Abstract
A total of 139 endophytic fungal isolates derived from 20 medicinal and aromatic plants were tested for their capacity to ferment rose oil distillation wastewater (RODW). Internal transcribed spacer sequence-based phylogenetic analysis affiliated the collected isolates to seven genera and nine different species, of which Alternaria alternata was the most widely represented. The fungal fermentation of RODW was evaluated through analysis of the sugar and phenolics content and phenolics composition. After cultivation in a small volume of RODW, the performed cluster analysis grouped the isolates in four distinct clusters according to their capacity to ferment RODW sugars and phenolics. Further larger volume shake-flask cultivation of a selected subset of fungi with a pronounced RODW fermentation capacity, outlined three groups of isolates with a distinct pattern and dynamics of sugars and phenolics fermentation. High-performance liquid chromatography analysis of RODW phenolics extracts following the fermentation by the selected fungal isolates showed two main types of changes in phenolics composition: (1) changes in the relative abundance of part of the RODW phenolic compounds, mainly due to utilization of sugar residues from the RODW phenolic glycosides and (2) biosynthesis of new phenolic compounds, most of which are specific to the endophytic fungal isolates used for RODW fermentation. The overall results of the study clearly demonstrate that endophytic fungal isolates represent a rich and untapped source for efficient fermentation of RODW and other agro-industrial phenolics-rich wastewaters and highlight their capacity for directional changes and modification of RODW phenolics composition towards further wastewater phenolics valorization.
Introduction
Rose oil distilled from the flowers of the oil-bearing rose, Rosa damascena Mill. f. trigintipetala Dieck, is one of the most valuable essential oils [Citation1]. The main part of the rose oil supplied to the world market is produced in two countries, Bulgaria and Turkey. The production of rose oil is based on traditional water-steam distillation from rose flowers followed by cohobation of the obtained water distillate. The industrial rose oil distillation generates large quantities of phenolics-rich rose oil distillation wastewater (RODW), which is either spread on waste lagoons near the distilleries or discharged in the drainage system, and represents a major environmental concern [Citation2–4]. On the other hand, RODW contains valuable bioactive phenolic compounds, which makes their extraction and valorization very attractive. Our recent research demonstrates an efficient extraction of the RODW phenolics following their successive adsorption and desorption on а macroporous resin [Citation2]. Furthermore, the obtained phenolics-enriched extract shows high biological activities, including inhibition of cell proliferation, migration and TNF-α-induced vascular endothelial growth factor (VEGF) secretion in immortalized human keratinocytes [Citation5,Citation6] and inhibition of tyrosinase activities [Citation7]. The characterization of the composition of RODW phenolics and phenolic extracts demonstrated the presence of mainly quercetin, kaempferol and phenylethyl alcohol glycosides as well as ellagic acid [Citation2,Citation3]. Besides phenolics, RODW contains high quantities of sugars, suggesting fungal or yeast fermentation could be efficiently applied for modification of RODW composition and phenolics biotransformation. Fungal fermentation has been successfully utilized for treatment of several types of phenolic-rich wastewater generated by the agro-industry, for example, the most frequently studied olive oil mill wastewater and wastewater from olive processing [Citation8–12], as well as winery wastes [Citation13], distillery wastewater [Citation14], and cork boiling wastewater [Citation15]. The reported fermentation and phenolics biotransformation of olive oil mill wastewater employed both fungal and yeast strains available in collections or indigenous isolates [Citation9,Citation11,Citation16–19].
The isolation and functional characterization of endophytic fungi from various plant species attract increasing attention and have been subject of a number of studies in recent years (for review, see [Citation20–25]). A significant part of this research has been directed towards characterization and utilization of the functional diversity of endophytic fungi from traditional medicinal plants, aiming to obtain novel pharmaceutically active biomolecules [Citation26–28]. Although the relatively high sugar and phenolic content of RODW makes it a possible substrate for fungal fermentation, to our knowledge the only work reported in this area until now is our recent study on RODW fermentation by the rhizospheric micromycete strain Trichoderma asperellum SL-45 [Citation29].
The present study explored the capacity of endophytic fungi isolated from local medicinal and aromatic plants to ferment RODW sugars and phenolics and to modify the phenolics composition. The study is an essential first step towards further application of fungal fermentation for diversification of the composition and valorization of RODW phenolics.
Materials and methods
Sampling of RODW and culture media
RODW liquid samples were collected during the 2014 rose oil distillation campaign from the cooling pool immediately after discharging the wastewater from the rose oil distillers. Volumes of 5 L were collected in plastic bottles and stored at –20 °C till use. Portions of RODW filtered through a 2 mm nylon mesh were used for preparation of RODW culture media by autoclaving at 121 °C for 20 min, without pH correction and with addition of 20 g/L agar to obtain solid RODW media. Prior to and after autoclaving, RODW had a pH value of 4.4. potato dextrose agar (PDA) media was routinely used as the culture medium for the isolation and growth of the plant endophytic fungi.
Isolation of plant endophytic fungi
Fully developed leaves of healthy medicinal and essential oil plants, without symptoms of disease, were collected from the accessions of the plant genetic resource collection of ‘Rosa Select’ Ltd., Cherganovo, Bulgaria. The collected leaves were sterilized by treatments with 70% ethanol for 30 s, followed by 0.1% HgCl2 for 7 min and were washed five times with sterile water. Leaf explants were dissected avoiding leaf veins and placed on sterile Petri dishes with solid PDA medium. Three plants from each plant species () were sampled, and 40 leaf explants from each sampled plant were used for in vitro cultures. The cultures were incubated at 26 °C and were observed periodically. A small part of the endophytic fungi growing at the edge of the plant explants were duly transferred separately on fresh PDA dishes and the explants were removed from the culture. The tips of the fungal hyphae from growing fungi samples were removed and placed on fresh PDA medium, and this procedure was repeated two to three more times to obtain a pure fungal isolate. The purity of each fungal culture was assessed by regular microscope examination. The obtained pure cultures from each endophytic fungal isolate were preserved at 4 °C and –80 °C for further use. Mycelia obtained after growth of the fungal isolates on 5 mL PDA liquid shaking cultures were collected in 1.5 mL plastic tubes, snap frozen in liquid nitrogen and stored at –80 °C for DNA extraction.
Table 1. Number of endophytic fungal isolates according to their taxonomic affiliation and plant source of isolation.
Molecular identification of the fungal isolates
Total DNA was extracted from the mycelia of the endophytic fungal isolates stored at –80 °C, after grinding with a mortar and pestle in 1.5 mL tubes and using GeneJET Genomic DNA Purification Kit (Thermo Scientific). The isolated DNA samples were used as templates for PCR amplification of the internal transcribed spacer (ITS) region using universal ITS1 and ITS4 primers [Citation30], Phusion High-Fidelity DNA Polymerase master mix (Thermo Scientific) and PCR conditions of: 30 s denaturation at 98 °C, followed by 35 cycles at 98 °C for 10 s, 57 °C for 30 s and 72 °C for 15 s and final extension at 72 °C for 3 min (Quanta Biotech QB-96). The ITS fragments were gel purified using a GeneJET Gel Extraction Kit (Thermo Scientific) and were directly sequenced or cloned into a pJET 1.2 vector using a CloneJet PCR Cloning kit (Thermo Scientific). Sequencing of the purified PCR fragments and plasmid DNAs containing the ITS regions was carried out by Macrogen Inc. (South, Korea) using the ITS or vector primers. The obtained sequences were assembled and manually edited using Vector NTI v. 10 (Life Technologies). The obtained ITS sequences were BLAST-search compared to the sequences deposited in GenBank to determine their close relatives and approximate phylogenetic affiliations. The obtained ITS sequences together with selected pools of the GenBank retrieved sequences were used for phylogenetic tree construction and analyses using MEGA 4.1 [Citation31].
Fungal isolate cultures on RODW
The studied fungal isolates were cultured in autoclaved RODW using volumes of 5 mL in polystyrene tubes and 100 mL in Erlenmeyer flasks. A small piece of agar with mycelium was added to 5 mL RODW medium and was grown at 26 °C, 190 rpm in a Clim-O-Shake thermostat shaker (Kuhner AG). Two-hundred-microlitre samples were taken on the 48th, 72nd h after the start of cultivation in order to monitor the changes in the reducing sugars and total phenolic content and the entire culture was finally sampled 96 h after the start. For shake-flask cultures, the overnight 5 mL cultures were added to 100 mL of autoclaved RODW in 500 mL flasks and were cultivated further for 120 h at 26 °C, 190 rpm. One-millilitre samples were taken every 24 h to monitor the changes in the reducing sugars and total phenolic content.
Analytical methods
All samples subjected to analysis of phenolics and sugar content were centrifuged in a Sigma 1-15PK centrifuge in Eppendorf tubes at 14,000 rpm for 20 min at 4 °C. The supernatants were transferred in a new tube and were analysed. Total phenolic content was determined by a modified Folin-Ciocalteau method [Citation32] adapted for an LKB 6060–006 microplate reader (LKB GmbH). Ten microlitres of sample were mixed with 790 µL distilled H2O and 50 µL Folin-Ciocalteu’s phenol reagent (Sigma) in a 96 Nunc® DeepWellTM plate (Sigma). After brief vortexing, the plate was incubated for 5 min at room temperature, followed by the addition of 150 µL 20% Na2CO3. The samples were again vortexed and incubated for 2 h at room temperature. Aliquots of 200 µL of each sample were transferred to an ELISA micro plate, and the absorption was measured at 670 nm. The phenolic content was calculated as gallic acid equivalents (GAE) according to a calibration curve. The reducing sugar content was determined by the DNS method [Citation33] adapted for an LKB 6060–006 microplate reader (LKB GmbH). One hundred microlitres of sample were mixed with 100 µL of DNS reagent in a 96 Nunc® DeepWellTM plate (Sigma). After vortexing, the samples were heated at 94 °C for 15 min followed by cooling on ice. One millilitre of ultrapure water was added to the cooled samples. Aliquots of 200 µL of each sample were transferred to an ELISA micro plate, and the absorption was measured at 540 nm. The reducing sugars content was calculated as mg/L D-glucose according to a calibration curve. All analyses were performed in three replicates.
Phenolics extraction
Phenolics were extracted from fermented RODW derived from shake flask cultures and collected after the lower level of reducing sugars was reached for each culture. The extraction was performed using 10 mL PD-10 columns (GE Healthcare) filled with 1 g of Sepabeads® SP-207 resin (Mitsubishi Chemical) purchased from Sigma. Prior to extraction, the resin was activated by addition of 4 mL of methanol, washing with ultrapure water, treatment with 8 mL of 1 mol/L NaOH and washing with ultrapure water three times. Phenolics extraction was carried out by resin adsorption after addition of 10 mL of sample to the column. The phenol-depleted liquid was removed and the resin was washed three times with 10 mL ultrapure water. The phenolics extract was obtained after two consecutive elutions with 2 mL of 95% ethanol. The two eluates were mixed giving a RODW extract sample for further HPLC-UV analysis.
HPLC-UV analysis
Two millilitres of each RODW extract sample were placed in a 15-mL Falcon tube and evaporated at 40 °C for 6 h using CentriVap Benchtop Vacuum Concentrator (Labconco) for ethanol removal. The remaining water extract was frozen in liquid nitrogen and freeze-dried using Martin Christ Alpha 1-2 LD Plus freeze drier. The dry RODW extracts were dissolved in 10% aqueous DMSO containing 3 mg/mL TBHQ (Sigma) as an internal standard to a final concentration of 1 mg/mL phenolics and were filtered through a 0.45-µm MS® nylon filter (Membrane Solutions). HPLC analysis of the extracts was carried out on a Hitachi Elite LaChrom HPLC system consisting of an L-2130 pump and L-2420 UV-Vis detector operated by EZ Chrom Elite ver. 3.3.2 SP2 (Agilent) and using H2O (A) and ACN (B) as mobile phases both containing 1% acetic acid. Twenty microliters of each sample were manually injected and analysed on an Inertsil® ODS-4 (4.6 × 250 mm, 5 µm, GL Sciences) column at room temperature using the following gradient programme: 0 min 10% B, 0–40 min to 46% B, 40–42 min to 100% B, 42–52 100% B isocratic, 52–54 to 10%B. UV absorbance was recorded at 254 nm. Compounds were identified based on co-chromatography with analytical standards (SIGMA) as well as compounds purified by preparatory HPLC from RODW extract and identified in a previous study [Citation2]. All chromatograms were integrated using the EZ Chrom Elite ver. 3.3.2 SP2 (Agilent) software. Peak areas were normalized to the area of the internal standard. The relative amounts of the identified compounds were expressed as percentage of the amount in the control RODW extract sample (RODW without fungal fermentation).
Statistical analysis
Cluster analysis was performed using SPSS ver. 25 (IBM). Scatter plots and charts were produced using Microsoft Excel 2016. Principal component analysis was performed using ClustVis [Citation34].
Results and discussion
Isolation and molecular identification of plant endophytic fungi
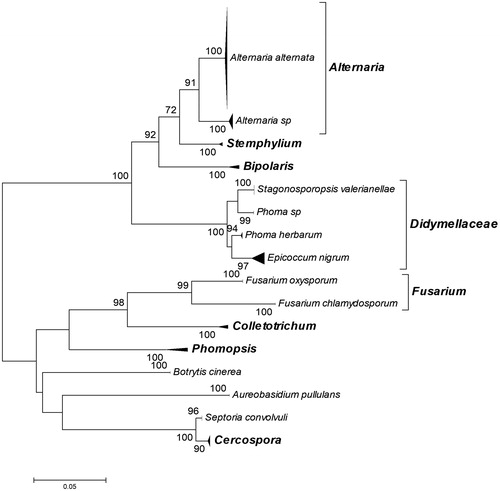
Our preliminary experiments on in vitro cultivation of leaf explants from the sampled medicinal plants directly on solid RODW media showed that the cultivated explants become necrotic and decay without appearance of growing endophytic fungi. Therefore, the primary isolation of the endophytic fungi was performed following cultivation of leaf explants on PDA agar medium. A total of 139 endophytic fungal isolates were collected after cultivation of leaf explants from 20 medicinal and essential oil plants on PDA medium (, Supplement S1). The total number of endophytic fungal isolates derived from each plant species varied from 2 to 12 isolates per species. No endophytic fungal growth was observed after cultivation of leaf explants from Ruta graveolens plants. The phylogenetic affiliation of the collected isolates was performed following sequence analysis of the ITS region, BLAST search and construction of a phylogenetic tree involving the analysed ITS sequences and such retrieved from the GenBank database after BLAST search. In the constructed phylogenetic tree, the analysed ITS sequences of the endophytic fungi were grouped in 16 distinct clusters, supported by high bootstrap values (). All observed clusters involved GenBank-retrieved sequences of characterized fungal strains and isolates affiliated to a particular fungal species or genus, which was used for the phylogenetic affiliation of the analysed endophytic fungal isolates. Accordingly, the collected endophytic fungal isolates were affiliated to nine fungal species and seven genera, (, Supplement S1). Closer observation of the constructed phylogenetic tree showed that the two small clusters of Phoma herbarum species and Phoma genus (Phoma sp.) were arranged separately within the large Didymellaceae cluster (). The observed separation of P. herbarum and Phoma sp. represent the well-known problems with the taxonomy of the broad and complex genus Phoma and the phylogeny of Didymellaceae, reported in other studies [Citation35,Citation36]. The largest number, over half of the collected endophytic fungal isolates, were affiliated to Alternaria alternata (83 isolates, 60%) and Alternaria sp. (11 isolates, 7.9%), (), supporting the observation that Alternaria species are frequently prevalent among the isolated endophytic fungi [Citation37,Citation38]. On the other hand, the majority of the fungal species and genera identified in the present study consisted of only one to four different isolates, e.g. Aureobasidium pullulans, Botrytis cinerea.
Figure 1. Neighbour-joining phylogenetic tree constructed from ITS sequences of the 139 endophytic fungal isolates collected in the present study and ITS sequences of characterized fungal strains retrieved from the GenBank database after BLAST search. Bootstrap values greater than 70% confidence are shown at the branching points (percentage of 1000 resamplings). All phylogroups are presented as subtree triangles. The subtrees were designated according to the taxonomic affiliation of the GenBank retrieved ITS sequence of each characterized fungal strain allocated in the subtree. Information and accession numbers of the GenBank retrieved ITS sequences involved in the subtree triangles of the phylogenetic tree are provided in Supplement S1b. The phylogenetic affiliation of each of the studied endophytic fungal isolates to the designated subtree and respective taxonomic group is provided in Supplement S1a.
Testing the RODW fermentation capacity of the endophytic fungal isolates
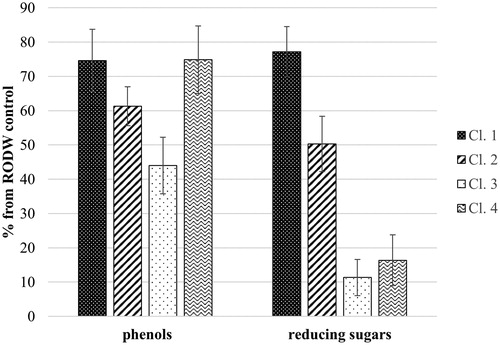
All 139 isolates of endophytic fungi were tested for their capacity to grow in liquid RODW medium and metabolize its sugars and phenolics. Five millilitres of RODW liquid media were inoculated with endophytic fungal mycelia and the levels of the total sugars and phenolics in the culture media were determined 96 h following inoculation of the cultures. Analysis of the results showed large variation in the ability of the tested 139 fungal isolates to grow and ferment RODW. The level of variation ranged from unchanged levels of the initial reducing sugar content in RODW (3054 ± 55 mg/L D-glucose equivalents) to a reduction down to 5% of the initial level. Similarly, the total phenolics content of samples from the cultures varied from its initial content in RODW (1490 ± 15 mg/L GAE) to a reduction down to 26% of the initial level. shows the distribution of the number of analysed isolates according to the level of total sugar content utilization in RODW after the fermentation. The predominant part of RODW fermentation samples showed high (0–20% of the starting RODW level) and relatively low (60–80% of the starting RODW level) utilization of total sugars in RODW fermented by, respectively, 39 and 41 of the tested isolates. The 40–60% and 80–100% intervals included 25 and 23 samples, respectively, whereas the 20–40% interval included the lowest number of 11 isolates. In terms of the total phenolic content, the highest number of fermentation samples (69 samples) showed moderate utilization of the total RODW phenolics (60–80% of initial levels), whereas the lowest number of 10 isolates was scored for the highest phenolics utilization (20–40% of initial RODW levels). The 40–60% and 80–100% intervals scored 42 and 18 isolates, respectively (). shows a scatter plot of the 139 tested fungal isolates based on data about the reducing sugar and total phenolic contents after RODW fermentation. Additional clustering analysis (Supplement S2) distributed the samples into 4 clusters designated as Cl.1, Cl.2, Cl.3 and Cl.4 in , including 60, 34, 34 and 11 isolates, respectively. further presents the average sugar and phenolics content of RODW after fermentation for each cluster, shown as percentage of the initial RODW levels prior to fermentation. Correspondingly, cluster Cl.1 included isolates with low capacity for both RODW sugar and phenolics fermentation. The isolates from Cl.2 showed moderate ability to ferment RODW with decline in sugars and phenolics levels of RODW down to 50 ± 8% and 61 ± 6% at the end of fermentation. Both the isolates from Cl.3 and Cl.4 demonstrated high ability to ferment RODW sugars, correspondingly down to 11 ± 5% and 16 ± 7% of the initial levels. The isolates from Cl.3 showed relatively high capacity for phenolics fermentation (down to 44 ± 8% from the initial), the fermentation of RODW by Cl.4 isolates resulted in a weaker decrease in the total phenolics level of RODW, down to 75 ± 10%, showing they preferentially ferment RODW sugar but not the phenolics.
Figure 2. Summary of the RODW sugars and phenolics fermentation by the tested endophytic fungal isolates in small volume RODW cultures after 96 h of cultivation. The number of RODW fermentation samples resulting in a reduction in the contents of reducing sugars (a) and total phenolics (b) in the fermented RODW presented as % of the initial RODW content and corresponding to the indicated intervals.
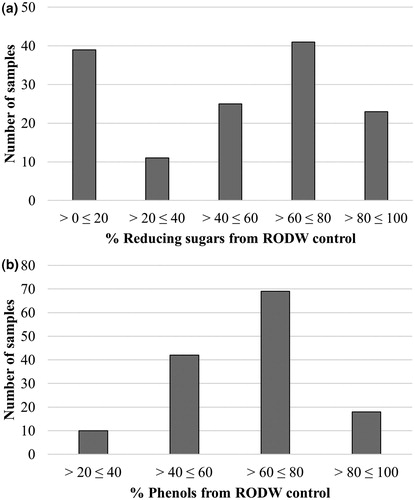
Figure 3. Scatter plot presenting the results of cluster analysis based on combined data for RODW sugar and phenolic content in small volume RODW cultures after 96 h of cultivation with endophytic fungal isolates. The levels of sugars and phenolics utilization at the end of fermentation are presented as % of the initial RODW content. The four clusters related to different fermentation impact are designated. The dendrogram constructed following the cluster analysis is presented in Supplement S2.
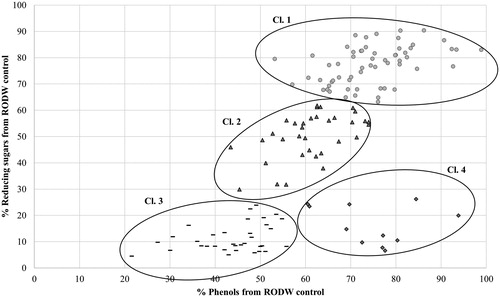
Figure 4. Average content of reducing sugars and total phenolics for the subsets of endophytic fungal isolates belonging to the clusters designated in . The contents of sugars and phenolics were measured at the end of small volume fermentations and are presented as % of the initial RODW content.
The examination of the fermentation data according to the phylogenetic affiliation of the studied endophytic fungal isolates showed that the isolates from Bipolaris sp., Botrytis sp., Septoria convolvuli and Stemphylium sp. were associated only with Cl.1, related to their limited capacity for RODW fermentation (Supplement S1). The tested isolates of A. alternata were associated with all four identified clusters, demonstrating a wide range RODW fermentation capacity of the analysed isolates from this species. This is consistent with the reported high fermentation and bio-transformation capacity of various Alternaria sp. isolates [Citation37,Citation39], as well as with the high degree of genetic variation of endophytic A. alternata isolates derived from single plant species [Citation38]. Variations of the RODW fermentation capacity and impact were observed between isolates affiliated to one and the same genus as well, e.g. the tested representatives of the two Fusarium species, Fusarium oxysporum and Fusarium chlamydosporum. Whereas all Fusarium isolates showed high ability to ferment RODW sugars, the isolate affiliated to F. chlamydosporum was more efficient in RODW phenolics fermentation compared to the moderate phenolics fermentation capacity of the F. oxysporum isolate (Supplement S1). Similarly, our experiments showed significant difference between the fermentation capacities of the tested isolates in the P. herbarum and Phoma sp. clusters (). Whereas all P. herbarum isolates showed low RODW fermentation capacity and were allocated in Cl.1, the two isolates from the Phoma sp. cluster were placed in Cl.3 and CL.4 related to high RODW sugar and moderate to high phenolics fermentation. The fermentation data of the studied Cercospora isolates showed a different pattern and they were placed in Cl. 1, Cl. 2 and Cl. 3 but not in Cl. 4, related to the variations in their ability to ferment RODW sugars but low capacity to ferment phenolics. The obtained results demonstrated high diversity within the tested pool of endophytic fungi in relation to their capacity to ferment RODW and impact the fermentation. The generated fermentation data offer a good starting point for further testing of various scenarios on RODW fungal fermentation and valorization. The results also point out that primary nonselective isolation of a larger number of endophytic fungal isolates followed by subsequent testing of their fermentation capacity towards specific phenolic-rich wastewater or another substrate is a more fruitful strategy in comparison to the direct selection for growth on the phenolics rich RODW medium, which was not successful in our study.
Dynamics of RODW sugars and phenolics fermentation by selected endophytic fungal isolates
The impact of endophytic fungal fermentation on RODW sugar and phenolics content was further evaluated in larger (100 mL) RODW shake flask cultures for a selected set of 37 isolates of which 35 isolates (belonging to Cl.3 and Cl.4) showed high degree of sugar utilization following 96 h of cultivation in small volume liquid RODW cultures and two isolates (Cl.2) showed a moderate degree of sugar utilization. All shake flask cultures were grown for a period of 120 h, and the sugar and phenolics contents were measured every 24 h. The obtained data for the dynamics of sugar and phenolics fermentation were used for PCA analysis. Three distinct groups of isolates were identified on the PCA score plot, Gr.1, Gr.2 and Gr.3, consisting of 25, 9 and 3 isolates, respectively (). The average data for dynamics of sugar and phenolics fermentation by members of each of the identified groups is presented in . As can be seen from , the RODW fermentation by all three groups showed relatively rapid depletion of the sugar content during the first 24 h, down to 84%, 77% and 64% for Gr.1, Gr.2 and Gr.3, respectively. The period after that clearly separated the Gr.3 cultures, which continued to utilize sugar at a high rate, depleting nearly the entire sugar content at the end of the cultivation period. In contrast, following the initial 24-period of relatively rapid sugar fermentation, Gr.1 and Gr.2 cultures showed no significant changes in the total sugar content over the next 24–72 h of cultivation. After that, both groups showed increased rate of sugar content depletion, higher for the Gr.2 isolates ().
Figure 5. PCA analysis based on data for RODW sugars and phenolics fermentation dynamics during shake flask fermentation. Groups of isolates with similar fermentation patterns are designated.
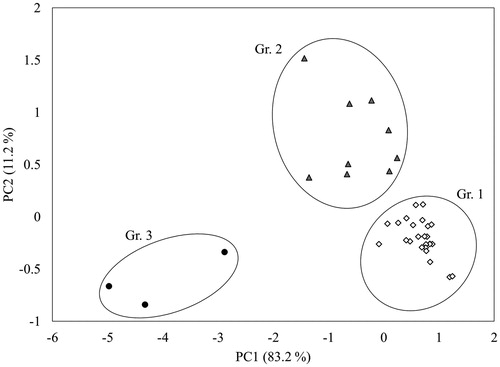
Figure 6. Dynamics of changes of sugars (a) and total phenolics (b) during shake flask RODW fermentation by selected fungal isolates. Average data for each group of isolates determined after PCA analysis shown in .
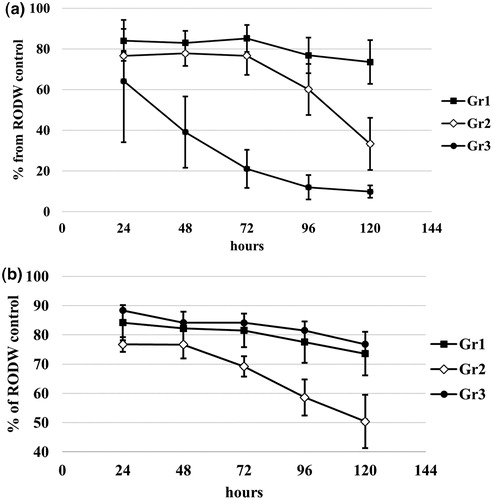
In terms of changes of the phenolics content during fermentation, Gr.1 and Gr.3 showed similar behaviour of relatively gradual reduction of the total phenolic content down to 73–76% from the initial RODW content, at the end of cultivation. At the same time, Gr.2 isolates showed continuous high rate of phenolics fermentation resulting in phenolics utilization down to 50% of the initial RODW content (). The results from this part of the study demonstrated that besides the diversity among the tested isolates in their overall capacity to ferment RODW sugars and phenolics, there are also significant differences in the fermentation dynamics, which has to be considered and employed when developing a strategy and optimization of the fermentation processes involving endophyte fungal fermentation in RODW processing and valorization.
Impact of fungal fermentation on RODW phenolics composition
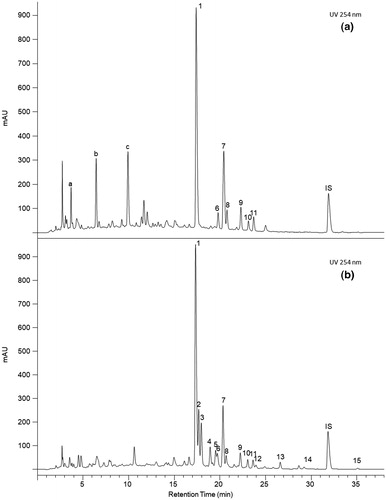
One of the key factors that determine the potential for industrial application of fungal fermentation in RODW valorization are the directional changes of RODW phenolics composition by a particular endophytic fungal isolate and generation of valuable bioactive compounds, through biotransformation and/or biosynthesis. Accordingly, HPLC/UV analysis of phenolic extracts of RODW fermented by 37 isolates cultivated in 100 mL flask cultures was carried out to assess the changes in the phenolics composition following fungal fermentation of RODW. Although the correct and detailed identification of the new phenolics compounds detected in the fermented RODW further requires an additional set of analytical methods, the comparison of the HPLC/UV chromatograms of phenolic extracts from RODW and RODW fermented by selected endophytic fungal isolates showed two main types of changes in the phenolics composition: (1) changes in the relative abundance of part of the RODW phenolics compounds, mainly due to depletion of sugar residues from the RODW phenolic glycosides, this often resulting in an increase in the respective aglycon and (2) biosynthesis of a significant amount of new phenolic compounds, most of which are specific to the particular endophytic fungal isolate used for RODW fermentation. Both types of changes are demonstrated on the chromatograms shown in .
Figure 7. HPLC/UV chromatograms of phenolic extract from RODW with (a) or without (b) 120 h of fermentation with isolate EF123 (A. alternata). (1) Elagic acid; (2) Hyperoside; (3) Isoquercitrin; (4) Kaempferol-3-O-rutinoside; (5) Kaempferol-3-O-galactoside; (6) Quercetin-O-methyl disaccharide; (7) Astragalin; (8) Quercitrin; (9) Kaempferol-3-O-xyloside; (10) Multiflorin B; (11) Kaempferol-3-O-arabinoside; (12) Kaempferol-3-O-rhamnoside; (13) Multiflorin A; (14) Quercetin; (15) Kaempferol; (a), (b) and (c) unidentified compounds.
The industrial rose oil distillation from R. damascena flowers is based on a genetically very narrow pool of rose varieties [Citation40], utilizing rose flowers with similar volatile composition [Citation41] and employing traditional and very similar essential oil production technology and practices [Citation1,Citation42]. Accordingly, the rose oil distillation industry releases large quantities of phenolics-rich RODW with very similar composition and parameters (our unpublished data). Our recent successful attempts for larger scale RODW phenolics extraction and reported biological activities of the obtained phenolic extracts suggest some possible applications [Citation5–7]; however, so far the potential for valorization of RODW phenolics and bioactive compounds remains unutilized. The results of the present study open a new wide avenue for valorization of RODW and similar types of phenolics-rich wastewaters from agroindustry. Indeed, the demonstrated high diversity among the tested endophytic fungal isolates in relation to their capacity for RODW sugars and phenolics fermentation, and dynamics of the fermentation process, makes them a valuable tool for directional modification of the RODW composition. Given the available large quantities of RODW and possibilities to carry out large-scale endophytic fungal fermentation of RODW at a relatively low cost, the results of the present study offer generation of a wide range of RODW phenolic extracts differing in composition, biological activities and possible application. On the other hand, the observed high rate and complexity of changes in RODW phenolic composition, following endophytic fungal fermentation, points out the need for application of adequate experimental approaches and methods for in-depth evaluation and characterization of the biological activities of the phenolics extract from fermented RODW, which is currently a main bottleneck for wider application of endophytic fungal fermentation for RODW valorization.
Conclusions
The results from the present study demonstrate that a large part of endophytic fungal isolates collected from medicinal and aromatic plants ferment RODW at different rates and dynamics in relation to the RODW sugar and phenolics content. Furthermore, the RODW fermentation by endophytic fungi results in significant changes of RODW phenolics composition involving both, changes in abundances of particular RODW compounds and biosynthesis of new phenolic compounds which are not typically present in RODW. All this suggests that the fungal endophytes isolated from medicinal and aromatic plants offer a valuable but still poorly explored source of fungal isolates for fermentation of RODW and directional changes and modifications of RODW phenolics composition. Taking into account the large RODW volumes generated by the rose oil industry and the low variation in RODW composition, the obtained results suggest that the endophytic fungal fermentation of RODW could be applied to expand the range of composition and biological activities of the RODW phenolics extracts towards their subsequent valorization.
Disclosure of interest
The authors report no conflict of interest.
Funding
Supplemental Material
Download PDF (281.6 KB)Supplemental Material
Download MS Excel (22.3 KB)This work was supported by grant IZEBZ0_143110/1 from the Swiss National Science Foundation (SNF) and grant D02-1148 from the Ministry of Education and Science of Bulgaria, within the frame of the Bulgarian-Swiss Research Program 2011–2016.
References
- Kovacheva N, Rusanov K, Atanassov I. Industrial cultivation of oil bearing rose and rose oil production in Bulgaria during 21st century, directions and challenges. Biotechnol Biotechnol Equip. 2010;24:1793–1798.
- Rusanov K, Garo E, Rusanova M, et al. Recovery of polyphenols from rose oil distillation wastewater using adsorption resins-a pilot study. Planta Med.. 2014;80:1657–1664.
- Schiber A, Mihalev K, Berardini N, et al. Flavonol glycosides from distilled petals of Rosa damascena Mill. Zeitschrift für Naturforschung C. 2005;60:379–384.
- Slavov A, Vasileva I, Stefanov L, et al. Valorization of wastes from the rose oil industry. Rev Environ Sci Biotechnol.. 2017;16:309–325.
- Wedler J, Rusanov K, Atanassov I, et al. A polyphenol-enriched fraction of rose oil distillation wastewater inhibits cell proliferation, migration and TNF-α-induced VEGF secretion in human immortalized keratinocytes. Planta Med. 2016;82:1000–1008.
- Wedler J, Weston A, Rausenberger J, et al. In vitro modulation of inflammatory target gene expression by a polyphenol-enriched fraction of rose oil distillation waste water. Fitoterapia. 2016;114:56–62.
- Solimine J, Garo E, Wedler J, et al. Tyrosinase inhibitory constituents from a polyphenol enriched fraction of rose oil distillation wastewater. Fitoterapia. 2016;108:13–19.
- Aggelis G, Ehaliotis C, Nerud F, et al. Evaluation of white-rot fungi for detoxification and decolorization of effluents from the green olive debittering process. Appl Microbiol Biotechnol. 2002;59:353–360.
- Aissam H, Penninckx MJ, Benlemlih M. Reduction of phenolics content and COD in olive oil mill wastewaters by indigenous yeasts and fungi. World J Microbiol Biotechnol.. 2007;23:1203–1208.
- Bevilacqua A, Cibelli F, Raimondo ML, et al. Fungal bioremediation of olive mill wastewater: using a multi-step approach to model inhibition or stimulation. J Sci Food Agric.. 2017;97:461–468.
- Jaouani A, Sayadi S, Vanthournhout M, et al. Potent fungi for decolourisation of olive oil mill wastewaters. Enzyme Microb Technol. 2003;33:802–809.
- Morillo J, Antizar-Ladislao B, Monteoliva-Sánchez M, et al. Bioremediation and biovalorisation of olive-mill wastes. Appl Microbiol Biotechnol.. 2009;82:25–39.
- Dias AA, Fernandes JM, Sousa RMO, et al. Fungal conversion and valorization of winery wastes. In: Mycoremediation and environmental sustainability. Cham: Springer; 2018. p. 239–252.
- Pant D, Adholeya A. Biological approaches for treatment of distillery wastewater: a review. Bioresour Technol. 2007;98:2321–2334.
- Mendonça E, Pereira P, Martins A, et al. Fungal biodegradation and detoxification of cork boiling wastewaters. Eng Life Sci.. 2004;4:144–149.
- Amaral C, Lucas MS, Sampaio A, et al. Biodegradation of olive mill wastewaters by a wild isolate of Candida oleophila. Int Biodeterior Biodegrad. 2012;68:45–50.
- Ntougias S, Baldrian P, Ehaliotis C, et al. Biodegradation and detoxification of olive mill wastewater by selected strains of the mushroom genera Ganoderma and Pleurotus. Chemosphere. 2012;88:620–626.
- Robles A, Lucas R, de CG, et al. Biomass production and detoxification of wastewaters from the olive oil industry by strains of Penicillium isolated from wastewater disposal ponds. Bioresour Technol. 2000;74:217–221.
- Sarris D, Giannakis M, Philippoussis A, et al. Conversions of olive mill wastewater-based media by Saccharomyces cerevisiae through sterile and non-sterile bioprocesses. J Chem Technol Biotechnol.. 2013;88:958–969.
- Khiralla A, Spina R, Yagi S, et al. Endophytic fungi: occurrence, classification, function and natural products. In: Hughes E, editor. Endophytic fungi: diversity, characterization and biocontrol. Hauppauge (NY): Nova Science Publisher’s Inc.; 2016. p. 1–39
- Nisa H, Kamili AN, Nawchoo IA, et al. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: a review. Microb Pathog. 2015;82:50–59.
- Sandhu SS, Kumar S, Aharwal RP, et al. Endophytic fungi: eco-friendly future resource for novel bioactive compounds. In: Endophytes: biology biotechnology. Cham: Springer; 2017. p. 303–331.
- Sudheep N, Marwal A, Lakra N, et al. Fascinating fungal endophytes role and possible beneficial applications: an overview. In: Plant-microbe interactions in agro-ecological perspectives. Singapore: Springer; 2017. p. 255–273.
- Tejesvi MV, Pirttilä AM. Endophytic fungi, occurrence, and metabolites. In: Physiology and genetics. Cham: Springer; 2018. p. 213–230.
- Yan L, Zhao H, Zhao X, et al. Production of bioproducts by endophytic fungi: chemical ecology, biotechnological applications, bottlenecks, and solutions. Appl Microbiol Biotechnol.. 2018;102:6279–6298.
- Bhagat J, Kaur A, Sharma M, et al. Molecular and functional characterization of endophytic fungi from traditional medicinal plants. World J Microbiol Biotechnol.. 2012;28:963–971.
- Huang W-Y, Cai Y-Z, Xing J, et al. A potential antioxidant resource: endophytic fungi from medicinal plants. Econ Bot. 2007; 61:14–30
- Kaul S, Gupta S, Sharma S, et al. The fungal endobiome of medicinal plants: a prospective source of bioactive metabolites. In: Medicinal plants and fungi: recent advances in research and development. Singapore: Springer; 2017. p. 167–228.
- Rusanova M, Rusanov K, Momchilova S, et al. Assessment the fermentation of rose oil distillation wastewater (RODW) by Trichoderma asperellum SL-45 as additional step for fungal biomass production, to the RODW phenolics extraction. Sofia: Ann Sofia Univ. 2018; Book 4, (in press).
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications. Academic Press Inc; 1990;18. p. 315–322.
- Tamura K, Dudley J, Nei M, et al. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599.
- Singleton VL, RO, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178.
- Miller G. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem; 1959;31:426–428.
- Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570.
- Aveskamp MM, de Gruyter J, Woudenberg JHC, et al. Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol. 2010;65:1–60.
- Chen Q, Jiang J, Zhang G, et al. Resolving the Phoma enigma. Stud Mycol. 2015;82:137–217.
- Eram D, Arthikala M-K, Melappa G, et al. Alternaria species: endophytic fungi as alternative sources of bioactive compounds. Ital J Mycol. 2018;47:40–54.
- Guo L, Xu L, Zheng W-H, et al. Genetic variation of Alternaria alternata, an endophytic fungus isolated from Pinus tabulaeformis as determined by random amplified microsatelites (RAMS). Fungal Divers. 2004;16:53–65.
- Lou J, Fu L, Peng Y, et al. Metabolites from Alternaria fungi and their bioactivities. Molecules. 2013;18:5891–5935.
- Rusanov K, Kovacheva N, Vosman B, et al. Microsatellite analysis of Rosa damascena Mill. accessions reveals genetic similarity between genotypes used for rose oil production and old Damask rose varieties. Theor Appl Genet.. 2005;111:804–809.
- Rusanov K, Kovacheva N, Rusanova M, et al. Low variability of flower volatiles of Rosa damascena Mill. plants from rose plantations along the Rose Valley, Bulgaria. Ind Crops Prod. 2012;37:6–10.
- Rusanov K, Kovacheva N, Rusanova M, et al. Traditional Rosa damascena flower harvesting practices evaluated through GC/MS metabolite profiling of flower volatiles. Food Chem. 2011;129:1851–1859.
