Abstract
This study examined the regulation of phenotype and function of natural killer (NK) cells by nasopharyngeal carcinoma (NPC) cells with metastasis-associated in colon cancer-1 (MACC1) overexpression. Thirty patients with NPC and 20 healthy subjects were included. Peripheral blood (5 mL) was collected, and NK cells were purified using Ficoll and negative separation. NPC HONE1 cells were transfected with pcDNA3.1-MACC1 or negative control plasmids, and culture supernatant was collected for co-culture with NK cells. Flow cytometry was used to determine the expression of activated and inhibitory receptors on the cells. Western blotting was used to determine the expression of proteins. The results showed that the ratio of NK cells in the peripheral blood of NPC patients was elevated, but the activity and tumor-killing effect of NK cells were reduced. MACC1 promoted the escape of HONE1 cells from killing by NK cells. HONE1 with overexpression of MACC1 down-regulated the expression of NKG2D in NK cells, inhibited the proliferation of NK cells and escaped the immune surveillance by NK cells. MACC1 down-regulated the expression of NKG2D in NK cells by up-regulating the expression of TGF-β1 in HONE1 cells, thereby inhibiting the activity of NK cells. MACC1 inhibited the cell cycle of NK cells via TGF-β1 pathway. The study demonstrated that MACC1 elevated the expression of TGF-β1 by NPC HONE1 cells, thereby inhibiting NKG2D expression and G1/S transition in NK cells. MACC1 helped NPC HONE1 cells avoid killing by NK cells.
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common head and neck malignant tumors in the clinic, and China has the highest incidence and mortality rate of NPC in the world [Citation1]. Studies show that about 80% of NPC cases around the world are in China [Citation2, Citation3]. NPC is occult and its early symptoms are atypical, including blood flow in nasal mucus, unilateral tinnitus, ear occlusion, hearing loss or headache [Citation4]. Most NPC patients have symptoms of cervical lymph node enlargement when they are diagnosed, suggesting metastasis of the tumor [Citation5]. At present, recurrence and metastasis are the main reasons for poor prognosis of NPC patients, and immune escape is one of the key reasons for tumor cell survival and metastasis.
Metastasis-associated in colon cancer-1 (MACC1) is a gene discovered after genomic analysis of human colon cancer tissue, metastatic tumor tissue and human normal tissue [Citation6, Citation7]. Further studies show that the MACC1 gene is highly expressed in a variety of tumors such as colon cancer, liver cancer, and esophageal cancer [Citation8, Citation9]. The protein encoded by MACC1 consists of four domains (ZU5, SH3 and 2 death domains at the hydroxyl terminus), which are closely related to cell signal transduction and apoptosis [Citation10]. MACC1 is a transcription factor that up-regulates c-Met expression, thereby activating HGF/c-Met signaling pathway and promoting tumor proliferation, invasion, metastasis and survival [Citation11]. It is reported that MACC1 is up-regulated in NPC and promotes tumor invasion and metastasis [Citation12]. However, it is not reported whether MACC1 regulates the immunological properties of tumor cells while promoting the metastatic potential of NPC cells.
Natural killer (NK) cells are the first line of defense against infection and tumour development [Citation13]. NK cells exert their biological functions in tissues after maturation [Citation14]. NK cells have been proved to have a strong tumor killing function [Citation15]. After knocking out NK cells in mice, the incidence of tumors is increased significantly [Citation16]. At clinical level, adoptive immunotherapy by NK cells has already been applied in clinical cancer treatment [Citation17]. In recent years, the immunotherapy strategy by Car-NK has been widely concerned by researchers [Citation18]. As a key component of tumor tissue microenvironment, tumor cells play important roles in microenvironment remodeling [Citation19], but their regulatory effects and mechanism of action on NK cells are not clear yet. In the present study, we examined the regulation of phenotype and function of NK cells by NPC cells with MACC1 overexpression and investigated the underlying mechanism of action at tissue and cellular levels.
Subjects and methods
Subjects
A total of 30 patients with NPC (21 males and 9 females; age range 31–68 years; mean age 47.6 years) who were treated at our hospital between May 2015 and January 2017 were included in the present study. None of the patients had history of malignant tumors, chemotherapy or radiotherapy. All patients had poorly differentiated or highly differentiated squamous cell carcinoma. Among all patients, 17 had cervical lymph node metastasis, and 13 had no lymph node metastasis. According to TNM staging and grading standards by Union for International Cancer Control (UICC) in 2003, 11 patients were at stage I, 6 patients were at stage II, 5 patients were at stage III, and 8 patients were at stage IV. In addition, 20 healthy subjects were included as a control group. Peripheral blood (5 mL) was collected from all patients and healthy subjects.
Cell culture and transfection
NPC HONE1 cell line (Call Bank, Chinese Academy of Sciences, Shanghai, China) was cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin and 100 IU/mL streptomycin under 37 °C, 5% CO2, and 70% humidity. The cells were passaged every 3 days.
On the day before transfection, HONE1 cells (2 × 105) in logarithmic growth were seeded into 24-well plates, and cultured in antibiotics-free RPMI-1640 medium supplemented with 10% FBS until reaching 60–70% confluency. In the first vial, 0.5 µg pcDNA3.1-MACC1 (Hanbio Biotechnology Co., Ltd., Shanghai, China) was mixed with 50 µL Opti Mem medium (Thermo Fisher Scientific, Waltham, MA, USA). In the second vial, 1 µL Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) was mixed with 50 µL Opti Mem medium. After standing still for 5 min, the two vials were combined for additional waiting at room temperature for 20 min. Then, the mixtures were added onto cells in respective groups. Six hours later, the medium was replaced with RPMI-1640 medium containing 10% fetal bovine serum. After cultivation for 48 h, the cells were collected for further assays.
Isolation, purification and culture of NK cells
To isolate peripheral blood mononuclear lymphocytes (PBMCs), 3 mL whole blood was gently added onto the surface of 3 mL Ficoll solution, followed by centrifugation at 2000×g and 4 °C for 20 min. The middle mist-like layer was lymphocytes, which was transferred to a new 15 mL tube. The separated lymphocytes were mixed with phosphate-buffered saline (PBS) to reach 10 mL, and centrifuged at 2000×g and 4 °C for 10 min before removing supernatant. Then, the lymphocytes were washed again with 5 mL PBS before being mixed with 1x BD buffer (Thermo Fisher Scientific, Waltham, MA, USA). NK cell negative separation kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to purify the cells according to the manufacturer’s manual. Briefly, isolated peripheral blood mononuclear lymphocytes were resuspended in 0.5 mL BD IMag buffer (MagniSortHuman NK Cell Enrichment Kit, BD Biosciences; Franklin Lakes, NJ, USA), and mixed with 200 μL biotin. After incubation at room temperature in dark for 30 min, the labelled cells were transferred to a new tube which was kept on magnet for 7 min. The supernatant was transferred to a new tube. This procedure was repeated three times, and NK cells without beads and biotin were obtained. The resulted NK cells were cultured in RPMI-1640 medium containing 10% FBS, 100 IU interleukin (IL)-2 and 100 IU IL-15 under 37 °C and 5% CO2 for 48 h before use.
Determination of killing effect of NK cells
HONE1 cells (2 × 104) were mixed with NK cells at a ratio of 1:4, and cultured in 24-well plates at 37 °C and 5% CO2 for 24 h. NK cells from healthy subjects were used as control. Then, the supernatant was collected and centrifuged at 12,000×g and 4 °C for 10 min. The resulted supernatant was stored at −80 °C and HONE1 cells in each well were collected for apoptosis analysis.
Detection of apoptosis by flow cytometry
Cells (1 × 106) were first washed with cold PBS twice, and then subjected to flow cytometry using ANXN V FITC APOPTOSIS DTEC KIT I (BD Biosciences, Franklin Lakes, NJ, USA) following the manufacturer’s manual for the detection of apoptosis. Cells with ANNEXIN V-positive values were early apoptotic cells, those with PI-positive values were necrotic cells, and those with double positive values were late apoptotic cells.
Lactate dehydrogenase (LDH) assay
The supernatant of co-cultured HONE1 cells and NK cells was collected at 24 h, and centrifuged at 12,000×g for 10 min. Afterwards, 120 μL of supernatant was used for LDH assay following the manufacturer’s manual (Beyotime, Shanghai, China).
Detection of NK cell surface receptors and effector molecules by flow cytometry
A total of 1 × 105 PBMCs were suspended in 100 μL RPMI-1640 medium, and fluorescence-labelled antibodies were added, including activated receptors p30, p44, p46 and NKG2D, as well as inhibitory receptors 158a, 158b and G2A. After incubation at room temperature in darkness for 30 min, the cells were washed with PBS three times, and centrifuged at 600×g for 5 min to collect NK cells. Finally, 200 μL of PBS was added to resuspend the labelled cells, which were subsequently used for flow cytometry.
Detection of cell cycle of NK cells by flow cytometry
NK cells (1 × 105) were washed with PBS twice, and subjected to flow cytometry using Cell Cycle Assay Kit (BD Biosciences, Franklin Lakes, NJ, USA) for the detection of cell cycles. Briefly, the cells were incubated with 200 μL liquid A at room temperature for 10 min, and then mixed with 150 μL liquid B before incubation at room temperature for 10 min. Then, 120 μL liquid C was added before incubation at room temperature in darkness for 10 min. Finally, the cells were used for flow cytometry, and the results were analyzed using ModFit software (Verity Software House, Topsham, ME, USA).
Western blotting
Before lysis, cells were trypsinized and collected. The cells were lysed with precooled Radio-Immunoprecipitation Assay (RIPA) lysis buffer (600 μL; 50 mmol/L Tris-base, 1 mmol/L EDTA, 150 mmol/L NaCl, 0.1% sodium dodecyl sulphate, 1% TritonX-100, 1% sodium deoxycholate; Beyotime Institute of Biotechnology, Shanghai, China) for 30 min on ice. The mixture was centrifuged at 12,000×g and 4 °C for 10 min. The supernatant was used to determine protein concentration by bicinchoninic acid (BCA) protein concentration determination kit (RTP7102, Real-Times Biotechnology Co., Ltd., Beijing, China). The samples were then mixed with 5× sodium dodecyl sulphate loading buffer before denaturation in boiling water bath for 10 min. Afterwards, the samples (5 μL) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 100 V. The resolved proteins were transferred to polyvinylidene difluoride membranes on ice (250 mA, 1 h) and blocked with 50 g/L skimmed milk at room temperature for 1 h. Then, the membranes were incubated with rabbit anti-human MACC1 polyclonal primary antibody (1:1000; ab106579; Abcam, Cambridge, UK), rabbit anti-human TGF-β1 monoclonal primary antibody (1:800; #3709; Cell Signaling Technology, Danvers, MA, USA) or mouse anti-human GAPDH monoclonal primary antibody (1:5000; Beyotime, Shanghai, China) at 4 °C overnight. After extensive washing with phosphate-buffered saline with Tween 20 for three times for 15 min, the membranes were incubated with goat anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5000; Santa Cruz, Dallas, TX, USA) for 1 h at room temperature before washing with phosphate-buffered saline with Tween 20 three times for 15 min. Then, the membrane was developed with enhanced chemiluminescence detection kit (Beyotime, Shanghai, China) for imaging. Image Lab v3.0 software (Bio-Rad, Hercules, CA, USA) was used to acquire and analyze imaging signals. The relative contents of target proteins were expressed against GAPDH.
Statistical analysis
The results were analyzed using SPSS 20.0 statistical software (IBM, Armonk, NY, USA). The data were expressed as means ± standard deviations. Data were tested for normality. Multigroup measurement data were analyzed using one-way ANOVA. In case of homogeneity of variance, Least Significant Difference and Student-Newman-Keuls methods were used; in case of heterogeneity of variance, Tamhane’s T2 or Dunnett’s T3 method was used. Comparison between two groups was carried out using Student’s t-test. P < 0.05 indicated statistically significant differences.
Results and discussion
Distant metastasis of tumor cells is the main cause of poor prognosis of NPC, and it is directly related to down-regulation or deletion of tumor-suppressor genes or over-activation of oncogenes [Citation4]. In past studies, a large number of oncogenes have been identified to be closely related to the prognosis, radiotherapy resistance and metastasis of NPC, but the regulation of the immunological characteristics of NPC cells by these oncogenes has rarely been reported [Citation20, Citation21]. Studies show that tumor tissues are infiltrated with a large number of natural and acquired immune cells, such as CD4+T cells, CD8+T cells, NK cells, macrophages, mast cells, and dendritic cells [Citation22, Citation23]. The distant metastasis of tumor cells requires evasion of tumor cells from surveillance by immune cells, and the roles of oncogenes in this process still need further studies.
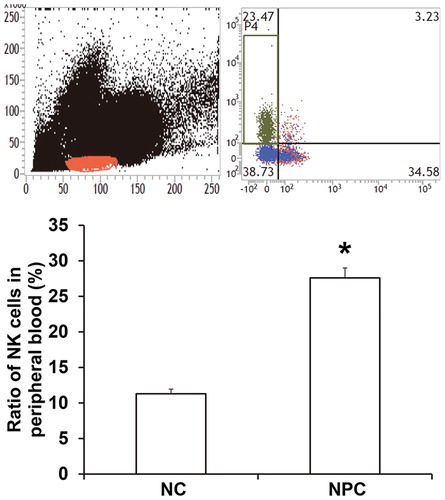
The ratio of NK cells in peripheral blood of NPC patients was elevated, but the activity and tumor-killing effect of NK cells were reduced
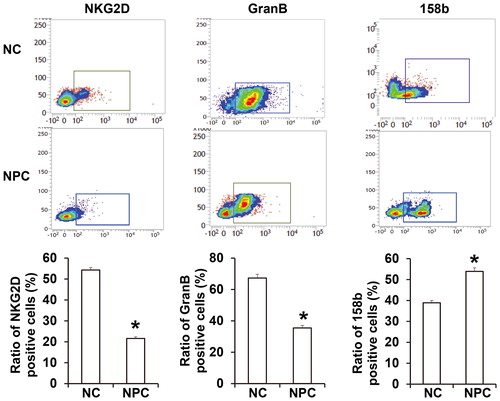
To determine the ratio and killing effect of NK cells in NPC tissues or peripheral blood, flow cytometry was performed. The data showed that the ratio of NK cells in peripheral blood from NPC patients (27.6% ± 1.3%) was significantly higher than that in healthy subjects (11.3% ± 0.65%) (P < 0.05; ). In addition, the percentage of NK cells with positive expression of activated receptor G2D from NPC patients was significantly lower than that from healthy subjects (P < 0.05), while the percentage of NK cells with positive expression of inhibitory receptor 158 b was higher than that in healthy subjects (P < 0.05) (). The ratio of NK cells with positive expression of GranB (35.5% ± 1.6%), a key effector molecule, in NPC patients was significantly lower than that in healthy subjects (67.3% ± 2.4%) (P < 0.05; ). The results suggest that the ratio of NK cells in peripheral blood of NPC patients is elevated, but the activity and tumor-killing effect of NK cells are reduced.
The tumor-killing effect of NK cells in peripheral blood from NPC patients was reduced
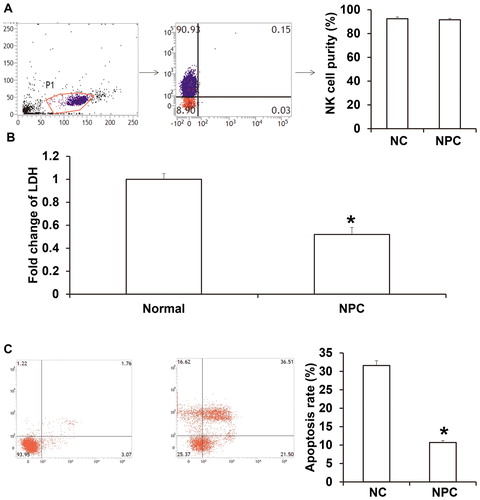
To examine the in vitro tumor-killing effect of NK cells in NPC patients, NK cells were isolated and co-cultured with HONE1 cells, and LDH assay was performed. The data showed that the purity of isolated CD3−CD56+ NK cells reached more than 90%, and the percentage of T cells was less than 1% (). Co-culture showed that LDH release in the supernatant of NK cells from NPC patients and HONE1 cells was significantly lower than that in the supernatant of NK cells from healthy subjects and HONE1 cells (P < 0.05) (). In addition, the apoptotic rate of HONE1 cells induced by NK cells from NPC patients (10.7% ± 0.54%) was significantly lower than that induced by NK cells from healthy subjects (31.6% ± 1.3%) (P < 0.05) (). The results indicate that the tumor-killing effect of NK cells in peripheral blood from NPC patients is reduced.
MACC1 promoted the escape of HONE1 cells from killing by NK cells
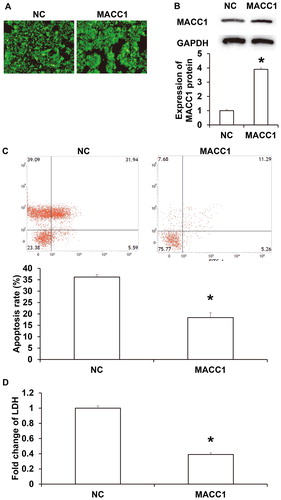
As an oncogene, MACC1 promotes the proliferation, migration and invasion of colon cancer cells, and has a negative correlation with the prognosis of colon cancer [Citation24]. It is reported that MACC1 is up-regulated in many other tumors, and can promote the proliferation, migration and invasion of tumor cells, such as liver cancer, gastric cancer and esophageal squamous cell carcinoma [Citation25, Citation26]. MACC1 is a transcription factor that can increase the expression of c-Met and activate the HGF/c-Met signalling pathway to regulate the proliferation, drug resistance and apoptosis of tumor cells [Citation27]. MACC1 is also highly expressed in NPC and is positively correlated with the occurrence and development of NPC. However, it has not been reported whether MACC1 promotes the immune escape of tumor cells. It is reported that MACC1 assists tumor cell metastasis by promoting epithelial mesenchymal transition of tumor cells. Tumor cells with epithelial mesenchymal transition have stronger migration and invasion abilities, and transform the microenvironment by secreting multiple cytokines or enzymes [Citation28, Citation29]. For example, breast cancer cells with epithelial mesenchymal transition can promote the transformation of TAM1 macrophages to TAM2 macrophages [Citation30]. To test whether overexpression of MACC1 promotes the escape of HONE1 cells from killing by NK cells, HONE1 cells were transfected with MACC1. Immunohistochemistry showed that GFP fluorescence was evenly distributed in HONE1 cells under 100x light microscope (). Western blotting showed that the expression of MACC1 protein in HONE1 cells transfected with MACC1 plasmid was significantly higher than that in untransfected HONE1 cells (P < 0.05) (). After culturing HONE1 cells with NK cells at a ratio of 1:4 for 12 h, the apoptotic rate of HONE1 cells with MACC1 overexpression (18.4% ± 1.1%) was significantly lower than that of HONE1 cells in the negative control group (36.2% ± 2.1%; ). LDH assay showed that the LDH level in the supernatant from co-cultured NK cells and HONE1 cells with overexpression of MACC1 was significantly higher than that in the control group (P < 0.05; ). The results suggest that MACC1 promotes the escape of HONE1 cells from killing by NK cells.
Figure 4. Effect of MACC1 on the escape of HONE1 cells from killing by NK cells. (A) Expression of GFP after transfection with MACC1 plasmid. (B) MACC1 protein expression determined by Western blotting. (C) Apoptosis of HONE1 cells in MACC1 group or NC group. *, P < 0.05 compared with NC group. (D) LDH secretion by HONE1 cells in MACC1 group or NC group. *, P < 0.05 compared with NC group.
HONE1 with overexpression of MACC1 down-regulated the expression of NKG2D in NK cells, inhibited the proliferation of NK cells and escaped the immune surveillance by NK cells
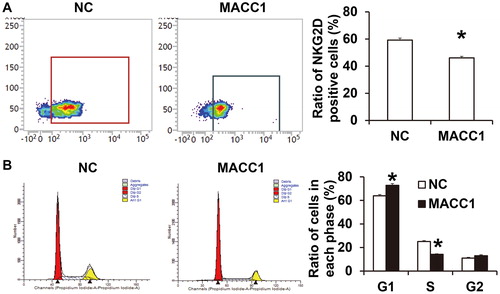
To test how MACC1 promotes the escape of HONE1 cells from killing by NK cells, NK cells were cultured with the supernatant of HONE1 cells in the negative control group or the MACC1 overexpression group, and flow cytometry was carried out to examine activated and inhibitory receptors on NK cell surface and killing effector molecules in the cells. The data showed that the ratio of NK cells with positive expression of activated receptor NKG2D after treatment with supernatant of HONE1 cells with overexpression of MACC1 was significantly lower than that in the negative control group (P < 0.05; ). Flow cytometry showed that the supernatant of HONE1 cells with overexpression of MACC1 increased the proportion of NK cells in G1 phase and decreased the proportion of NK cells in S phase compared with the negative control group (P < 0.05 for both; ). The results indicate that HONE1 with overexpression of MACC1 down-regulates the expression of NKG2D in NK cells, inhibits the proliferation of NK cells, and escapes the immune surveillance by NK cells. NKG2D is a member of C type lectin receptors, and is expressed in the form of homologous dimer on cell surface [Citation31]. NKG2D activates NK cell killing activity through binding with its ligand major histocompatibility complex class I chain-related gene A (MICA) or MICB [Citation32]. NKG2D is one of the most important activated receptors in NK cells, but its regulatory mechanism is still unclear. NKG2D in NK cells is regulated by miRNA molecules. For example, miR-30c promotes the killing activity of NK cells by up-regulating NKG2D expression [Citation33]. In addition, miR-1245 can directly down-regulate the expression of NKG2D in NK cells, thereby reducing the activity of NK cells [Citation34].
Figure 5. Effect of HONE1 cells with MACC1 overexpression on biological functions of NK cells. (A) Ratio of NK cells with positive expression of NKG2D after treatment with supernatant of HONE1 cells without or with overexpression of MACC1. Flow cytometry was used to detect NKG2D expression. *, P < 0.05 compared with NC group. (B) Ratio of NK cells at different cell cycles after treatment with supernatant of HONE1 cells without or with overexpression of MACC1. Flow cytometry was used to detect cell cycles. *, P < 0.05 compared with NC group.
MACC1 down-regulated the expression of NKG2D in NK cells by up-regulating the expression of TGF-β1 in HONE1 cells, thereby inhibiting the activity of NK cells
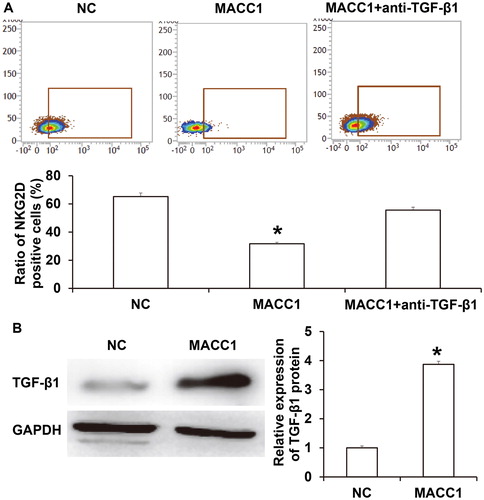
TGF-β1 is the main inhibitor of NK cells. Many tumor cells can secrete TGF-β1, a main inhibitor of NK cells, which down-regulates the expression of NKG2D in NK cells, thereby inhibiting NK cell activity [Citation35]. To examine the mechanism by which MACC1 down-regulates the expression of NKG2D in NK cells, rabbit anti-human TGF-β1 antibody was added onto NK cells together with the supernatant of HONE1 cells with overexpression of MACC1. The data showed that the ratio of NK cells with positive NKG2D expression in the MACC1 + anti-TGF-β1 group was significantly higher than that in the MACC1 group (P < 0.05; ). Western blotting showed that TGF-β1 expression in HONE1 cells with overexpression of MACC1 was significantly higher than that in HONE1 cells in the negative control group (P < 0.05; ). The results suggest that MACC1 down-regulated the expression of NKG2D in NK cells by up-regulating the expression of TGF-β1 in HONE1 cells, thereby inhibiting the activity of NK cells.
Figure 6. MACC1 regulation of NKG2D via TGF-β1. (A) Ratio of NK cells with positive expression of NKG2D. Expression of NKG2D was detected by flow cytometry. *, P < 0.05 compared with NC group. (B) Expression of TGF-β1 in HONE1 cells with or without overexpression of MACC1. Western blotting was used to detect protein expression. *, P < 0.05 compared with NC group.
MACC1 inhibited the cell cycle of NK cells via TGF-β1 pathway
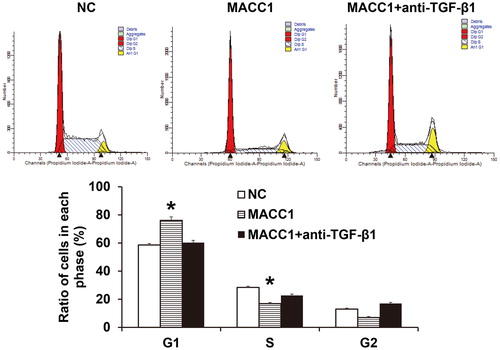
To determine the mechanism by which MACC1 inhibited NK cell cycle, rabbit anti-human TGF-β1 antibody was added onto NK cells together with the supernatant of HONE1 cells with overexpression of MACC1. The data showed that the arrest of NK cells in G1 phase by supernatant of HONE1 cells with overexpression of MACC1 was reversed by rabbit anti-human TGF-β1 antibody to levels similar to the negative control group (). The result indicates that MACC1 inhibited the cell cycle of NK cells via the TGF-β1 pathway.
Conclusions
The present study demonstrated that MACC1 down-regulated the expression of activated receptor NKG2D in NK cells by up-regulating TGF-β1 in HONE1 cells. In addition, decreased expression of NKG2D inhibited the tumor-killing activity of NK cells, and facilitated immune escape of NPC cells.
Ethical approval and consent to participate
All procedures performed in this study were approved by the Ethics Committee of Southwest Medical University. Written informed consent was obtained from all patients or their families.
Consent for publication
Written informed consents for publication of any associated data and accompanying images were obtained from all subjects or their parents, guardians or next of kin.
Acknowledgements
The authors wish to thank their department and research team for their help and dedication.
Availability of data and materials
The datasets used and/or analyzed during this current study are available from the corresponding author upon reasonable request.
Disclosure Statement
The authors declare that they have no competing interests.
Funding
The study was supported by Science and Technology Project of Luzhou City under grant number 2016-s-67.
References
- Feng M, Huang Y, Fan X, et al. Prognostic variables for temporal lobe injury after intensity modulated-radiotherapy of nasopharyngeal carcinoma. Cancer Med. 2018;7:557–564.
- Zhou M, Zhou G, Hu S, et al. Tanshinone IIA suppress the proliferation of HNE-1 nasopharyngeal carcinoma an in vitro study. Saudi J Biol Sci. 2018;25:267–272.
- He J, Xu J, Wu P, et al. Rapid detection of quantum dot immune chromatography nasopharyngeal carcinoma EBNA1 antibody. Oncol Lett. 2018;15:3562–3565.
- Wu JH, Tang JM, Li J, et al. Upregulation of SOX2-activated lncRNA ANRIL promotes nasopharyngeal carcinoma cell growth. Sci Rep. 2018;8:3333.
- Chan SL, Ng LS, Goh X, et al. Time course and clinical characterization of cisplatin-induced ototoxicity after treatment for nasopharyngeal carcinoma in a South East Asian population. Head Neck. 2018;40:1425–1433.
- Stein U, Walther W, Arlt F, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15:59–67.
- Stein U, Smith J, Walther W, et al. MACC1 controls Met: what a difference an Sp1 site makes. Cell Cycle. 2009;8:2467–2469.
- Qian LQ, Li XQ, Ye PH, et al. Downregulation of MACC1 inhibits the viability, invasion and migration and induces apoptosis in esophageal carcinoma cells through the phosphatase and tensin homolog/phosphoinositide 3-kinase/protein kinase B signaling pathway. Oncol Lett. 2017;14:4897–4905.
- Wang J, Wang W, Cai H, et al. MACC1 facilitates chemoresistance and cancer stem celllike properties of colon cancer cells through the PI3K/AKT signaling pathway. Mol Med Rep. 2017;16:8747–8754.
- Wu J, Zhang D, Li J, et al. MACC1 induces autophagy to regulate proliferation, apoptosis, migration and invasion of squamous cell carcinoma. Oncol Rep. 2017;38:2369–2377.
- Rohr UP, Herrmann P, Ilm K, et al. Prognostic value of MACC1 and proficient mismatch repair status for recurrence risk prediction in stage II colon cancer patients: the BIOGRID studies. Ann Oncol. 2017;28:1869–1875.
- Meng F, Li H, Shi H, et al. MACC1 down-regulation inhibits proliferation and tumourigenicity of nasopharyngeal carcinoma cells through Akt/beta-catenin signaling pathway. PLoS One. 2013;8:e60821.
- Hong Y, Zhao XY, Yu XX, et al. The impact of donor characteristics on the invariant natural killer T cells of granulocyte-colony-stimulating factor-mobilized marrow grafts and peripheral blood grafts. Transpl Immunol. 2018;48:55–59.
- Burrello C, Garavaglia F, Cribiu FM, et al. Short-term oral antibiotics treatment promotes inflammatory activation of colonic invariant natural killer T and conventional CD4(+) T Cells. Front Med (Lausanne). 2018;5:21.
- Rebuli ME, Pawlak EA, Walsh D, et al. Distinguishing human peripheral blood NK cells from CD56dimCD16dimCD69 + CD103+ resident nasal mucosal lavage fluid cells. Sci Rep. 2018;8:3394.
- Glasner A, Levi A, Enk J, et al. NKp46 receptor-mediated interferon-gamma production by natural killer cells increases fibronectin 1 to alter tumor architecture and control metastasis. Immunity. 2018;48:396–398.
- Vari F, Arpon D, Keane C, et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood. 2018;131:1809–1819.
- Dabitao D, Hedrich CM, Wang F, et al. Cell-specific requirements for STAT proteins and Type I IFN receptor signaling discretely regulate IL-24 and IL-10 expression in NK Cells and macrophages. J Immunol. 2018;200:2154–2164.
- Ko A, Han SY, Choi CH, et al. Oncogene-induced senescence mediated by c-Myc requires USP10 dependent deubiquitination and stabilization of p14ARF. Cell Death Differ. 2018; 25:1050–1062.
- Wu J, Hann SS. Functions and roles of long-non-coding RNAS in human nasopharyngeal carcinoma. Cell Physiol Biochem. 2018;45:1191–1204.
- Yoshizaki T, Kondo S, Endo K, et al. Modulation of the tumor microenvironment by Epstein-Barr virus latent membrane protein 1 in nasopharyngeal carcinoma. Cancer Sci. 2018;109:272–278.
- Shibahara I, Hanihara M, Watanabe T, et al. Tumor microenvironment after biodegradable BCNU wafer implantation: special consideration of immune system. J Neurooncol. 2018;137:417–427.
- Ravi R, Noonan KA, Pham V, et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat Commun. 2018;9:741.
- Wang N, Zhang Y, Liang H. microRNA-598 inhibits cell proliferation and invasion of glioblastoma by directly targeting metastasis associated in colon cancer-1. Oncol Res. 2018; 26:1275–1283.
- Juneja M, Kobelt D, Walther W, et al. Statin and rottlerin small-molecule inhibitors restrict colon cancer progression and metastasis via MACC1. PLoS Biol. 2017;15:e2000784. [cited 2019 Feb18]
- Ma L, Zhou Y, Luo X, et al. Long non-coding RNA XIST promotes cell growth and invasion through regulating miR-497/MACC1 axis in gastric cancer. Oncotarget. 2017;8:4125–4135.
- Chandrasinghe P, Stebbing J, Warusavitarne J. The MACC1-SPON2 axis: a new biomarker and therapeutic target in colorectal cancer. Oncogene. 2017;36:1474–1475.
- Chae YK, Chang S, Ko T, et al. Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci Rep. 2018;8:2918.
- Li S, Xu F, Zhang J, et al. Tumor-associated macrophages remodeling EMT and predicting survival in colorectal carcinoma. Oncoimmunology. 2018;7:e1380765.
- Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–620.
- Gharagozloo M, Rezaei A, Kalantari H, et al. Decline in peripheral blood NKG2D + CD3 + CD56+ NKT cells in metastatic colorectal cancer patients. Bratisl Lek Listy. 2018;119:6–11.
- Apithy MJ, Charbonnier A, Desoutter J, et al. Impact of MICA and NKG2D polymorphisms in HLA-fully matched related and unrelated hematopoietic stem cell transplantation. Bone Marrow Transplant. 2018;53:918–922.
- Ma Y, Gong J, Liu Y, et al. MicroRNA-30c promotes natural killer cell cytotoxicity via up-regulating the expression level of NKG2D. Life Sci. 2016;151:174–181.
- Espinoza JL, Nguyen VH, Ichimura H, et al. A functional polymorphism in the NKG2D gene modulates NK-cell cytotoxicity and is associated with susceptibility to Human Papilloma Virus-related cancers. Sci Rep. 2016;6:39231.
- Otegbeye F, Ojo E, Moreton S, et al. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS One. 2018;13:e0191358.