentiation and is associated with cardiac septum formation [Citation35]. In the downregulated group, both the miR-17-92 and miR-106b-25 clusters were involved in the TGF-beta signalling pathway (). A wide spectrum of cellular functions such as proliferation, differentiation, apoptosis, and migration are regulated by TGF-beta family members, which is essential in several different cell types for normal heart development [Citation36]. Meanwhile, the miR-503/424 cluster was involved in the cell cycle pathway and the MAPK (mitogen activated protein kinase) signalling pathway, which may affect the function of cardiac myocytes during heart morphogenesis.
Dynamic microRNA expression profile during heart development
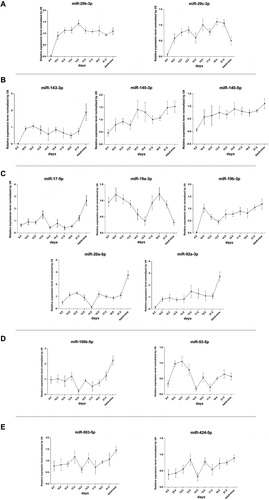
In our study, tissue samples of the ASD group were derived from infants with ASD aged 6–12 months, while the normal control samples were obtained from fetal atrial septa at a gestational age of 30–33 weeks. Because induced abortion was not recommended to any pregnancy in which the fetus was diagnosed with ASD alone, it was impossible to obtain atrial septum tissues from fetuses with ASD. These led us to use unpaired stage samples between the case and control groups. Thus, it was important to exclude the differences in miRNA expression levels throughout normal cardiac morphogenesis by investigating the miRNA expression dynamics during heart development. We used the C57BL/6 mouse as a model, and examined the expression profiles of miRNAs in the upregulated and downregulated clusters by qRT-PCR at continuous embryonic heart development stages, including E8.5, E10.5, E12.5, E13.5, E14.5, E15.5, E17.5, E18.5, E21.5 and adult mice. According to the parallel stages of heart development in mice and humans, atrial septum formation starts at 7.5 weeks in humans and at E11.5 days in mice [Citation37]. Based on this comparison, we determined that a human gestational age of 30–33 weeks is parallel to mouse embryonic stages beyond E17.5, as we have previously reported [Citation38]. In the upregulated cluster, miR-29b-3p showed stable expression and miR-29c-3p was downregulated during the heart development process (), allowing us to exclude differences caused by developmental stages. However, three miRNAs in the miR-143/145 cluster showed an increased expression trend (), which suggested that the observed upregulation of the miR-143/145 cluster in the ASD group is a result of normal heart development and there is no correlation with the occurrence of CHD. In the downregulated clusters, excluding miR-19a-3p, the rest of the miRNAs in the miR-17-92, miR-106b-25 and miR-503/424 clusters were increasing during the E17.5 to adulthood period (). Therefore, the observed differences were not caused by normal heart development, and they may have a close relationship with the occurrence of CHD by affecting different biological processes during heart morphogenesis.
Figure 4. Expression profile of miRNAs during C57BL/6 mice heart development: miR-29 cluster (a), miR-143/145 cluster (b), miR-17-92 cluster (c), miR-106b-25 cluster (d) and miR-503/424 cluster (e).
There is a paucity of research on miRNA expression profiles in ASD. Song et al. [Citation39] reported that hsa-let-7a, hsa-let-7b and hsa-miR-486 were significantly upregulated in the plasma of children with ASD. A miRNA SNP study indicated that the miR-196a2 (rs11614913 T > C) homozygous CC variant and the C allele are negatively associated with ASD [Citation40]. A familial ASD study showed that a new miR-139-5p target site may be involved in CHD [Citation22]. In our study, we found that the miR-17-92 and mir-106b-25 clusters were downregulated in the ASD group. Some early studies have reported that targeted deletion of the miR-17-92 cluster can cause neonatal lethality resulting from VSDs and lung hypoplasia [Citation41], and dysregulation of this cluster can lead to lethal hypertrophic cardiomyopathy and arrhythmogenesis [Citation42]. miR-17-92 plays a role in BMP signalling during the development of the cardiac outflow tract [Citation43]. Furthermore, the miR-106b-25 cluster is a paralogue cluster of miR-17-92, and the loss of both can lead to the development of exacerbated cardiac failings, such as VSDs [Citation41]. Meanwhile, the miR-29 cluster is important for regulating extracellular matrix expression during pathological remodelling in cardiac tissue [Citation44]. However, it has also been reported that the miR-29 cluster is associated with CHD, including TOF and VSD [Citation45]. Therefore, considering the relationship between miRNA clusters and CHD, it is probable that aberrant expression of the above dysregulated microRNA clusters in ASD patients may affect atrial septal morphogenesis.
Limitations of the study
This investigation has some limitations. First, we used dynamic mouse heart development to exclude the dysregulation of miRNAs caused by the unpaired stage samples between the human ASD and control groups. This may affect the accuracy of the miRNA differential expression. Second, this study needs to be verified in a larger, independent population to confirm the dysregulation of these microRNAs in ASD. Future studies are needed to overcome these limitations.
Conclusions
Here, we performed a miRNA microarray between atrial septum tissues from ASD and control groups. Advanced bioinformatic analyses indicated that a strong dysregulation of the miRNA expression profiles was present in ASD patients. The results of this study showed that upregulation of miRNA cluster miR-29 and downregulation of clusters miR-17-92, miR-106b-25 and miR-503/424 may be associated with ASD. We have identified some dysregulated miRNAs in ASD patients that may disrupt target genes involved in cardiac development during heart septum morphogenesis. Identification of these dysregulated miRNAs will allow a better understanding of the pathogenesis of ASD and further improve the treatment of CHD.
Acknowledgments
We are most grateful to the participants who have so willingly joined in this study. And we sincerely thank all hospital staff for participating in and supporting this study.
Disclosure statement
The authors declare that they have no conflict of interest.
Funding
This work was supported by grants from the National Natural Science Foundation of China [under grants nos. 31160230 and 81560060].
References
- Mitchell SC, Korones SB, Berendes HW. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation. 1971;43:323–332.
- Van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011; 58:2241–2247.
- Hinton RB. Genetic and environmental factors contributing to cardiovascular malformation: a unified approach to risk. J Am Heart Assoc. 2013 ;[cited 2019 Jan 03]; 2:e000292.
- Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009; 119:480–486.
- Geva T, Martins JD, Wald RM. Atrial septal defects. Lancet. 2014; 383:1921–1932.
- Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001 ;107:[cited 2019 Jan 03]; E32.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116:281–297.
- Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014; 15:509–524.
- John B, Enright AJ, Aravin A, et al. Human MicroRNA targets. PLoS Biol. 2004 ;2:e363. [cited 2019 Jan 03];
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005; 120:15–20.
- Shivdasani RA. MicroRNAs: regulators of gene expression and cell differentiation. Blood. 2006; 108:3646–3653.
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009; 136:215–233.
- Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015; 87:3–14.
- Barwari T, Joshi A, Mayr M. MicroRNAs in cardiovascular disease. J Am Coll Cardiol. 2016; 68:2577–2584.
- Liang Y, Ridzon D, Wong L, et al. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8:166., [cited 2019 Jan 03];
- Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res. 2009; 104:724–732.
- Batkai S, Bar C, Thum T. MicroRNAs in right ventricular remodelling. Cardiovasc Res. 2017; 113:1433–1440.
- Hagiwara S, Kantharidis P, Cooper ME. MicroRNA as biomarkers and regulator of cardiovascular development and disease. Curr Pharm Des. 2014; 20:2347–2370.
- Shah P, Bristow MR, Port JD. MicroRNAs in heart failure, cardiac transplantation, and myocardial recovery: Biomarkers with therapeutic potential. Curr Heart Fail Rep. 2017; 14:454–464.
- Hoelscher SC, Doppler SA, Dreßen M, et al. MicroRNAs: pleiotropic players in congenital heart disease and regeneration. J Thorac Dis. 2017; 9:S64–S81.
- Smith T, Rajakaruna C, Caputo M, et al. MicroRNAs in congenital heart disease. Ann Transl Med. 2015;3:333 [cited 2019 Jan 03];
- Wang Y, Du X, Zhou Z, et al. A gain-of-function ACTC1 3'UTR mutation that introduces a miR-139-5p target site may be associated with a dominant familial atrial septal defect. Sci Rep. 2016[Cited 2019 Jan 03].6:25404.
- Hsu SD, Lin FM, Wu WY, et al. miRTarBase: a database curates experimentally validated microRNA-target interactions. Nucleic Acids Res. 2011; 39:D163–D169.
- Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet. 2000; 25:25–29.
- Dennis G, Sherman BT, Hosack DA, et al. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [cited 2019 Jan 03];
- Thum T, Catalucci D, Bauersachs J. MicroRNAs: novel regulators in cardiac development and disease. Cardiovasc Res. 2008; 79:562–570.
- Fabbri M, Bottoni A, Shimizu M, et al. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA. 2011; 305:59–67.
- Wang W, Li R, Meng M, et al. MicroRNA profiling of CD3+ CD56+ cytokine-induced killer cells. Sci Rep. 2015;5:9571–9503. [cited 2019 Jan
- Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006; 313:1922–1927.
- Sedmera D, Thompson RP. Myocyte proliferation in the developing heart. Dev Dyn. 2011; 240:1322–1334.
- Sun C, Kontaridis MI. Physiology of cardiac development: from genetics to signaling to therapeutic strategies. Curr Opin Physiol. 2018; 1:123–139.
- Liang D, Xu X, Deng F, et al. miRNA-940 reduction contributes to human tetralogy of Fallot development. J Cell Mol Med. 2014; 18:1830–1839.
- Li J, Cao Y, Ma XJ, et al. Roles of miR-1-1 and miR-181c in ventricular septal defects. Int J Cardiol. 2013; 168:1441–1446.
- Liu N, Bezprozvannaya S, Williams AH, et al. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008; 22:3242–3254.
- Chen D, Zhang Z, Meng Y. Systematic tracking of disrupted modules identifies altered pathways associated with congenital heart defects in down syndrome. Med Sci Monit. 2015; 21:3334–3342.
- Arthur HM, Bamforth SD. TGFbeta signaling and congenital heart disease: insights from mouse studies. Birth Defects Res A Clin Mol Teratol. 2011; 91:423–434.
- Krishnan A, Samtani R, Dhanantwari P, et al. A detailed comparison of mouse and human cardiac development. Pediatr Res. 2014; 76:500–507.
- Wang W, Niu Z, Wang Y, et al. Comparative transcriptome analysis of atrial septal defect identifies dysregulated genes during heart septum morphogenesis. Gene. 2016; 575:Pt 1): 303–312.
- Song Y, Higgins H, Guo J, et al. Clinical significance of circulating microRNAs as markers in detecting and predicting congenital heart defects in children. J Transl Med. 2018[Cited 2019 Jan 3].16:42.
- Yu K, Ji Y, Wang H, et al. Association of miR-196a2. miR-27a, and miR-499 Polymorphisms with Isolated Congenital Heart Disease in a Chinese Population. Genet Mol Res. 2016[Cited 2019 Jan 3].15.
- Ventura A, Young AG, Winslow MM, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008; 132:875–886.
- Danielson LS, Park DS, Rotllan N, et al. Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. Faseb J. 2013; 27:1460–1467.
- Bai Y, Wang J, Morikawa Y, et al. Bmp signaling represses Vegfa to promote outflow tract cushion development. Development. 2013; 140:3395–3402.
- Kriegel AJ, Liu Y, Fang Y, et al. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics. 2012; 44:237–244.
- Zhu S, Cao L, Zhu J, et al. Identification of maternal serum microRNAs as novel non-invasive biomarkers for prenatal detection of fetal congenital heart defects. Clin Chim Acta. 2013; 424:66–72.