Abstract
Mastitis is a deleterious disease of dairy cattle, and its incidence is closely related with the innate immune response, including the complement system. Mannose-binding lectin-associated serine protease 2 (MASP2) is a functional central enzyme in the complement system. Here, we aimed to study single nucleotide polymorphisms (SNPs) of MASP2 and its relationship with mastitis and milk production in Chinese Holstein cattle. We found three novel SNPs, namely g.14047A > C, g.14248T > C and g.14391C > T, in MASP2. The SNP g.14047 A > C (GAA (Glu)>GAC (Asp)) at position 608aa of MASP2 was correlated with the CH50 level and the protein percentage. Meanwhile, the SNP g.14248 T > C (ATT (Ile) > ATC (Ile)) at position 675aa was also correlated with the CH50 value and the milk production. The haplotype combinations H2H2 and H2H4 showed a significant relationship with the CH50, SCS and the milk production abilities, respectively. Based on the SNPs identified in this gene, several genotypes and haplotypes, respectively, were found in Chinese Holstein cattle. The genotypes AC (g.14047 A > C) and TT (g.14248 T > C) and the haplotype combinations H2H2 and H2H4 may be used as molecular markers for breeding mastitis-resistant dairy cattle. The haplotype combination H1H2 should be gradually phased out in breeding programmes.
Keywords:
Introduction
Mastitis is one of the most harmful diseases to dairy cows. It is closely related to decreased quantity and quality of milk production by dairy cows; it reduces the long-term lactation of dairy cows, and causes harm to human health [Citation1]. The resistance of cows to infectious diseases is genetically determined. More recently, an approach involving the improvement of bovine genetics through molecular marker selective breeding has become widely accepted. It is of great importance to select useful genetic markers for mastitis resistance and to identify cattle with high resistance.
The first line of defense against foreign infections is usually the body's innate immune system. The bovine complement system, which is present in the serum and milk, plays an important defensive role against infections in the mammary gland [Citation2–4]. Mannose-binding lectin-associated serine protease 2 (MASP2) is the central protease in the complement system. Studies have indicated that MASP2 polymorphisms and MASP2 serum levels are associated with various diseases. For example, MASP2 could be used as a prognostic biomarker for colorectal cancer [Citation5] and liver cancer [Citation6]. MASP2 has also been associated with rheumatoid arthritis [Citation7], and the SNPs (rs2273346 and rs6695096) of MASP2, with the susceptibility to tuberculosis [Citation8]. Besides, MASP2 might also be an acute-phase protein, and its plasma level might serve as a new reference index in the diagnosis of upper respiratory tract infection in children [Citation9]. Among sepsis patients, the MASP2 levels in neonates were higher than those in healthy controls, but in older patients, the MASP2 levels were lower than in healthy controls [Citation10]. MASP2 levels appear to have a dual effect in HIV and HCV/HBV co-infection. Low levels of MASP2 may increase the susceptibility to infection [Citation11, Citation12]. MASP2 has been associated with the development risk of ischemic stroke, and the rs147270785*A alleles in MASP2 can be used as a protective factor for ischemic stroke [Citation13]. However, the role of MASP2 and its polymorphisms in Chinese Holstein cattle are unknown.
Herein, we investigated the relationship of MASP2 polymorphisms with mastitis, milk production traits and the hemolytic activity of the complement pathway in Chinese Holstein cattle. The MASP2 single nucleotide polymorphisms (SNPs) associated with mastitis in dairy cows was further analyzed.
Materials and methods
Animals
A total of 648 Chinese Holstein cattle were selected randomly from three dairy farms in Jinan Agriculture Development Area, P. R. China. Genomic DNA, serum samples and milk samples were collected. These cattle included 31 sire families with 24–55 daughters from each sire. The following six traits were analyzed: somatic cell score (SCS), ACH50, CH50, 305-day milk yield, fat percentage and protein percentage.
Ethics of experimentation
All animal experiments were conducted according to the ethics guidelines of Heze Medical College. And all efforts were made to minimize animal suffering.
DNA and serum samples
Blood samples were collected from the jugular vein and serum samples were isolated after centrifugation. The genomic DNA was extracted from blood by a genomic DNA purification kit (Blood Genome DNA Extraction Kit, TaKaRa, Dalian, China). The content of DNA was measured spectrophotometrically and then adjusted to 50 ng/μL.
Polymorphism and genotyping screening
Genomic DNA samples from 48 cattle were used for analysis of MASP2 polymorphisms. By using a candidate-gene strategy, we screened the polymorphisms of MASP2 over all exons (∼2.2 kb), 5′-flanking regions (∼2.0 kb), 3′-untranslated regions (∼0.5 kb) and about 2.7 kb intron sequences. PCR primers () were designed with Primer 5.0 software based on the sequence of bovine MASP2. The PCR system (total volume 25 μL) contained 50 ng of genomic DNA, 2.5 μL 10× PCR buffer, 0.8 μL 10 μmol/L of each primer, 0.8 μL 50 mmol/L of Mg2+, 0.6 μL of 10 μmol/L deoxyribonucleoside triphosphates (dNTP), and 0.5 μL of 5 U/μL TaqDNA polymerase (TaKaRa, Dalian, China). The PCR procedure was performed in a Eppendorf Mastercycler nexus PCR instrument at the following conditions: 94 °C, 5 min; 35 cycles of (94 °C, 30 s; 58 °C, 30 s; 72 °C, 40 s); and then 72 °C, 8 min. The PCR products were directly sequenced using an ABI PRISMTM3730 DNA Sequencer (Applied Biosystems) and Big Dye terminator v3.1 Sequencing Kit (Shanghai Sangon, Shanghai, China). The sequence data were analyzed with the DNAMAN software (Version 4.0, Lynnon Corporation, Quebec, Canada).
Table 1. Primers for PCR.
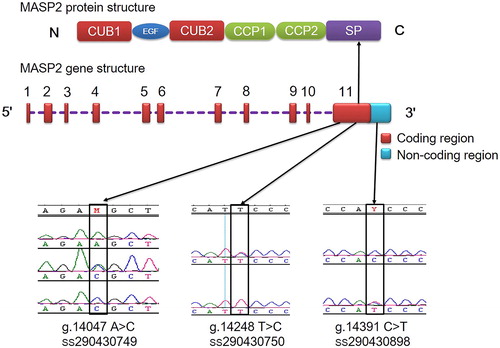
From the sequencing results, only three SNPs (g.14047 A > C, g.14248 T > C, g.14391 C > T) were detected on the eleventh exon of MASP2. And these SNPs were genotyped by PCR restriction fragment length polymorphism (PCR-RFLP), respectively. The SNP (g.14047 A > C) was genotyped by employing created restriction site PCR (CRS-PCR) and the primers containing two nucleotides mismatched, respectively, enabling the use of restriction enzymes for discriminating sequence variations. The detection results of allelic variation at the SNPs sites were based on the electrophoretic pattern of the restriction enzyme-treated PCR products.
Somatic cell score (SCS) assay
There is a close genetic correlation between SCS and mastitis, which makes SCS a good marker for mastitis. In general, lower SCS mainly reflects a lower incidence of mastitis. Therefore, indirect selection, typically using SCSs as indicators of resistance to mastitis, has been widely used [Citation14, Citation15].
In this study, milk samples were firstly collected and analyzed for Somatic Cell Count (SCC) according to the standardized methods defined by the official paper of technical specification of the joint progeny testing in China youth Holstein bulls (Dairy Association of China). The SCC was evaluated monthly for each lactation period by using the instrument Fossomatic 5000 (Foss Electric, Denmark) in the laboratory of the Dairy Herd Improvement (DHI) center (OX Biotechnology, Shandong, China). And then, the SCS is calculated using the following equation: SCS (cells/mL) = log2 (SCC/100) + 3 [Citation16].
Classical complement pathway hemolytic titer (CH50) assay
CH50 assay was performed according to the previously described methods [Citation2]. Briefly, the rabbit red blood cells (RRBCs) were washed with 1 mL phosphate buffered saline (pH = 7.4) and intramuscularly injected into three adult goats once a week and totally for 6 weeks. Then the goats were bled 3 days after the final boost to collect antiserum. Goat antiserum was first heated at 56 °C for 30 min to inactivate the endogenous complement, and then the serum was diluted at a ratio of 1:100 with 10 mmol/L EDTA-GVB (pH = 7.4, containing 5 mmol/L sodium barbiturate, 146 mmol/L NaCl and 0.1% gelatin) and mixed with an equal volume of RRBC suspension (109 cells/mL EDTA-GVB) with a constant swirling. The mixture was then incubated at 37 °C for 30 min. Then 1.3 mL bovine serum, which was diluted 75-fold with gelatin veronal buffer (GVB, pH = 7.4, containing 0.1% gelatin, 0.15 mmol/L CaCl2 and 0.5 mmol/L MgCl2) was added and incubated at 37 °C for 2 h. Finally, the samples were centrifuged at 1500×g for 5 min, and the absorbance of the supernatants at 414 nm was spectrophotometrically measured to determine the balance point of the hemolysis rate.
The preparation of RRBC suspension
First, the RRBCs were washed thrice with EDTA-GVB to replace Alsever's solution and adjust the concentration of RRBC suspension to 5%. Then 0.1 mL of suspension was diluted to 1.5 mL and the optical density at 414 nm (OD414) was evaluated; distilled water was used as margin. The density of RRBC was adjusted to 109 cells/mL and the result was calculated as follows: Vf = Vi×OD414/0.740, where Vi is the initial volume of 5% suspension and Vf is the last volume.
The preparation of sensitization RRBC
At first, goat antiserum was inactivated at 56 °C for 30 min. Then the goat antiserum was diluted 1:100 with EDTA-GVB and mixed with the RRBC suspension at a ratio of 1:1. The mixture was incubated at 37 °C for 30 min, and the sensitized cells were washed with glucose gelatin veronal buffer (GGVB, pH 7.8 containing 0.1% gelatin, 0.15 mmol/L CaCl2, 0.5 mmol/L MgCl2 and 2.5% glucose) three times. The concentration was adjusted to 5 × 108 cells/mL and kept at 4 °C.
CH50 evaluation
The serum samples were diluted with GVB at a ratio of 1:75. Different volumes of the diluted sera ranging from 0.4 to 1.0 mL were dispensed into 1.5 mL tubes, and the total volume was adjusted to 1.3 mL with EDTA-GVB. Then 0.2 mL sensitization RRBC suspension was added (5 × 108 cells/mL), including blank control and 100% of hemolysis, incubated at 37 °C for 2 h, and then centrifuged at 1000×g for 5 min. The absorbance of the supernatants was measured spectrophotometrically at 414 nm (OD414). Then the CH50 values (units/mL) were calculated according to the reported method [Citation17].
Alternative complement pathway hemolytic titer (ACH50) assay
An ACH50 assay was performed according to the method of Liu et al. [Citation2]. To evaluate ACH50, 648 serum samples were diluted with GVB (containing 5 mmol/L sodium barbiturate, 149 mmol/L NaCl, and 0.1% gelatin) at the ratio of 1:50. Different volumes of the diluted sera ranging from 0.25 to 0.1 mL were added into 5 mL tubes, respectively. Then 0.1 mL of RRBC was added into each tube and the volume was adjusted to 3.5 mL with EGTA-Mg2+-GVB (pH = 7.3, containing 10 mmol/L EGTA, 4 mmol/L Mg2+, and GVB). The mixture was incubated at 37 °C for 90 min and centrifuged at 1000×g for 5 min. The absorbance of the supernatant was measured spectrophotometrically at 414 nm (OD414). Then the calculation of ACH50 (units/mL) was performed as previously described [Citation17].
Data analysis
The Hardy–Weinberg equilibrium was tested by using gene frequencies obtained from simple gene counting, and the chi-squared test was used for comparing observed and expected values. Polymorphism information content (PIC), heterozygosity (He), and effective number of alleles (Ne) were calculated by TFPGA software described by Miller [Citation18]. The haplotype frequencies and linkage disequilibrium analyses of the SNPs were estimated by SHEsis software [Citation19]. Association analyses were performed by the General Linear Model (GLM) procedure from the SAS software (version 8.0) to compare relationships between milk performance parameters, SCS, CH50, ACH50 of serum and a different genotype or haplotype. The fixed effects of farms, genotypes, season of birth and parity were included as independent variables, while the animal additive genetic effect and permanent environmental effects on individual cows were handled as random effects in the linear model, as follows: Yijkl = μ + Gi + Sj + Hk + Pl + eijkl. Where Yijkl is the observed value of each milk trait; μ is the mean; Gi is the fixed effect of the genotype or haplotype; Sj is the fixed effect of the season; Hk is the fixed effect of the farm (k = 1 to 10); Pl is the fixed effect of parity (l = 1 to 4); eijkl is the random residual error. Multiple comparisons were performed by Duncan’s method. A value of P < 0.05 was regarded as a significant difference.
Results and discussion
Single nucleotide polymorphism of MASP2
The characteristics of mastitis resistance are regulated by multiple innate immune genes in dairy cows. Among them, the lectin pathway is an important component. Many studies have indicated the association of SNPs of lectin pathway in the complement genes with different responses to mastitis resistance. For instance, the mutations in the mannan binding lectin 1, mannan binding lectin 2 and complement C4 genes have been associated with mastitis resistance in the Chinese Holstein cattle [Citation4, Citation20, Citation21]. Moreover, MASP2 is another central protease of the lectin pathway. Wu et al. [Citation22] detected the SNP (G553A) in the third exon (CUB1 domain) of MASP2, and analyzed the association between this SNP and SCS in the Chinese Holstein cattle. They showed that the cows with genotype GG had significantly lower SCS than those with other genotypes. However, here in our study, we did not find this SNP (G553A) but only three other SNPs (g.14047 A > C, g.14248 T > C and g.14391 C > T) were detected (). SNPs g.14047 A > C and g.14248 T > C are located on the eleventh exon, while SNP g.14391 C > T is within the 3′ UTRs 107 nucleotides downstream of the stop codon UAA. The SNP g.14047 A > C (GAA (Glu) > GAC (Asp) at aa position 608) was identified as a non-synonymous mutation, and SNP g.14248 T > C (ATT (Ile) > ATC (Ile) at aa position 675) was identified as a synonymous mutation. Moreover, the SNPs g.14248 T > C and g.14391 C > T in 648 bovines with different PCR-RFLP patterns were sequenced and the results showed that these two SNPs were always linked in the following manner: (1) animals homozygous at position 14248 were always homozygous at position 14391, and heterozygotes were heterozygous at all two positions; (2) the individuals had either T at position 14248, and C at 14391, or C at position 14248 and T at 14391. Therefore, we concluded that these two SNPs g.14248 T > C and g.14391 C > T were linked completely in the tested populations. This region could be inherited as a unit. These mutations were confirmed by sequencing in the reverse direction, and were submitted to GeneBank of NCBI (National Center for Biotechnology Information).
Phenotype analysis
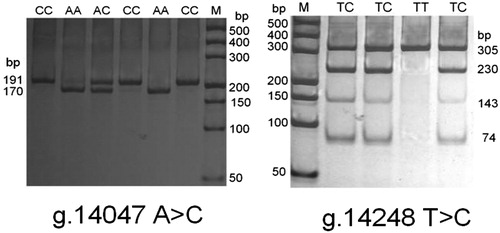
In this study, we detected SNPs in 648 Chinese Holstein cattle via PCR-RFLP, CRS-PCR and DNA sequencing techniques. Digestion of the PCR fragment by Alu1 (containing the g.14047A > C locus) generated fragments with lengths of 170 and 21 bp for genotype AA; 191 bp for genotype CC; and 191, 170 and 21 bp for genotype AC, respectively. Digestion with FOK1 (containing g.14248T > C and g.14391C > T loci) generated fragments with lengths of 305 bp for genotype TT; 305, 230, 143 and 74 bp for genotype TC; and we did not detect the CC genotype among the 648 Chinese Holstein cattle (, ).
Table 2. Primers and PCR-RFLP test of SNP genotypes in bovine MASP2.
Hardy–Weinberg equilibrium
The allele and genotype frequencies of the three SNPs in bovine MASP2 are shown in . The genotypes AA, TT and CC were the dominant genotypes of g.14047 A > C, g.14248 T > C and g.14391 C > T, respectively. The alleles A, T and C were the predominant alleles of g.14047 A > C, g.14248 T > C and g.14391 C > T, respectively. He, Ne and PIC analyses indicated that g.14047 A > C met the Hardy–Weinberg equilibrium (P > 0.05), indicating its stability by long-term artificial selection in our samples of Chinese Holstein cattle. However, the g.14248 T > G SNP was found in only two genotypes and deviated from the Hardy–Weinberg equilibrium, thus indicating that the distribution of the genotype frequency may have stratification in our samples of Chinese Holstein cattle. The genotype distribution showed that the g.14047 A > C SNP had moderate polymorphism (0.25 < PIC < 0.5) and the g.14248 T > G SNP had low polymorphism (PIC < 0.25).
Table 3. Genetic indices of the three SNPs.
Relationship of genotypes and haplotypes of MASP2 with milk production traits, SCS, CH50 and ACH50
The effects of the three loci of MASP2 on milk production traits (fat percentage, protein percentage and 305-day milk yield), CH50, ACH50 and SCS scores are summarized in . The SAS system analysis revealed that the polymorphism at g.14047A > C correlated with CH50 and protein percentage. Cows with the homozygous genotype CC had significantly higher CH50 than those with the genotypes of AC and AA (P < 0.05), and the cows with genotypes AC and CC had higher protein percentage than those with genotype AA (P < 0.05). Thus the genotype AC of g.14047A > C was the superior genotype for mastitis-resistance and may improve milk quality for Chinese Holstein cattle. The reason may be that the SNP g.14047 A > C at the aa position 608 was identified as a non-synonymous mutation and resulted in an amino acid substitution of glutamic by aspartic acid in the serine protease (SP) domain. The SP domain is at the center of the catalytic ability and is important for MASP2 to cleave C4 and C2. The SP domain itself is sufficient for auto activation and is able to cleave the C2 substrate as efficiently as the intact molecule. Although the g.14047 A > C SNP is not located in the active sites (His482, Asp531 and Ser632) of MASP2, this amino acid substitution of glutamic with aspartic acid might considerably affect the folding or stability of the protein and possibly lead to a change of the enzymatic activity of MASP2. It is also possible that the amino acid change in this position causes a modification in the catalytic region of the enzyme, thus altering the enzymatic activity of MASP2.
Table 4. Relationships of SCS, ACH50, CH50 and milk production with different genotypes.
However, the SNP g.14248 T > C (ATT (Ile) > ATC (Ile)) at aa position 675 was identified as a synonymous mutation. The polymorphism g.14248T > C was identified only in two genotypes and correlated with CH50 and milk production traits. The cows with genotype TT had lower CH50 value (P < 0.05) and higher 305-day milk yield than those with the genotype of TC (P < 0.01). Thus the TT genotype might be useful as a molecular and genetic marker of mastitis in Chinese Holstein dairy cows. Some scientists have found that this rare silent mutation could affect the protein function in some cellular environments [Citation23], which may be because of the dimensional structure change of MASP2 resulting from the C allele, thus affecting the function. This needs to be further studied and verified in the future. Furthermore, we found that g.14248T > C and g.14391 C > T were linked. Therefore, this influence may come from g.14391 C > T, which is located in the 3′ UTR, 107 nucleotides downstream of the stop codon UAA. There is evidence that 3′UTR plays a pivotal role in the regulation of mRNA 3′ end formation, stability, degradation, nuclear export, subcellular localization and translation. Particularly, 3′UTRs are rich in cis-acting regulatory elements [Citation24]. Juszczuk-Kubiak et al. [Citation25] showed that the SNP in the 3′UTR of the bovine calpain small subunit gene was associated with meat quality. The mutations g.14248 T > C and g.14391 C > T may affect gene regulation and selective splicing regulation, although g.14248 T > C will not affect the amino acid sequence [Citation26], and subsequently affect the serum concentration and activity of MASP2. The biological mechanism needs to be further investigated.
Two SNPs (g.14047A > C and g.14248T > C) of MASP2 were used for haplotype reconstruction, including: H1: ACT, H2: ATC, H3: CCT and H4: CTC. The estimated frequencies were: 0.024, 0.573, 0.067 and 0.336 for H1–H4, respectively. Among these four haplotypes, H2 showed the highest frequency and H1 showed the lowest frequency. The SNP g.14248T > C labeled two genotypes and thus g.14047A > C and g.14248T > C produced six combined haplotypes. The relationships of combined haplotypes with various milk production traits, ACH50, CH50 and SCS are shown in . The analysis of the haplotype combination showed a significant positive relationship with CH50, SCS and milk production traits. The cows with haplotype combinations H2H2 and H2H4 had lower SCS and CH50 (P < 0.05) and higher 305-day milk yield than those with other haplotype combinations. Thus the haplotype combinations H2H2 and H2H4 might be useful as molecular and genetic markers of mastitis and high-producing milk trait of Chinese Holstein dairy cows. The cows with haplotype combination H1H2 showed significantly higher SCS but lower 305-day milk yield than the ones with haplotype combinations H2H2 (P < 0.01), H2H4 (P < 0.01), H3H4 (P < 0.01) and H4H4 (P < 0.01). This indicates a negative correlation of SCS with 305-day milk yield. This might be due to association of the increased SCS in the dairy cows with mastitis. As a result, their mammary gland cells might be damaged or destroyed, thereby leading to dysfunctional secretion and subsequent decline in milk yield [Citation22]. Thus, the ones with haplotype combination such as H1H2, which do not have beneficial effects on milk production, should be gradually phased out in breeding programmes [Citation27]. The milk fat percentage of five genotypes and six haplotype combinations had no statistically significant difference, which is consistent with the result of Needs and Anderson [Citation28].
Table 5. Relationships between ACH50, SCS, CH50 and milk production with different haplotype combinations.
Conclusions
The correlation analysis results suggest that the MASP2 gene has a significant relationship with mastitis and milk quality. The genotypes AC (g.14047A > C), TT (g.14248 T > C) and the haplotype combinations H2H2 and H2H4 may be used as molecular and genetic markers for breeding mastitis-resistant dairy cattle. The haplotype combination H1H2 should be gradually phased out in the breeding programmes. However, more samples and larger studies are needed to further verify this. Moreover, the underlying mechanisms also need to be further studied.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Seegers H, Fourichon C, Beaudeau F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Res. 2003;34:475–491.
- Liu J, Ju Z, Li Q, et al. Mannose-binding lectin 1 haplotypes influence serum MBL-A concentration, complement activity, and milk production traits in Chinese Holstein cattle. Immunogenetics. 2011;63:727–742.
- Wang X, Ju Z, Huang J, et al. The relationship between the variants of the bovine MBL2 gene and milk production traits, mastitis, serum MBL-C levels and complement activity. Vet Immunol Immunopathol. 2012;148: 311–319.
- Yang Y, Li Q, Ju Z, et al. Three novel single-nucleotide polymorphisms of complement component 4 gene (C4A) in Chinese Holstein cattle and their associations with milk performance traits and CH50. Vet Immunol Immunopathol. 2012;145: 223–232.
- Ytting H, Christensen IJ, Steffensen R, et al. Mannan-binding lectin (MBL) and MBL-associated serine protease 2 (MASP-2) genotypes in colorectal cancer. Scand J Immunol. 2011;73: 122–127.
- Ding W, Qiu Q, Liu G, et al. Metadata checklist: identification of CHI3L1 and MASP2 as a biomarker pair for liver cancer through integrative secretome and transcriptome analysis. OMICS. 2014;18: 658–661.
- Goeldner I, Skare T, Boldt AB, et al. Association of MASP-2 levels and MASP2 gene polymorphisms with rheumatoid arthritis in patients and their relatives. PLoS One. 2014;9:e90979.
- Chen M, Liang Y, Li W, et al. Impact of MBL and MASP-2 gene polymorphism and its interaction on susceptibility to tuberculosis. BMC Infect Dis. 2015;15:151.
- Xiong S, Zhao N, Qiu Y, et al. Plasma levels of mannan-binding lectin-associated serine protease 2 in children with upper respiratory tract infection. J South Med Univ. 2015;35:888–893.
- Swierzko AS, Szala-Pozdziej A, Kilpatrick DC, et al. Components of the lectin pathway of complement activation in paediatric patients of intensive care units. Immunobiology. 2016; 221:657–669.
- Boldt AB, Beltrame MH, Catarino SJ, et al. A dual role for Mannan-binding lectin-associated serine protease 2 (MASP-2) in HIV infection. Mol Immunol. 2016;78:48–56.
- Silva AA, Catarino SJ, Boldt A, et al. Effects of MASP2 haplotypes and MASP-2 levels in hepatitis C-infected patients. Int J Immunogenet. 2018; 45: 118–127.
- Tsakanova G, Stepanyan A, Nahapetyan K, et al. Serine proteases of the complement lectin pathway and their genetic variations in ischaemic stroke. J Clin Pathol. 2018;71:141–147.
- Chen R, Yang Z, Ji D, et al. Polymorphisms of the IL8 gene correlate with milking traits, SCS and mRNA level in Chinese Holstein. Mol Biol Rep. 2011; 38: 4083–4088.
- Ruegg PL. Mastitis in dairy cows. Vet Clin North Am Food Anim Pract. 2012;28:xi–xii.
- Rupp R, Boichard D. Genetic parameters for clinical mastitis, somatic cell score, production, udder type traits, and milking ease in first lactation Holsteins. J Dairy Sci. 1999;82:2198–2204.
- Oswald IP, Lantier F, Bourgy G, et al. Classical and alternative pathway haemolytic activities of ovine complement: variations with age and sex. Vet Immunol Immunopathol. 1990;24:259–266.
- Miller M. Tools for Population Genetic Analysis (TFPGA) 1.3. 1997: A Windows Program for the Analysis of Alloozyme and Molecular Population Genetic Data. http://www.marksgeneticsoftware.net/_vti_bin/shtml.exe/tfpga.htm.
- Shi Y, Lin H. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15: 97–98.
- Wang C, Liu M, Li Q, et al. Three novel single-nucleotide polymorphisms of MBL1 gene in Chinese native cattle and their associations with milk performance traits. Vet Immunol Immunopathol. 2011;139: 229–236.
- Zhao Z, Wang C, Li Q, et al. Novel SNPs of the mannan-binding lectin 2 gene and their association with production traits in Chinese Holsteins. Genet Mol Res.. 2012;11:3744–3754.
- Wu J, Bai J, Li L, et al. Genetic polymorphisms of the BMAP-28 and MASP-2 genes and their correlation with the somatic cell score in Chinese Holstein cattle. Genet Mol Res. 2015;14:1–8.
- Hunt R, Sauna ZE, Ambudkar SV, et al. Silent (synonymous) SNPs: should we care about them?. Methods Mol Biol. 2009;578:23–39.
- Chen J, Férec C, Cooper DN. A systematic analysis of disease-associated variants in the 3' regulatory regions of human protein-coding genes I: general principles and overview. Hum Genet. 2006;120:1–21.
- Juszczuk-Kubiak E, Flisikowski K, Wicińska K. A new SNP in the 3'UTR region of the bovine calpain small subunit (CAPNS1) gene. Mol Biol Rep. 2010;37:473–476.
- Niu D, Yang Y. Why eukaryotic cells use introns to enhance gene expression: Splicing reduces transcription-associated mutagenesis by inhibiting topoisomerase I cutting activity. Biol Direct. 2011;6:1–10.
- Hughes AL, Packer B, Welch R, et al. Effects of natural selection on interpopulation divergence at polymorphic sites in human protein-coding Loci. Genetics. 2005;170:1181–1187.
- Needs EC, Anderson M. Lipid composition of milks from cows with experimentally induced mastitis. J Dairy Res. 1984;51:239–249.