Abstract
New bacterial strain BN66 was isolated by selective enrichment, identified as Bacillus cereus and proved to degrade crude oil, together with biosurfactant synthesis. Free and cryogel immobilized Bacillus cereus cells were involved in a crude oil degradation process. The studied strain degraded 93% of the aliphatic fraction for 48 h. We immobilized cells in two types of cryogels synthesized from high molar mass polyacrylamide or acrylamide precursors and explored the degradation capability and possibility for re-use of the preparations. Reusability tests revealed that the oil degradation ability of immobilized cells was stable after 47 days (28 °C and shaker speed 120 rpm) and the degradation rate of immobilized cells was maintained at a high level up to the 20th cycle of operation. The matrices obtained from high molar mass polyacrylamide appeared to be more suitable due to their ability to keep the cells within the carrier. The cells immobilized in cryogels exhibited more effective degradation for 22 active cycles at semicontinuous mode of operation compared to only three cycles performed by free cells.
Introduction
Crude oil and its processing products are some of the most common anthropogenic contaminants. Petroleum hydrocarbons include a big group of chemicals that have caused a major concern due to their widespread distribution into the environment and harmful effects to humans [Citation1]. Oil spills cause a great hazard to terrestrial and marine ecosystems [Citation2]. Fortunately, unlike higher organisms, some microorganisms possess the catabolic capacity to use petroleum hydrocarbons as a carbon and energy source. Bioremediation has been identified as a potential emerging technology for removing different spills, where physical washing and collection could not help. Highly hazardous oily materials can be mineralized to harmless products using suitable microorganisms [Citation3,Citation4]. A limiting factor for successful bioremediation of oil polluted sites is the low aqueous solubility and strong adsorptive capacity of the hydrophobic contaminants to soil [Citation5]. So, hydrocarbons presented in crude oil require solubilization before being degraded by microorganisms. One way to enhance their solubility is to apply surface active agents such as biosurfactants [Citation6]. They are amphipathic molecules with a hydrophilic and a hydrophobic domain that accumulate at interfaces, can form micelles, lower the surface tension and thereby enhance the solubility of poorly soluble compounds. One way of improving the survival and retention of the bioremediation agents in the contaminated sites is to use bacterial cells in an immobilized form [Citation7]. Most of the reported immobilization approaches are either based on nonspecific adsorption of bacterial cells or on physical entrapment of cells in gels or micro-holes or retention of cells within the network of a polymer matrix [Citation8]. Besides the application in industrial processes, the immobilization techniques are the basis for making a number of biotechnological products with applications in bioremediation, diagnostics, bioaffinity chromatography and biosensors.
In this paper, we report the selection of a new biosurfactant-producing bacterial strain with crude-oil degrading activity. Fabrication of polyacrylamide cryogels containing Bacillus cereus cells for crude oil degradation was accomplished. The macroporous cryogels possess good chemical, biological and physicomechanical characteristics that provide high exploitation life of these materials and much longer degradation ability of the immobilized cells as compared to free cells. To the best of our knowledge, this is the first paper describing the use of polyacrylamide cryogels as carriers of newly isolated strain Bacillus cereus BN66 for possible application in bioremediation of oil polluted sites.
Materials and methods
Isolation of a bacterial strain degrading crude oil
Strain BN66 was isolated by selective enrichment from soils polluted by hydrocarbons near a gas station (Sofia, Bulgaria). Enrichment was conducted with 2 g of soil in 250-mL flasks containing 50 mL of mineral salt medium (MSM) with 1% crude oil (Lukoil Neftochim Burgas Oil Refinery, based in Burgas, Bulgaria) as a carbon source. MSM contained: K2HPO4·3H2O (4.8 g L−1); KH2PO4 (1.5 g L−1); (NH4)2SO4 (1.0 g L−1); Na3(C6H5O7)·2H2O (0.5 g L−1); MgSO4·7H2O (0.2 g L−1); yeast extract (0.1 g L−1), supplemented with trace element solution with the following composition: CaCl2·2H2O (2.0 g L−1); MnCl2·4H2O (0.4 g L−1); NiCl2·6H2O (0.4 g L−1); ZnSO4·7H2O (0.4 g L−1); FeCl3·6H2O (0.2 g L−1); Na2MoO4·2H2O (0.2 g L−1). The medium was shaken at 120 rpm for 7 days, 5 mL of the suspension were transferred to 50 mL of fresh medium and incubated for further 7 days at 28 °C. The resultant suspension (2 mL) was placed on MSM agar plates with crude oil to obtain pure cultures. The fastest growing single colonies were selected and tested for their ability to degrade 1% crude oil in MSM solution. Strain BN66 was selected for further study. All hydrocarbons used to elucidate the degradation capacity of strain BN66 were of analytical grade and were products of Fluka (Buchs, Switzerland).
Biosurfactant-producer screening and taxonomic identification
Surface tension measurement and emulsifying activity were used to screen biosurfactant producers from the extracted crude oil degraders. Samples of the culture media of the selected strains were centrifuged at 8000g for 20 min. Surface tension (ST) of the supernatant fluid of the culture was measured by the ring method using a DU Nouya ring tensiometer (Kruss T 10, Hamburg, Germany). The emulsifying activity of the culture supernatant was estimated by adding 0.5 mL of sample fluid and 0.5 mL of kerosene to 4.0 mL of distilled water. The tube was vortexed for 10 s, held stationary for 1 min, and then visually examined for turbidity of a stable emulsion. Emulsification index (EI24) was determined as described by Cooper and Goldenberg [Citation9]. Emulsifying activity was expressed as the percentage of the total height occupied by the emulsion. A variety of hydrocarbons including hexadecane and crude oil were used as test substrates.
Preliminary identification of strain BN66 was carried out on the basis of Gram reaction, cell morphology and identified to the species level following directions of Bergey’s Manual of Determinative Bacteriology [Citation10]. Confirmation was obtained through determination of the 16S rDNA sequence. DNA was extracted from a pure culture by standard salting out procedure [Citation11].
Determination of the 16S rRNA gene sequence was performed by DNA extraction from a pure culture. The extracted DNA was amplified by polymerase chain reaction (PCR) using primers for 16S rRNA of Bacillus cereus ATCC 10987 (GenBank accession number NC-003909) designed by us. We used the forward primers 221 F1 (5’ cgc att agc tag ttg gtg a 3’) and 537 F2 (5’ ccc tgg tag tcc acg ccg ta 3’) and the reverse primers 1190 R1 (5’ ggc atg atg att tga cgt cat 3’) and 1479 R2 (5’ ggc atg atg att tga cgt cat 3’). The PCR was performed in the following mixture: about 100 ng bacterial DNA, 10 pmol of each primer, 0.2 mmol L−1 of each deoxyribonucleoside triphosphate (dNTP), 1X supplied PCR buffer (including 1.25 mmol L−1 MgCl2, 0.5 U ExPrime Taq (Genet Bio, Chungnam, Korea) and ddH2O up to final volume of 25 μL. The cycling conditions were as follows: initial denaturation at 95 °C for 5 min; 30 cycles denaturation at 95 °C for 45 s, annealing at 58 °C for 45 s and synthesis at 72 °C for 90 s; final extension at 72 °C for 5 min. The obtained amplification products were separated by 2% agarose gel electrophoresis and then sequenced using the same primers as those in the PCR. The sequencing reaction was performed by BigDye terminator sequencing kit (Applied Biosystems, Foster City, CA, USA) and analyzed by automated sequencer ABI 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Microbial growth on hydrocarbons and biodegradation
Liquid cultures were grown in duplicate in 250-mL flasks containing 50 mL MSM (pH 7.2) and different hydrocarbons: hexadecane, benzene, toluene, and naphtalene. Flasks were incubated on a rotary shaker (120 rpm) at 28 °C. Hydrocarbons were used individually as sole sources of carbon and energy at final concentration of 10 g L−1. Uninoculated control flasks were prepared to detect losses due to abiotic processes. All cultures were grown in duplicate. Growth with hydrocarbons used as carbon sources was determined while monitoring the optical density at 610 nm (OD610) or as dry biomass. When growth was expressed as dry biomass, 10 mL of the culture were mixed with two volumes of dichloromethane and centrifuged at 10000 g for 15 min; the pellet was washed twice with 10 mL water and dried to a constant weight. Biodegradation was measured as substrate depletion. Whole cultures were extracted with equal volumes of hexane and residual hydrocarbons were quantified by gas chromatography using a Hewlett-Packard model 5859 instrument equipped with a flame ionization detector. Strain BN66 was grown in 50 mL MSM solution with 1% crude oil as a carbon and energy source. The resultant preparations were inoculated with 2% (v/v) overnight glucose-grown preculture of the strain. Cultures were incubated on a rotary shaker (120 rpm) at 28 °C. Since crude oils were not homogeneously distributed in the shake flasks, destructive sampling of whole cultures was carried out. Thus 50 mL samples were used to extract total petroleum hydrocarbons with equal volumes of n-hexane. The composition of the crude oils was analyzed by gas chromatography mass spectroscopy analyses (GC/MS). The GC/MS were recorded on a Hewlett Packard 6890 GC system plus 5973 MSD (Hewlett Packard, Palo Alto, CA, USA) instrument operating in EI mode at 70 eV. Sample volumes of 1 μL were injected into an HP5-MS column (30 m × 0.25 mm × 0.25 µm). The temperature programme was 100–280 °C at 10 °C min−1 and 10-min hold at 280 °C. The flow rate of the carrier gas (He) was 0.8 mL min−1. Split ratio was 1:50. TPHs were measured as the sum of all peak areas on the chromatogram and calculated as a percentage from the sum of peak areas of the uninoculated control.
Cryogel preparation and determination of gel fraction yield and degree of swelling
Acrylamide (AAm), polyacrylamide (PAAm, molar mass = 5 × 106 – 6 × 106 g mol−1), H2O2 (30 vol.% water solution), poly(ethylene glycol) diacrylate (PEGDA, molar mass ∼ 575 g mol−1) were purchased from Aldrich and used without purification. PAAm (0.5 g) was dissolved in 10 mL distilled water under stirring to obtain homogeneous aqueous solution (5 wt.%). The initiator H2O2 (5 wt.% with respect to polymer) were added under stirring at room temperature and the resulting homogeneous solution was poured into Teflon dishes (20 mm diameter) forming a 4 mm thick layer. Then, samples were kept in a freezer at - 20 °C for 2 h. Finally, the frozen system was irradiated with full spectrum UV-vis light with a “Dymax 5000-EC” UV curing equipment with 400 W metal halide flood lamp for 5 min (irradiation dose rate = 5.7 J cm−2 min−1; input power = 93 mW cm−2).
Cryogels from acrylamide (5 wt.%) were prepared by the procedure described above, except that AAm and PEGDA (10 wt.% to monomer) were added instead of polymer. Rheological measurements were performed on a Haake RheoStress 600 rheometer with a parallel plate sensor system and Peltier temperature controller. The elastic modulus was determined by frequency sweep measurements performed in the 0.03 – 1 Hz frequency range at 25 °C in Controlled deformation mode at γ = 0.005. The gel fraction (GF) yield of cryogels was determined gravimetrically. First, the cryogels were extracted in distilled water for 6 days at room temperature. After that, the samples were freeze-dried, weighed and the GF yield was calculated by the relationship GF (%) = (weight of freeze-dried sample/initial weight of polymer (monomer and crosslinking agent)) × 100.
Disks of freeze dried cryogel were weighted and then immersed in distilled water at room temperature and an equilibrium water uptake was reached. The surface of the cryogel was blotted by filter paper prior to weighing. The degree of swelling (DS) was calculated as DS = (weight of wet sample/weight of freeze-dried sample). The experimental errors of the GF yields and the DS calculations were in the range 2–3%.
Re-use of the immobilized preparations
Inocula with cell density (OD610) of 0.4 were employed in the processes of biodegradation. The pH values were 6.7–6.8 all the time due to the buffering activity of the nutrient medium used. The freeze dried cryogels were immersed in 7 mL of cell suspension. Cells were harvested by centrifugation at 8000g and resuspended in phosphate buffer (0.06 mol L−1, pH 7.0 at 20 °C) to obtain a cell density of 64 × 109 g−1 carrier material. Cells were immobilized into the cryogel pores by soaking freeze dried disks – three pieces in cell suspension in a 250-mL Erlenmeyer flask till 95% of the suspension was taken by the matrix. At each cycle, MSM was supplemented to 50 mL with fresh sterile medium without cell transfer, and only crude oil (1%) was added. Cultures were incubated during the long-term semicontinuous biodegradation processes while shaking (120 rpm) at 28 °C. Scanning electron microscopy (SEM) studies were carried out. The gels with and without cells were frozen, fractured and freeze dried in an “Alpha 1-2 Freeze Drier” (Martin Christ) at –55 °C and 0.02 mbar for 24 h. The interior morphology of the gels was studied by using a Philips 515 scanning electron microscope operating at 25 kV. Before observations, the gel specimens were fixed on a glass substrate and coated with gold for 60 s.
Data analysis
All data are presented as mean values with standard deviation (±SD). The significance of differences between the treatments was evaluated by one-way analysis of variance (ANOVA) and a Bonferroni post hoc test, using InStat (GraphPad Software Inc., La Jolla, CA, USA). Values of P < 0.05 were considered significant.
Results and discussion
Characterization of strain BN66, hydrocarbon utilization, detection of biosurfactant production
Among the isolated single colonies, strain BN66 was found to have a high level (more than 90%) of crude oil degrading activity and was selected for further study. The strain BN66 was gram-positive; the cells were spore-forming motile rods with dimensions of 1–4 μm. Comparative sequence analysis of the 16S rRNA in the GenBank database revealed that the bacterial strain BN66 showed total accordance (100% similarity) with the published sequence for Bacillus cereus ATCC 14579 (GenBank accession number NC-004722).
As shown in , BN66 could utilize all tested n-alkanes (C6–C19) for a period of 7 days. Dense growth resulted from use of C14–C19, while only slight growth occurred on short-chain n-alkanes from hexane to nonane. C5–C9 alkanes were degraded to 95%, while C10–C15 and C16–C19 alkanes were totally degraded. Out of the tested aromatics, benzene, toluene and biphenyl were degraded to 84, 90 and 70%, respectively; whereas the percent of degradation of naphtalene, anthracene and phenanthrene was 95, 58 and 52%. Thus, the isolated strain was found to be useful for enhancing the biotreatment of oil.
Table 1. Hydrocarbon utilization by Bacillus cereus BN66 accomplished in 7 days.
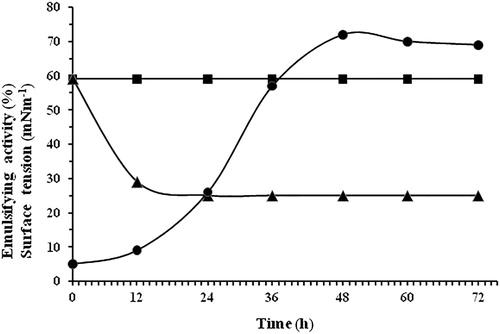
When strain BN66 was grown with 1% crude oil as the sole carbon source over a 12 h period, the surface tension of the culture dropped rapidly to reach its lowest point of 29 mN m−1 and did not change till the end of the cultivation (). The lowered surface tension of the culture medium and the determination of the emussification index (EI24) indicated biosurfacatant production.
Crude oil degradation by free and cryogel immobilized bacterial cells. Synthesis and characterization of cryogels for immobilization
Polyacrylamide cryogels from two different precursors, high molar mass linear polymer (PAAm cryogel) and monomer (AAm cryogel), were obtained by the combination of cryotropic technique and photo-crosslinking as described elsewhere [Citation12] and assessed as carriers of Bacillus cereus cells. Firstly, the aqueous solutions of polymer (monomer) and photoinitiator were frozen at −20 °C and, then, irradiated with UV light for 5 min. Below the freezing point, most of the water is frozen and forms ice crystals, while the non-freezable, bound water and the dissolved substances are accumulated in a non-frozen liquid microphase [Citation13]. In the case of PAAm cryogels, H2O2 generates hydroxyl radicals upon irradiation, which react with the polymer molecules and give rise to macroradicals. The polymer network forms in the liquid microphase by intermolecular recombination of two or more macroradicals. Since ice crystals act as porogens, after thawing, the obtained material comprised a heterogeneous structure of large interconnected pores (80 – 100 µm) surrounded by thin walls (). The pore channels of cryogels were filled mainly with free water.
Figure 2. SEM micrographs of polyacrylamide cryogels obtained from polymeric precursor (A) and monomer (B).
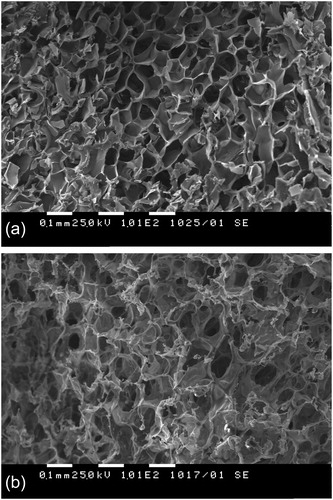
AAm cryogels were obtained by simultaneous free radicals’ polymerization of acrylamide and crosslinking reactions both initiated by the UV light. The crosslinking agent PEGDA was the determining factor for obtaining a three-dimensional network instead of linear macrochains. Although AAm cryogels possessed identical macroporous structure () with PAAm cryogels, the two materials exhibited different viscoelastic properties. The cryogels based on high molar mass polymer were softer than AAm gels. In fact, the elastic modulus, G’, of PAAm cryogels was approximately one order of magnitude lower as compared to the gels synthesized from AAm (). A substantial difference was also found for another two basic parameters of the cryogels, GF yield and degree of swelling. The GF yields of the PAAm and AAm cryogels were 73% and 99%, whereas the DS of the PAAm and AAm gels were 35 and 23, respectively. The higher swelling and lower elastic modulus suggest the existence of a looser polymer network in PAAm cryogels than in AAm cryogels. Most probably, the use of a crosslinking agent (10 wt.% PEGDA) resulted in increased number of crosslink points and cross-linking density, giving enhanced GF yield and stiffness of material. We should point out that the pore size of the cryogels, which is in the range of 100 µm, and the fact that the freeze-drying process preserves the open-porous structure of cryogels (), allow easy immobilization of cells into the cryogel matrix. Thus, Bacillus cereus cells were entrapped into both polyacrylamide gels by soaking freeze-dried disks into cell suspension till 95% of the suspension was taken by the matrix (). Remarkably, in the case of cryogels synthesized from high molar mass polymer (OD610 0.2), cell leakage was negligible, whereas with the other matrix (AAm cryogel) there was pronounced cell leakage (OD610 1.6). Based on this result, the matrix based on high molar mass polymer was chosen in our further investigations.
Figure 3. Elastic modulus of polyacrylamide cryogels obtained from polymeric precursor (PAAm) and monomer (AAm).
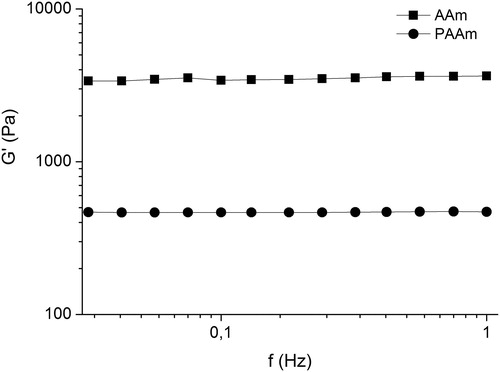
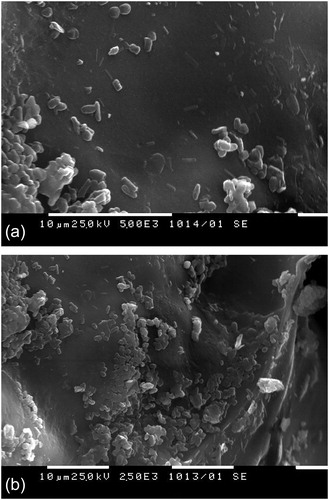
Figure 4. SEM micrographs of polyacrylamide cryogels obtained from polymeric precursor (A) and monomer (B) with immobilized cells.
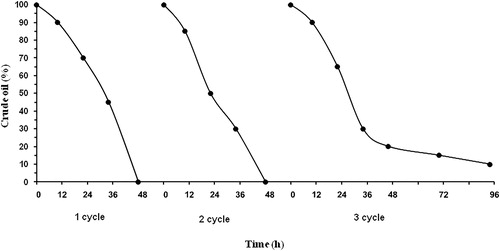
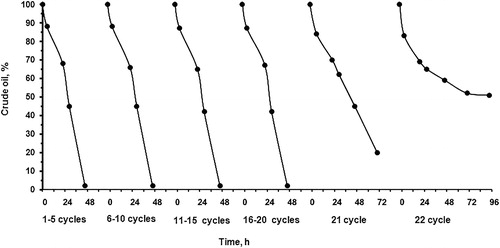
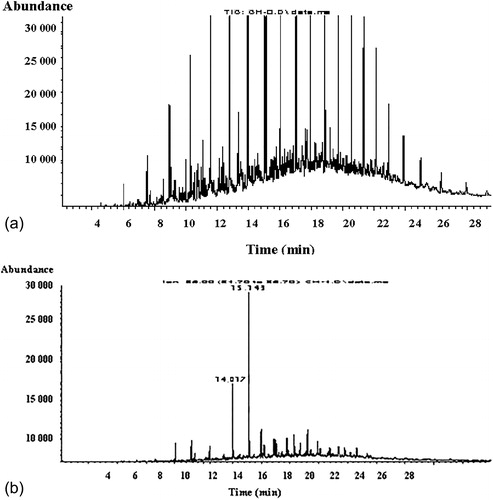
The ability of the strain to utilize the main classes of hydrocarbons was further confirmed through gas mass chromatographic analyses of cultures grown on 1% (v/v) crude oil. The chromatographic profile of the aliphatic fraction () showed that the n-alkanes were almost totally degraded (93%) by the free cells after only 2 days. The main residual hydrocarbons were the recalcitrant methylated alkanes 2,6,10,14-tetramethylpentadecane (pristane) and 2,6,10,14-tetramethylhexadecane (phytane). To mineralize the whole added crude oil quantity (3 g L−1), the process took three cycles of operation by the free cells (). Then, the experiments were performed at a semicontinuous mode of operation in 22 active runs with the immobilized preparations. The end of each run was marked when the whole quantity of crude oil was depleted. The obtained results confirmed that the method of immobilization appeared to be very effective for a long period of time—47 days at high rate and stability ().
Figure 5. Gas mass chromatographic profile of the aliphatic fraction before biodegradation (A) and after biodegradation (B).
Bacteria belonging to the genus Bacillus are frequently involved in bioremediation and other biotechnological processes [Citation14]. Bacillus strains capable of degrading hydrocarbons have been isolated from different contaminated sites. Thus, Zhuang et al. [Citation15] and Calvo et al. [Citation16] reported some naphtalene degrading strains of Bacillus naphthovorans and Bacillus pumilis isolated respectively from oil contaminated tropical marine sediments and oil sludge. Bacillus strains are capable of degrading also phenol [Citation17], cresols [Citation18], polychlorinated biphenyls [Citation19] and others. The degradation potential of B. cereus has been also examined for several pollutants, such as azo dyes [Citation20], polycyclic aromatic hydrocarbons [Citation21], 2,4-dichlorphenol [Citation22].
In this study, the newly isolated strain BN66 was identified by morphological, biochemical and 16S rRNA sequence analyses as a member of the genus Bacillus, being 100% related to Bacillus cereus. Strain BN66 degraded effectively representatives of the main classes of hydrocarbons and complex hydrocarbon mixtures such as crude oil. It has been widely documented that many hydrocarbon-degrading bacteria optimize the uptake of hydrophobic substrates by producing biosurfacatants [Citation23]. The members of genus Bacillus are known to produce some of the most potent surfactants of lipopeptide origin [Citation24]. For example, a B. cereus strain produces plipastatins, a family of lipopeptides with antifungal activity [Citation25,Citation26]. Our results undoubtedly indicate that B. cereus BN66 also produced biosurfactants when grown on crude oil, thus enhancing hydrocarbon degradation.
Immobilization of viable microbial cells, which can be achieved by attachment, aggregation or entrapment, localizes the cells into a defined region and allows the repeated use of their catalytic activity. The high immobilization efficiency of the cells in an immobilized form and the high affinity between the matrix and the substrates lead to effective degradation. We achieved better performance with the polyacrylamide cryogels obtained from the polymeric precursor. The degradation rate increased as a result of increased availability of the substrates for the cells and a better interaction between the substrates and the immobilized cells. Quek et al. [Citation27] reported the immobilization and performance of Rhodococcus sp. F92 on polyurethane foam (PUF) in the bioremediation of petroleum hydrocarbons. The immobilized cells could degrade a variety of petroleum products. In another study, R. corynebacterioides QBT immobilized on chitin and chitosan flakes increased significantly the crude oil biodegradation [Citation28]. Xu and Lu [Citation29] demonstrated that oil removal in a crude-oil–contaminated soil was increased by application of hydrocarbon-degrading bacteria immobilized on peanut hull powder as the biocarrier. The oil-degrading ability of the immobilized bacterial consortium in cocopeat, rice hull powder and sodium alginate capsules was compared by Nunal et al. [Citation30].
The use of sodium alginate, activated carbon, and calcium chloride was reported to give good bacterial colonization and bioremediation by supplying an available surface area to support bacterial growth and adsorbing of sufficient substrates to make a direct contact between bacterial cells and petroleum hydrocarbon for a better performance in crude oil biodegradation. Five strains of bacteria, namely, Exiguobacterium sp. ASW-1, Pseudomonas aeruginosa strain ASW-2, Alcaligenes sp. ASW-3, Alcaligenes sp. ASS-1 and Bacillus sp. ASS-2, isolated from the Zhejiang coast in China, performed well in degrading 75.1% crude oil (1%, w/v) in 7 days by application of calcium alginate—activated carbon embedding carrier [Citation31]. The efficiencies of free and immobilized microbial consortia in the degradation of different types of petroleum hydrocarbons were investigated to show that the biodegradation rates of naphthalene, phenanthrene, pyrene and crude oil reached about 80%, 30%, 56% and 48% under the optimum environmental conditions of free microbial consortia after 7 days. Of the carriers used, semi-coke was the best immobilizing carrier followed by walnut shell and activated carbon. The degradation rate of the immobilized microbial consortium (47%) was higher than that of a free microbial consortium (26%) [Citation32]. In our case, only 2 days were needed by the free cells of the strain to degrade the n-alkanes almost totally (93%). The efficiency of the immobilization procedure was confirmed also by the high rate of degradation of the aliphatic fraction (almost 100% after 20 cycles of operation) using polyacrylamide cryogels.
The choice of carrier is an important issue and depends on the purpose for which the immobilized material will be used. The number of cells attached to the support depends on the kind of support. In our experiments, a key point for the good cell performance up to 22 runs of degradation was the lack of cell leakage. It seems that the cryogel matrices obtained from a high molar mass precursor possess internal structure that holds the cells inside the carrier. Most probably, due to the higher swelling of the looser PAAm network (DS 35), the cryogel walls partly closed the interconnected pores containing cells and, thus, prevented cell leakage. In the case of AAm cryogels, the incorporation of the crosslinking agent resulted in increased crosslinking density and lower swelling ability (DS 23), which preserved the large pore size after soaking in the cell suspension. In this material, the cells can easily escape the carrier in aqueous media. It was demonstrated that immobilized cells can be more effective, perform better and faster and can act for a longer period in bioremediation processess.
On the other hand, most known biosurfactants possess different biological activities [Citation33]. Recent studies showed that metabolites from a Bacillus cereus strain exhibit anticancer properties [Citation34]. In our case, the biosurfactants produced by the investigated strain are suitable to be tested for biological activity, as potential antitumour agents. Initial studies are under way.
Conclusions
Isolation, identification and characterization of a biosurfactant-producing strain capable of degrading crude oil hydrocarbons were performed. The cells immobilized in polyacrylamide cryogels were used for crude oil biodegradation. We demonstrated that immobilized cells of the selected strain can be much more effective for bioremediation purposes. Immobilization of viable microbial cells possessing the capability to degrade harmful pollutants can be identified as a potential emerging technology for removing different spills, as highly hazardous oily materials can be mineralized to harmless products.
Disclosure statement
The authors have no financial conflicts of interest to declare.
Additional information
Funding
References
- Al-Hawash B, Dragh A, Li S, et al. Principles of microbial degradation of petroleum hydrocarbons in the environment. Egypt J Aquatic Res. 2018; 44:71–76. https://doi.org/10.1016/j.ejar.2018.06.001
- Atlas RM, Hazen TC. Oil biodegradation and bioremediation: a tale of the two worst spills in US history. Environ Sci Technol. 2011; 45:6709–6715. DOI:10.1021/es2013227
- Kumari B, Singh SN, Singh DP. Characterization of two biosurfactant producing strains in crude oil degradation. Process Biochem. 2012; 47:2463–2471.
- Ismail S, Dadrasnia A. Biotechnological potential of Bacillus salmalaya 139SI: a novel strain for remediating water polluted with crude oil waste. PLoS One. 2015;10:e0120931. [cited 2019 Apr 15]; https://doi.org/10.1371/journal.pone.0120931
- Barathi S, Vasudevan N. Utilization of petroleum hydrocarbons by Pseudomonas fluorescens isolated from a contaminated soil. Environ Int. 2001; 26:413–416. https://doi.org/10.1016/S0160-4120(01)00021-6
- Zhang Y, Miller RM. Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant). Appl Environ Microbiol. 1992; 58:3276–3282.
- Bayat Z, Hassanshahian M, Cappello S. Immobilization of microbes for bioremediation of crude oil polluted environments: a mini review. Open Microbiol J. 2015; 9:48–54.
- Christova N, Petrov P, Kabaivanova L. Biosurfactant production by entrapped in cryogels Pseudomonas aeruginosa BN10 cells. Z Naturforsch. 2013; 68c:47–52.
- Cooper DJ, Goldenberg BG. Surface-active agents from two bacillus species. Appl Environ Microbiol. 1987; 53:224–229. https://doi.org/0099-2240/87/020224-06$02.00/0
- Holt JG, Krieg NR, Sneath PHA. Bergey’s Manual of Determinative Bacteriology. 9th Ed. Baltimore, MD: Lippincott Williams & Wilkins; 1994. ISBN-10:9780683006032
- Miller L, Dykes L, Polesky A. A simple salting-out procedure for extracting DNA from nucleated cells. Nucleic Acids Res. 1988;16:1215.
- Petrov P, Petrova E, Tsvetanov CB. UV-assisted synthesis of super-macroporous polymer hydrogels. Polymer. 2009; 50:1118–1123. https://doi.org/10.1016/j.polymer.2008.12.039
- Lozinsky VI. Cryogels on the basis of natural and synthetic polymers: preparation, properties and application. Russ Chem Rev. 2002; 71:489–511. http://dx.doi.org/RC020489
- Doddamani HP, Ninnekar HZ. Biodegradation of phenanthrene by a Bacillus species. Curr Microbiol. 2000; 44:11–14. https://doi.org/10.1007/s002840010083
- Zhuang WQ, Tay JH, Maszenan AM, et al. Bacillus naphtovorans sp. nov. from oil-contaminated tropical marine sediments and its role in naphthalene biodegradation. Appl Microbiol Biotechnol. 2002; 58:547–553. https://doi.org/10.1007/s00253-001-0909-0
- Calvo C, Toledo FL, González-López J. Surfactant activity of a naphthalene degrading Bacillus pumilis strain isolated from oil sludge. J Biotechnol. 2004; 109:255–262. https://doi.org/10.1016/j.jbiotec.2004.01.009
- Subramanian R. Kinetics of growth and catechol production by Bacillus stearotermophilus. Masters Abstr Int. 1992; 30:1405–1412.
- Duffner FM, Kirchner U, Bauer MP, et al. Phenol/cresol degradation by the thermophilic Bacillus thermoglucosidasius A7: cloning and sequence analysis of five genes involved in the pathway. Gene. 2000; 256:215–221. https://doi.org/10.1016/S0378-1119(00)00352-8
- Kim AA, Pestsov GV, Yadgarov KT, et al. Microorganisms degrading polychlorinated biphenyls. Appl Biochem Mircrobiol. 2004; 40:60–62. https://doi.org/10.1023/B:ABIM.0000010354.07292.ca
- Kherha MS, Saini HS, Sharma DK, et al. Comparative studies on potential of consortium and constituent pure bacterial isolates to decolorize azo dyes. Water Res. 2005; 39:5135–5141.
- Kazunga C, Aitken MD, Gold A, et al. Fluoranthene-2,3- and -1,5-diones are novel products from the bacterial transformation of fluoranthene. Environ Sci Technol. 2001; 35:917–922.
- Matafonova G, Shirapova G, Zimmer C, et al. Degradation of 2, 4-dichlorphenol by Bacillus sp. Isolated from an aeration pond in the Baikalsk pulp and paper mill (Russia). Int Biodeterior Biodegrad. 2006; 58:209–212.
- Hommel RK. Formation and physiological role of biosurfactants produced by hydrocarbon-utilizing microorganisms. Biodegradation. 1990; 1:107–119. https://doi.org/10.1007/BF00058830
- Singh P, Cameotra SS. Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol. 2004; 22:142–146.
- Nishikiori T, Naganawa H, Muraoka Y, et al. Plipastatins: new inhibitors of phospholipase A2, produced by Bacillus cereus BMG302-fF67. II. Structure of fatty acid residue and amino acid sequence. J Antibiot. 1986; 39:745–754. https://doi.org/10.7164/antibiotics.39.745
- Umezawa H, Aoyagi T, Nishikiori T, et al. Plipastatins: new inhibitors of phospholipase A2, produced by Bacillus cereus BMG302-fF67. I. Taxonomy, production, isolation and preliminary characterization. J Antibiot. 1986; 39:737–744. https://doi.org/10.7164/antibiotics.39.737
- Quek E, Ting YP, Tan HM. Rhodococcus sp. F92 immobilized on polyurethane foam shows ability to degrade various petroleum products. Biores Technol. 2006; 97:32–38.
- Gentili AR, Cubitto MA, Ferrero M, et al. Bioremediation of crude oil polluted seawater by a hydrocarbon degrading bacterial strain immobilized on chitin and chitosan flakes. Int Biodeterior Biodegrad. 2006; 57:222–228.
- Xu Y, Lu M. Bioremediation of crude oil-contaminated soil: comparison of different biostimulation and bioaugmentation treatments. J Hazard Mater. 2010; 183:395–401.
- Nunal SN, Santander de Leon, et al. Bioremediation of heavily oil-polluted seawater by a bacterial consortium immobilized in cocopeat and rice hull powder. Biocontrol Sci. 2014;19:11–22. SM, Bacolod E, https://doi.org/10.4265/bio.19.11
- Chen Q, Li J, Liu M, et al. Study on the biodegradation of crude oil by free and immobilized bacterial consortium in marine environment. PLoS One. 2017; 12:3–12.
- Shen T, Pi Y, Bao M, et al. Biodegradation of different petroleum hydrocarbons by free and immobilized microbial consortia. Environ Sci Processes Impacts. 2015;17:2022–2033.
- Cameotra SS, Makkar RS. Recent applications of biosurfactants as biological and immunological molecules. Curr Opin Microbiol. 2004; 7:262–266. https://doi.org/10.1016/j.mib.2004.04.006
- Vijaya Kumar ML, Thippeswamy B, Vasanth Raj P. Cytotoxicity and anticancer studies of Bacillus cereus and Bacillus pumilus metabolites targeting human cancer cells. Appl Biochem Microbiol. 2014; 50:619–623. https://doi.org/10.1134/S0003683814060088