Abstract
Hydrazones have versatile properties that make them promising for a range of possible applications. In this study, we examined a library of 17 aroylhydrazones derived from nicotinic acid hydrazide (1-12) and isonicotinic acid hydrazide (A-E) created by us for their biological activity. The antiproliferative activity of the compounds was investigated on non-tumour MCF-10A cells and cancer cell lines, MCF-7 and MDA-MB-231. Four compounds were selected as most active in cell growth inhibition of the tumour cell lines. These compounds, 5, 11, C and E, were tested on four additional cell lines: non-tumour BJ and cancer cell lines, HeLa, HepG2 and HT-29. Compounds 5 and E exhibited the highest selectivity index on cancer cell lines MDA-MB-231, HeLa and HepG2. High selectivity to MCF-7 cells was demonstrated with compound 5. Compound C was very selective to HepG2 cells as well as to MDA-MB-231 but to a lesser degree. Compound 11 showed selectivity against MDA-MB-231. The obtained results allow assessing the structure–activity relationship of the compounds and provide insight into the further development of this group of aroylhydrazones as more potent and selective anti-neoplastic agents.
Introduction
Over the last decades, hydrazones have been a subject of intensive investigations due to their versatile properties and possible applications in the field of analytical, organic and medicinal chemistry [Citation1–3]. Due to the presence of an azomethine group (>C = N-NH-CO) hydrazones are considered as a special group of organic compounds, widely employed as ligands in coordination chemistry [Citation4,Citation5]. In addition, hydrazones and their metal chelates have a growing importance because of the wide spectrum of their biological activities such as analgesic [Citation6], antimicrobial [Citation7–10], anti-inflammatory [Citation11], antifungal [Citation12], anticonvulsant [Citation13], antitubercular [Citation14,Citation15] and anticancer [Citation2,Citation10,Citation16–21].
In particular, aroylhydrazones, which are easily formed by the condensation of aromatic hydrazides (aroylhydrazides) with carbonyl compounds, are of special interest as they possess especially high and selective antiproliferative activities [Citation22–24]. Among the aroylhydrazones, especially effective anti-proliferative agents are the hydrazones derived by condensation reaction of salicylaldehyde and different acid hydrazides. For example, one of the compounds, salicylaldehyde benzoylhydrazone (SBH), has been shown to inhibit DNA synthesis and cell growth in a variety of cultured human and rodent cells [Citation25]. Various derivatives of SBH with a number of functional groups have been created in recent years, in order to discover new bioactive compounds with high antitumour activity and minimal toxicity [Citation26–33].
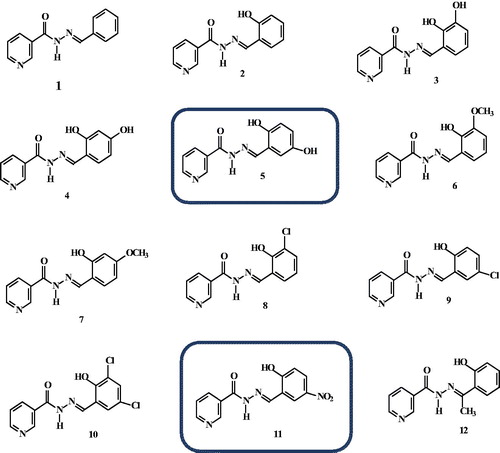
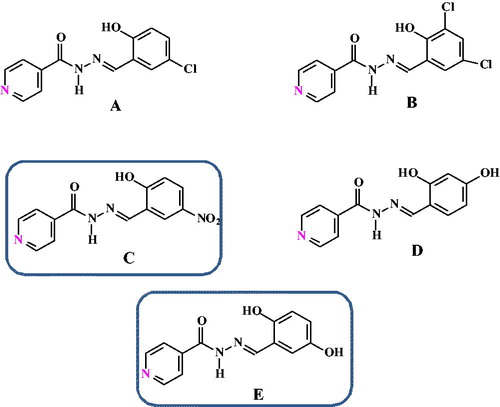
In this regard, as part of our study on the analytical application of hydrazones and Schiff bases we prepared and characterized a series of differently substituted aroylhydrazones, both derived from nicotinic acid 1-12 () [Citation34–41] and isonicotinic acid hydrazide A-E ().
As a next step, this study aimed to evaluate the biological activity of these compounds. To characterize these analogues, we examined their: (1) antiproliferative activity; (2) selectivity to tumour cells. All compounds were tested on non-tumour MCF-10A cells and on two cancer cell lines, MCF-7 and MDA-MB-231. Four compounds were selected as most active in inhibiting the growth of these tumour cell lines. These four compounds were tested on another four cell lines: non-tumour BJ and cancer cell lines HeLa, HepG2 and HT-29. These studies and the results obtained are important to resolve the structure–activity relationships that are essential for the development of more potent biologically active aroylhydrazones.
Materials and methods
Chemistry
All the reagents and chemicals used were of analytical and high purity grade. The solvents were purified and dried according to standard procedures. Salicylaldehyde was obtained from Merck (Germany), o-vanillin, nicotinic acid and iso-nicotinic acid hydrazides were purchased from Fluka (Switzerland); benzaldehyde, 2,4-dihydroxybenzaldehyde, 2-hydroxy-4-chlorobenzaldehyde and 3,5-dichloro-2-hydroxy benzaldehyde purchased from Sigma (Switzerland).
All hydrazones were synthesized in the University of Zagreb, according to previously described procedures [Citation29,Citation34, Citation42–45]. They were obtained by the condensation reaction of equimolar amounts of the corresponding aroylhydrazide (nicotinic hydrazide for 1-12 and isonicotinic hydrazide for A-E) and differently substituted aldehydes or ketones The reactions were carried out in dry alcohol (ethanol or ethanol) under argon atmosphere at 85 °C for 20 h. The solvent was evaporated and the solids were suspended in CH2Cl2, filtered (G-3), rinsed with CH2Cl2 (EtOH was used for 4, 5, 11 and 12), and dried at 50 °C over 24 h.
All obtained hydrazones were characterized by standard analytical procedures (UV/VIS, NMR, MS, HPLC, X-ray) [Citation34,Citation37–42]. The physico-chemical properties of the compounds 1–12 were investigated and reported [Citation32,Citation36,Citation38,Citation39,Citation41].
Cell cultures
MCF-10A (human mammary epithelial cell line), MCF-7 (human breast adenocarcinoma cell line) and MDA-MB-231 (human breast adenocarcinoma cell line) were cultured in Dulbecco Modified Eagle’s medium (DMEM; Gibco, Austria) supplemented with 10% foetal bovine serum (Gibco, Austria), 100 U/mL penicillin (Lonza, Belgium) and 0.1 mg/mL streptomycin (Lonza, Belgium) under a humidified 5% CO2 atmosphere at 37 °C. The complete growth medium for MCF-10A was additionally supplemented with: human insulin, cholera toxin, epidermal growth factor, hydrocortisone at the final concentrations recommended by Merck. Plastic flasks supplied by Greiner, Germany, were used to grow the cells. Cells were trypsinized using Trypsin-EDTA (Gibco, Austria) when they reached approximately 80% confluence.
In the experiments, cells in the exponential phase of growth were treated with Trypsin-EDTA prior to seeding into 96-well plates (Greiner, Germany) in a concentration of 2 × 103 cells/well. The cells were incubated for 24 h post seeding (under a humidified 5% CO2 atmosphere at 37 °C) to allow them to attach to the wells. Four cell lines were used in additional experiments: HeLa (human epithelioid cervix carcinoma), HepG2 (human hepatocellular carcinoma), HT-29 (human colorectal adenocarcinoma) and BJ (human fibroblast cell line). They were processed in the same manner as described above.
Antitumour activity
The cells were treated with the compounds in a wide concentration range (4–2000 µmol/L). Untreated cells were used as controls. Empty wells were blank controls. Cell viability was measured by a colourimetric assay based on tetrazolium salt MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma Chemical Co.). The MTT assay is based on the protocol first described by Mosmann [Citation46]. In this assay, living cells reduce the yellow MTT to insoluble purple formazan crystals. The compounds were dissolved in dimethyl sulphoxide (DMSO). The final concentration of DMSO in the samples did not affect the viability of the cells. The assay was performed 72 h after treatment with the compounds. For this purpose, MTT solution was prepared at 5 mg/mL in PBS and was filtered through a 0.22 µm filter. Then 1 mL of MTT solution was added to 10 mL DMEM and 100 µL of this solution were added into each well, including the cell-free blank wells. Then the plates were further incubated for 3 h to allow MTT to be metabolized. The supernatant was removed and 100 µL/well DMSO/ethanol (1/1) was added. The plates were placed in a microtiter plate shaker for 10 min at room temperature to thoroughly mix the purple formazan into the solvent. An ELISA plate reader (TECAN, Sunrise TM, Grodig/Salzburg, Austria) was used for reading the results. Optical density (OD) was determined at a wavelength of 540 nm and a reference wavelength of 620 nm. Cell viability determined by MTT assay was expressed as:
% viability = ((OD sample – OD blank control)/(OD control – OD blank control)) × 100
The effects of all tested hydrazones were compared to the activity of the referent cytostatic oxaliplatin (Ox), which is a drug widely used in the clinic [Citation47,Citation48].
Selectivity index (SI)
In the present study, the degree of selectivity of the synthetic compounds is expressed as selectivity index (SI). SI = IC50 of pure compound in a normal non-tumour cell line/IC50 of the same pure compound in a cancer cell line, where IC50 is the half maximal inhibitory concentration of the compounds over cell viability [Citation49].
Statistical analysis
Cell antiproliferative activity determined by MTT assay is expressed as percent of live cells versus the negative control group (untreated cells). The results are presented as mean values with standard deviation (±SD). The data were analysed for significance by one-way analysis of variance (ANOVA) and then compared by Bonferroni multiple comparison tests using GraphPad Prism Software, Version 3.00 (San Diego, CA, USA). Asterisks represent levels of statistical significance: p ≤ 0.001 (***), p ≤ 0.01 (**) and p ≤ 0.05 (*).
Results and discussion
A common route was used for the synthesis of the investigated aroylhydrazones. The aroylhydrazones 1–12 () and A-E () contain different substituents (e.g. 2-Cl, 3-Cl, -OH, NO2) at the salicilyc aroyl part. We also varied the position of the pyridine N atom (e.g. at position 2 or 3), while keeping hydrazone spacer between the pyridine and salicylaldehyde moieties.
The antiproliferative activity of the compounds was tested over different cancer cell lines and non-tumour cells. The effects were compared to the activity of Ox, a referent cytostatic, which is widely used in the clinic [Citation47,Citation48].
The results of the antiproliferative study of the compounds 5, 11, C and E on non-tumour MCF-10A cells and cancer cell lines MCF-7 and MDA-MB-231 are shown in . The cells were exposed to different concentrations (ranging from 4 to 2000 µmol/L) of the compounds for 72 h. The treatment of the cell lines with the compounds resulted in a concentration-dependent reduction in the number of the viable cells ().
Figure 3. Effect of compounds 5, 11, C and E on the growth of MCF-7, MDA-MB-231 and MCF-10A cells after 72 h of treatment. Cell antiproliferative activity determined by MTT assay is expressed as per cent of viable cells versus the control group (C, control) and is presented as mean ± SD (n = 6), ***p < 0.001, **p < 0.01, *p < 0.05, ANOVA-test.
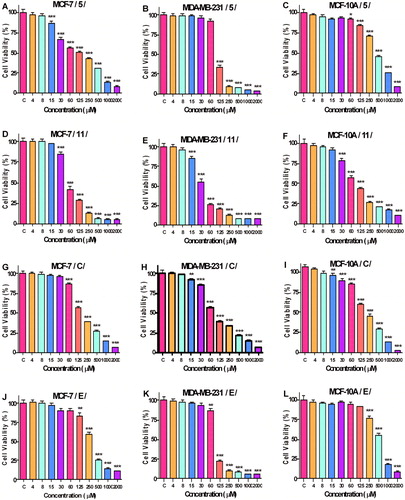
The antiproliferative activity of the compounds based on the half maximal inhibitory concentrations (IC50 values) is shown in . Compound 11 had a stronger inhibitory effect on the growth of the three cell lines compared to its parent compound. Compounds 5, C and E also showed promising results.
Table 1. Antiproliferative activity of the hydrazones on MCF-7, MDA-MB-231 and MCF-10A cells after 72 h of treatment (MTT-dye reduction assay).
The main problem of the drugs in clinical use for cancer treatment is selectivity. The selectivity index (SI) is an important measure to identify substances with promising biological activity. As the SI demonstrates the relation between IC50 of the respective pure compound on a non-tumour cell line and its IC50 on a cancer cell line, the greater the SI value is, the more selective the compound is to tumour cells. An SI value of less than 2 indicates general toxicity of the pure compound [Citation50]. Based on this, the SI data shown in indicate that some compounds exhibit a high degree of antiproliferative selectivity. The substances 11, 5, C, E showed a higher cell growth inhibitory effect with the cancer cells MDA-MB-231 and MCF-7 in comparison with the non-tumour human mammary epithelial cell line MCF-10A. SI of compound 5 was 3.619 (MCF-10A/MCF-7) and 4.237 (MCF-10A/MDA-MB-231). High SI was found for compound E against MDA-MB-231 cells: 5.803. For comparison, the SI value of oxaliplatin (Ox) against MCF-7 and MDA-MB-231 were 1.009 and 1.670, respectively ().
Table 2. Selectivity index of the hydrazones under investigation.
In order to investigate more closely the antiproliferative activity of the compounds with the highest selectivity index, additional cell lines were used: HeLa, HepG2 and HT-29 (cancer cell lines) and BJ (non-tumour cell line). The IC50 values of compounds 5, 11, C and E are shown in . The highest cell growth inhibitory effect was observed with the substances 11 and C in the cell line HepG2. In this case, the IC50 value of 11 was 14.46 µmol/L and the IC50 value of C was 19.11 µmol/L (whereas the respective value of Ox was 2.39 µmol/L). These results are comparable with previously reported activities of aroylhydrazones [Citation32]. Hristova-Avakumova et al. [Citation32] investigated the cytotoxic activity of 3-methoxy aroylhydrazone on HepG2 (hepatocellular carcinoma cell line), HEK-293 (human embryonic kidney cell line) and SH-SY-5Y (human neuroblastoma cells). Their compounds also showed selectivity for tumour cell lines. The IC50 values that they report for HepG2 cells are in micromolar concentrations in the range of 3.7–13.2 μmol/L [Citation32].
Table 3. Antiproliferative activity of the hydrazones in HeLa, HepG2, HT-29 and BJ cells after 72 h of treatment (MTT-dye reduction assay).
In our experiments, the selectivity index of compounds 5, 11, C, E and Ox to the additional cell lines () showed that none of these substances showed considerable SI on HT-29 cells. It may be concluded that compounds 5, 11, C and E do not exhibit selective antiproliferative activity against the cells of this cancer cell line. We observed a considerably higher SI value for compounds 5 and E on HepG2 cells, as well as for compound E on HeLa cells. The SI value in the latter case was 5.319, even higher than the SI of oxaliplatin (4.594). The SI of oxaliplatin with HepG2 and HT-29 cells was not reached by any of the compounds tested.
Table 4. Selectivity index of the hydrazones under investigation.
Taken together, the results from the antiproliferative activity screening of a library of aroylhydrazones (1-12 and A-E) indicate that the compounds 5 and E exhibit highest SI for the cancer cell lines MDA-MB-231, HeLa and HepG2. High selectivity to MCF-7 cells was demonstrated with compound 5. Compound C was selective to MDA-MB-231 and HepG2 cells. Compound 11 showed selectivity against MDA-MB-231. These compounds would be of interest for further study for the needs of pharmacology.
In terms of structure–activity relationship, we demonstrated the importance of the substituents at the salicylidene part of the molecule. For example, the introduction of substituents into the aromatic nucleus led to an increase in the cell growth inhibition activity of the compounds to varying degrees. The presence of two hydroxyl groups in positions 2 and 5 (substances 5 and E), as well as the introduction of the nitro group in position 5 (substances 11 and C) increased the selectivity of the compounds for the cancer lines. However, the introduction of a chlorine atom at position 5 (substance 9) reduced the selectivity versus selectivity of substances 5 and 11. It should also be noted that the position of the nitrogen atom in the aromatic nucleus in nicotinic or isonicotinic residue was not relevant for the activity of the compounds tested.
Conclusions
The results obtained with all tested substances from a library of aroylhydrazones showed that their biological activity depended particularly on the substituents at the salicylidene part of the molecule. The compounds of interest that showed selectivity for cancer cell lines have two hydroxyl groups in positions 2 and 5 in the aromatic nucleus (substances 5 and E) and a nitro group in position 5 (substances 11 and C). A modification that reduced the selectivity versus selectivity of substances 5 and 11 is a chlorine atom at position 5 (substance 9). The obtained results give insight into the structure–activity relationships of the tested group of compounds and lay the ground for further development of more potent and selective antitumour agents.
Acknowledgements
The authors are grateful to Dr. N. Poje (Division of Analytical Chemistry, Department of Chemistry, Faculty of Science, University of Zagreb) for her assistance in preparation of isonicotinoyl derivatives.
Funding
This study was financially supported by the National Science Fund, Ministry of Education and Science of Republic of Bulgaria under grant number DN19/17 and the Croatian Science Foundation under grant number IP-2014-09-4841.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Tamara Pajpanova https://orcid.org/0000-0001-6278-1816
References
- Suvarapu LN, Seo YK, Baek SO, et al. Review on analytical and biological applications of hydrazones and their metal complexes. Eletron J Chem. 2012;9:1288–1304.
- Verma G, Marella A, Shaquiquzzaman M, et al. A review exploring biological activities of hydrazones. J Pharm Bioall Sci. 2014;6:69–80.
- Rollas S, Küçükgüzel ŞG. Biological activities of hydrazone derivatives. Molecules. 2007;12:1910–1939.
- Stadler AM, Harrowfield J. Bis-acyl-/aroyl-hydrazones as multidentate ligands. Inorg Chim Acta. 2009;362:4298–4314.
- Shakdofa MME, Shtaiwi MH, Morsy N, et al. Metal complexes of hydrazones and their biological, analytical and catalytic applications: a review. Main Group Chem. 2014;13:187–218.
- Júnior WB, Alexandre-Moreira MS, Alves MA, et al. Analgesic and anti-inflammatory activities of salicylaldehyde 2-chlorobenzoyl hydrazone (H(2)LASSBio-466), salicylaldehyde 4-chlorobenzoyl hydrazone (H(2)LASSBio-1064) and their zinc(II) complexes. Molecules. 2011;16:6902–6915.
- Hollo B, Magyari J, Radovanovic VZ, et al. Synthesis characterisation and antimicrobial activity of bis(phthalazine-1-hydrazone)-2,6-diacetylpyridine and its complexes with CoIII, NiII, CuII and ZnII. Polyhedron. 2014;80:142–150.
- Aslan HG, Karacan N. Aromatic sulfonyl hydrazides and sulfonyl hydrazones: antimicrobial activity and physical properties. Med Chem Res. 2013; 22:1330–1338.
- Ajani OO, Obafemi CA, Nwinyi OC, et al. Microwave assisted synthesis and antimicrobial activity of 2-quinoxalinone-3-hydrazone derivatives. Bioorg Med Chem. 2010;18:214–221.
- Kumar P, Narasimhan B. Hydrazides/hydrazones as antimicrobial and anticancer agents in the new millennium. Mini-Rev Med Chem. 2013;13:971–987.
- Parrilha GL, Vieira RP, Rebolledo AP, et al. Binuclear zinc(II) complexes with the anti-inflammatory compounds salicylaldehyde semicarbazone and salicylaldehyde-4-chlorobenzoyl hydrazone H2LASSBio-1064. Polyhedron. 2011;30:1891–1898.
- Nfor EN, Husian A, Majoumo-Mbe F, et al. Synthesis, crystal structure and antifungal activity of a Ni(II) complex of a new hydrazone derived from antihypertensive drug hydralazine hydrochloride. Polyhedron. 2013;63:207–213.
- Sinha R, Sara U, Khosa R, et al. Nicotinic acid hydrazones: a novel anticonvulsant pharmacophore. Med Chem Res. 2011;20:1499–1504.
- Dandawate P, Vemuri K, Khan EM, et al. Synthesis, characterization and anti-tubercular activity of ferrocenyl hydrazones and their β-cyclodextrin conjugates. Carbohydr Polym. 2014;108:135–144.
- Pinheiro AC, Kaiser CR, Nogueira TCM, et al. Synthesis and antitubercular activity of new L-serinyl hydrazone derivatives. Med Chem. 2011;7:611–623.
- Chaston TB, Watts RN, Yuan J, et al. Potent antitumor activity of novel iron chelators derived from di-2-pyridylketone isonicotinoyl hydrazone involves fenton-derived free radical generation. Clin Cancer Res. 2004;10:7365–7374.
- Congiu C, Onnis V. Synthesis and biological evaluation of novel acylhydrazone derivatives as potential antitumor agents. Bioorg Med Chem. 2013;21:6592–6599.
- Alagesan M, Bhuvanesh NSP, Dharmaraj N. Potentially cytotoxic new copper(II) hydrazone complexes: synthesis, crystal structure and biological properties. Dalton Trans. 2013;42:7210–7223.
- Becker EM, Lovejoy DB, Greer JM, et al. Identification of the di-pyridyl ketone isonicotinoyl hydrazone (PKIH) analogues as potent iron chelators and anti-tumour agents. Br J Pharmacol. 2003;138:819–830.
- Savini L, Chiasserini L, Travagli V, et al. New α-(N)-heterocyclichydrazones: evaluation of anticancer, anti-IV and antimicrobial activity. Eur J Med Chem. 2004;39:113–122.
- Richardson DR. Iron chelators as therapeutic agents for the treatment of cancer. Crit Rev Oncol Hematol. 2002;42:267–281.
- Singh RK, Singh AK, Siddiqui S, et al. Synthesis, molecular structure, spectral analysis and cytotoxic activity of two new aroylhydrazones. J Mol Struct. 2017;1135:82–97.
- Lovejoy DB, Richardson DR. Novel “hybrid” iron chelators derived from aroylhydrazones and thiosemicarbazones demonstrate selective antiproliferative activity against tumor cells. Blood. 2002;100:666–676.
- Richardson DR, Kalinowski DS, Lau S, et al. Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochim Biophys Acta. 2009;1790:702–717.
- Johnson DK, Murphy TB, Rose NJ, et al. Cytotoxic chelators and chelates 1. Inhibition of DNA synthesis in cultured rodent and human cells by aroylhydrazones and by a copper(II) complex of salicylaldehyde benzoyl hydrazone. Inorg Chim Acta. 1982;67:159–165.
- Nair RS, Kuriakose M, Somasundaram V, et al. The molecular response of vanadium complexes of nicotinoyl hydrazone in cervical cancers - A possible interference with HPV oncogenic markers. Life Sci. 2014;116:90–97.
- Baker E, Richardson DR, Gross S, et al. Evaluation of the iron chelation potential of hydrazones of pyridoxal, salicylaldehyde and 2-hydroxy-1-naphthylaldehyde using the hepatocyte in culture. Hepatology. 1992;15:492–501.
- Richardson DR, Milnes K. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents II: the mechanism of action of ligands derived from salicylaldehyde benzoyl hydrazone and 2-hydroxy-1-naphthylaldehyde benzoyl hydrazone. Blood. 1997;89:3025–3038.
- Macková E, Hrušková K, Bendová P, et al. Methyl and ethyl ketone analogs of salicylaldehyde isonicotinoyl hydrazone: novel iron chelators with selective antiproliferative action. Chem Biol Interact. 2012;197:69–79.
- Nikolova-Mladenova B, Momekov G, Ivanova D, et al. Design and drug-like properties of new 5-methoxysalicylaldehyde based hydrazones with anti-breast cancer activity. J Appl Biomed. 2017;15:233–240.
- Nikolova-Mladenova B, Halachev N, Iankova R, et al. Synthesis, characterization and cytotoxic activity of new salicylaldehyde benzoylhydrazone derivatives as potential anti-proliferative agents. Arzneimittelforschung 2012;61:714–718.
- Hristova-Avakumova N, Yoncheva K, Nikolova-Mladenova B, et al. 3-Methoxy aroylhydrazones – free radicals scavenging, anticancer and cytoprotective potency. Redox Report. 2017;22:408–417.
- Nikolova-Mladenova B, Momekov G, Ivanov D. Synthesis and physicochemical characterization of new salicylaldehyde benzoyl hydrazone derivative with high cytotoxic activity. Pharmacia. 2011; LVIII:4–44.
- Galić N, Perić B, Kojić-Prodić B, et al. Structural and spectroscopic characteristics of aroylhydrazones derived from nicotinic acid hydrazide. J Mol Struct. 2001;559:187–194.
- Galić N, Rubčić M, Magdić K, et al. Solution and solid-state studies of complexation of transition-metal cations and Al(III) by aroylhydrazones derived from nicotinic acid hydrazide. Inorg Chim Acta. 2011;366:98–104.
- Galić N, Dijanošić A, Kontrec D, et al. Structural investigation of aroylhydrazones in dimethylsuplhoxide/water mixtures. Spectrochim Acta Part A. 2012;95:347–753.
- Budimir A, Benković T, Tomišić V, et al. Hydrolysis and extraction properties of aroylhydrazones derived from nicotinic acid hydrazide. J Solution Chem. 2013;42:1935–1948.
- Stražić D, Benković T, Gembarovski D, et al. Comprehensive ESI-MS and MS/MS analysis of aromatic hydrazones derived from nicotinic acid hydrazide. Int J Mass Spectrom. 2014;371:54–64.
- Galić N, Brođanac I, Kontrec D, et al. Structural investigations of aroylhydrazones derived from nicotinic acid hydrazide in solid state and in solution. Spectrochim Acta A. 2013;107:263–270.
- Benković T, Kenđel A, Parlov-Vuković J, et al. Aromatic hydrazones derived from nicotinic acid hydrazide as fluorimetric pH sensing molecules: structural analysis by computational and spectroscopic methods in solid phase and in solution. Spectrochim Acta A. 2018;190:259–267.
- Benković T, Kenđel A, Parlov-Vuković J, et al. Multiple dynamics of aroylhydrazone induced by mutual effect of solvent and light - spectroscopic and computational study. J Mol Liq. 2018;255:18–25.
- Benković T, Kontrec D, Tomišić V, et al. Acid–base properties and kinetics of hydrolysis of aroylhydrazones derived from nicotinic acid hydrazide. J Solution Chem. 2016;45:1227–1245.
- Ding S, Li W. Dioxomolybdenum(VI) complexes of hydrazones of two substituted salicylaldehydes: synthesis, structures, and catalytic properties. J Coord Chem. 2013;66:2023–2031.
- Borhade S. Synthesis, characterisation and spectrophotometric determination of Fe(II) complex of 2,4-dihydroxybenzaldehyde isonicotinoyl hydrazone{(E)-N’-(2,4-dihydroxy benzylidene)isonicotinohydrazide, it’s application & biological activity. Der Chemica Sinica. 2011;2:64–71.
- Xu J, Shu Y, Hu P. Crystal structure of N'-(3,5-dichloro-2-hydroxybenzylidene)-isonicotinohydrazide, C13H9Cl2N3O2. Z Kristallogr NCS. 2011;226:63–64.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;16:55–63.
- Alcindor T, Beauger N. Oxaliplatin: a review in the era of molecularly targeted therapy. Curr Oncol. 2011;18:18–25.
- Göschl S, Schreiber-Brynzak E, Pichler V, et al. Comparative studies of oxaliplatin-based platinum(iv) complexes in different in vitro and in vivo tumor models. Metallomics. 2017;9:309–322.
- Badisa RB, Lambert AT, Ikediobi CO, et al. Selective anticancer activity of pure licamichauxiioic-B acid in culture cell lines. Pharmaceut Biol. 2006;44:14–145.
- Koch A, Tamez P, Pezzuto J, et al. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J Ethnopharmacol. 2005;101:95–99.