Abstract
The aim of this study was to evaluate the possible role of MMP-7 serum levels as biomarkers for brain tumours. This study included 50 patients with meningiomas, other benign tumours, high-grade gliomas, brain metastases and other non-tumour diseases and 41 control individuals. The MMP-7 serum levels were measured by enzyme-linked immunosorbent assay (ELISA). We significantly found higher serum levels of MMP-7 in patients with benign brain tumours (2.33 ± 0.37 [SEM] ng/mL, p = 0.006) and brain metastases (2.54 ± 0.33 ng/mL, p = 0.0001) compared to controls (1.48 ± 0.09 ng/mL). Glioblastoma (GBM) patients had serum MMP-7 levels comparable to those of the controls (1.44 ± 0.13 ng/mL, p = 0.901) but significantly lower than those of the patients with benign tumours (p = 0.018) and brain metastases (p = 0.001). In patients with benign tumours, there was a positive correlation with borderline significance between serum MMP-7 levels and leukocyte counts (ρ = 0.538, p = 0.058). No difference was found (p = 0.448, paired-samples t-test) when comparing the MMP-7 levels in the serum samples obtained at admission and 4–7 days after surgery of some of the patients (n = 7). According to our results, the MMP-7 serum levels might be a useful serum biomarker for benign brain tumours and for brain metastases but not for glioblastoma.
Introduction
Brain tumours could be benign (such as meningioma and low-grade gliomas – astrocytomas I to II grade) and malignant (like high-grade gliomas – astrocytoma III to IV grade, ‘multiform glioblastoma’). Brain tumours are also subdivided into primary (all of the above) and secondary ones (metastases) [Citation1,Citation2]. Symptoms could include headache, vomiting, seizures, mental changes and muscle weakness [Citation3–6]. Tumours are diagnosed by computed tomography (CT), magnetic resonance imaging (MRI) and biopsy [Citation7,Citation8]. Treatment includes surgery, chemotherapy, radiotherapy and targeted therapy [Citation9,Citation10].
The brain is a frequent localization of metastases from cancers with different tissue origin [Citation11]. So far, the effectiveness of all current (multimodal) therapeutic approaches has been poor, and the life expectancy from the time of glioblastoma multiforme (GBM) diagnosis is 14 months on average [Citation12].
It has been shown that membrane-bound or secreted proteases, such as matrix metalloproteinases (MMPs), are involved in the expansion and invasion of gliomas via extracellular matrix (ECM) degradation [Citation12]. MMPs are produced within the brain and their production may be increased by substances such as pro-inflammatory cytokines and β-amyloid [Citation13].
The MMP family comprises more than 25 zinc-dependent proteases that cleave the ECM and cell-surface proteins and are involved in tissue remodelling in a variety of physiological and pathological conditions, including cancers. MMPs can activate and increase the bioavailability of a vast range of non-matrix proteins, such as cytokines, chemokines, soluble receptors and antimicrobial peptides [Citation14–16].
MMP-7 is primarily produced by non-injured, non-inflamed mucosal epithelium of many organs, but it is highly up-regulated by injury or exposure to bacteria resulting in stimulation of cell migration [Citation17,Citation18]. Substrates of MMP-7 are a variety of ECM components: elastin, type IV collagen, fibronectin, vitronectin, aggrecan and proteoglycans. Cleavage of other non-ECM targets results in the release of growth factors (e.g. heparin-binding epidermal growth factor [HB-EGF]), or receptors (epidermal growth factor receptor [EGFR]), as well as in ectodomain shedding of cell-surface molecules (Fas ligand and E-cadherin) [Citation18,Citation19].
In this respect, the aim of this study was to evaluate the possible role of MMP-7 serum levels as biomarkers for brain tumours.
Subjects and methods
Patients and controls
The group of patients consisted of 50 individuals: 32 males (64%) and 18 females (36%), with tumour diseases (benign and malignant) and others with non-tumour brain diseases. The age of all patients varied between 25 and 81 years (median age of 65 years). Three of the patients (6%) were with non-tumour, non-traumatic intracranial hemorrhage; 13 (26%) with benign brain tumours (11 with meningioma, 1 with oligodendroglioma grade II and 1 with Schwannoma); 14 patients (28%) were with malignant brain pathologies – high-grade gliomas, GBM, and 20 patients (40%) had brain metastases of different primary origin (lung, colorectal, breast, gastric or unknown origin).
The control group consisted of 41 individuals non-affected by any type of cancers or brain diseases: 13 males (31.7%) and 28 females (68.3%). The age of the controls varied between 26 and 76 years (median age of 52 years).
Ethics statement
Informed consent was given by all tested subjects prior to sample collection. The work was approved by the Ethics Committee at Medical Faculty, Trakia University, Stara Zagora, Bulgaria.
Measurement of MMP-7 serum concentrations
Venous blood was collected at the admission of the patients at the ward before the brain surgery. For seven patients, blood was also obtained 4–7 days after the surgery. Serum was separated by centrifugation and stored at −20 °C until the assay. The enzyme-linked immunosorbent assay (ELISA) was used for MMP-7 concentration measurement (R&D Systems®, Inc.). The results were expressed as optical density (OD) at 450 nm and the concentration was calculated in ng/mL according to the OD of the standards, using a standard curve.
Statistical analyses
Statistical analyses were performed using SPSS 16.0 for Windows (SPSS Inc.). Continuous variables were analyzed for normality of the distribution using one-sample Kolmogorov–Smirnov D-test. Student’s t-test or one-way analysis of variance (ANOVA) with LSD post-hoc analysis were used for comparison of continuous variables with normal distribution between independent groups and paired-samples t-test when comparing the continuous variable between dependent groups (before and after surgery). The Mann–Whitney U test or Kruskal–Wallis H test were used when variables with non-normal distribution were compared. Factors with p < 0.05 were considered statistically significant.
Results and discussion
The migration of tumour cells requires cell/matrix and cell/cell adhesion, together with degradation of ECM components. Different proteinases have been found to play a pivotal role in this process [Citation16,Citation20].
In our study, we significantly found higher MMP-7 serum levels in patients with non-tumour brain diseases (2.09 ± 0.26 [SEM] ng/mL, p = 0.045), with benign brain tumours (2.33 ± 0.37 [SEM] ng/mL, p = 0.006) and with brain metastases (2.54 ± 0.33 ng/mL, p = 0.0001) compared to controls (1.48 ± 0.09 ng/mL; ).
Figure 1. MMP-7 serum levels in non-tumour brain diseаses, benign brain cancers and brain metastases compared to controls.
Note: The data are presented as mean values with standard error of the mean (±SEM). *p = 0.006 controls versus benign brain tumours; **p = 0.0001 controls versus brain metastases.
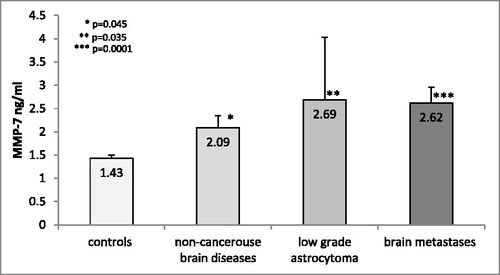
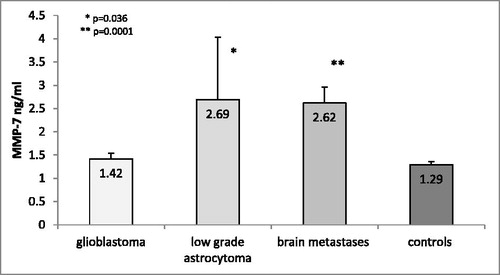
Surprisingly, our results showed commensurate serum levels of MMP-7 in patients with GBM and control individuals (1.44 ± 0.13 ng/mL vs. 1.48 ± 0.09 ng/mL, p = 0.901; ). Patients with benign tumours and with metastases had significantly higher MMP-7 levels compared to those with malignant glioblastoma (p = 0.018 and p = 0.001, respectively; ).
Figure 2. MMP-7 serum levels in GBM patients compared to other brain tumours and controls.
Note: The data are presented as mean values with standard error of the mean (±SEM). *p = 0.018 glioblastoma versus benign brain tumours; **p = 0.001 glioblastoma versus brain metastases.
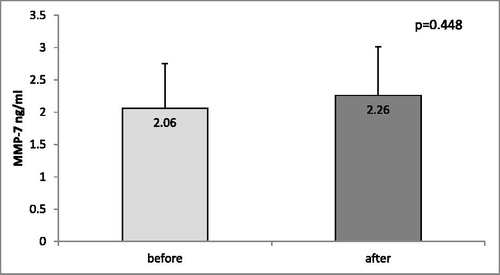
Next, we compared the MMP-7 levels in the blood samples from seven patients (five with GBM and two with brain metastases) before surgery and 4–7 days after the surgical therapy. When comparing the MMP-7 serum levels in those patients before and after surgery, we found no differences (2.06 ± 0.69 vs. 2.26 ± 0.75, p = 0.448, paired-samples t-test; ).
Figure 3. MMP-7 serum levels in samples (n = 7) collected before and after surgery of patients with GBM and brain metastases.
Note: The data are presented as mean values with standard error of the mean (±SEM).
Some recent researches have revealed a connection between the expression and/or serum levels of MMP-7 and different brain disorders, including contusional traumatic brain injury [Citation21], glioblastoma [Citation22], human brain gliomas [Citation23] and mood disorders [Citation24]. There is evidence that microglia is able to release MMP-7, and studies have suggested that in some neurological conditions the increased level of MMP-7 might be implicated in the alteration of the synaptic function [Citation25].
Tight junctions between microvascular endothelial cells within the brain prevent the direct entry of macromolecules and blood-borne cells, forming the BBB (blood–brain barrier) [Citation26]. The migration of cancer cells through the BBB is a key event during brain metastasis [Citation27] and it is known that MMPs are involved in pathogen clearance, monocyte migration, BBB permeability and neuroimmune processes [Citation28]. MMP-7 activity towards substrates associated to the epithelial cell membrane, such as β4-integrin, TNF-α, Fas ligand, heparin-binding EGF, IGF-binding proteins and plasminogen could promote epithelial cell migration, proliferation and apoptosis [Citation18,Citation29]. It is also shown that MMP-7 mediates shedding of E-cadherin (epithelial cadherin), the main calcium-dependent cell–cell adhesion protein in most epithelia [Citation18]. Moreover, the reduction or loss of E-cadherin was shown to correlate with increased malignancy in tumours and invasiveness in carcinoma cell lines in vitro [Citation30].
Besides ECM remodeling, MMPs regulate the activity or bioavailability of a variety of cytokines, growth factors, together with their receptors to aid tumour formation and progression [Citation31]. On the other hand, MMP-7 expression is regulated by TGF-β: in human glioma cell lines, TGF-β has been shown to stimulate the expression of MMP-7 and to facilitate the invasive behaviour of the cells [Citation18,Citation32]. Some studies show that MMP-7 is expressed in glioblastomas [Citation22,Citation33,Citation34]. A few surveys have shown the connection between NFAT1 (nuclear factor of activated T cells), ALDH1A1 (aldehyde dehydrogenase 1A1) and MMP-7. Both (NFAT1 and ALDH1A1) are overexpressed in high-grade glioma and they correlate with low differentiation, invasiveness and poor prognosis. Moreover, high levels of NFAT1 and ALDH1A1 might lead to or result from high levels of MMP-7 and mRNA [Citation35].
The expression levels of miR-320a and miR-663 are also connected to MMP-7. These miRNAs are decreased in high-grade glioma cells [Citation36]. The overexpression of miR-663 has been shown to suppress the proliferation and invasion of glioma cells [Citation37]. The overexpression of miR-320a suppresses G1/S phase transition, proliferation, migration and invasiveness of glioma cells that might occur via indirect stimulation of the tumour suppressor protein p21 and decreasing of Smad2, Smad4, MMP2, MMP7 and cyclinD1 expressions [Citation38].
Our results show no differences in MMP-7 serum levels between patients with GBM and control individuals, which are in contrast to the aforementioned literature data. The reason might be the variations in gene expression due to the gene variants. As the genes encoding MMPs are highly polymorphic, the low MMP-7 levels might be due to some functional single nucleotide polymorphisms (SNPs) affecting the transcriptional activity. Such functional SNPs might be the promoter MMP7 -181A > G (rs11568818) SNP: it has been shown that the G variant allele has greater basal transcriptional activity than the A allele in the human monocyte/macrophage cell line U937 in vitro [Citation39]. Since we did not genotype the studied groups, further studies with larger groups and with genotype analysis should be conducted.
The MMPs activity is tightly regulated on the level of activation and inhibition. MMPs are produced in inactive forms (pro-enzymes) which need to be activated by removal of the pro-peptide masking the catalytic site [Citation15,Citation29]. Tissue inhibitors of matrix metalloproteinases (TIMPs) are specific inhibitors of MMPs. There are four TIMPs (TIMP-1, TIMP-2, TIMP-3 and TIMP-4) have been identified in vertebrates. These TIMPS inhibit all MMPs tested so far, except that TIMP-1 fails to inhibit MT1-MMP [Citation40]. In this respect, we might consider that other factors are involved in the regulation of MMP-7 concentration and activity.
The circulating levels of MMPs are suggested to depend both on the local tissue expression and on the level of expression by the blood cells. Several studies prove that activated monocytes and macrophages are an important source of MMPs, including also of MMP-7 [Citation28,Citation41,Citation42]. In our study, we found a positive correlation, although with borderline significance (ρ = 0.538, p = 0.058) between MMP-7 levels and leukocyte number in patients with benign brain tumours, such as meningioma.
In a study, Harter et al. [Citation43] found higher leukocyte number in patients with brain metastases. In our study, the patients with glioblastoma and metastases had no significantly higher leukocyte numbers (11.06 ± 1.27 × 109/L; and 11.41 ± 0.82 × 109/L, respectively), compared to the patients with benign brain tumours (9.91 ± 1.14 × 109/L). However, the high variation in the serum MMP-7 in the different types of malignant brain tumours suggests that MMP-7 might be released by other cell types besides leukocytes. Immunohistochemistry analysis of MMP-7 expression could be useful in determining the type of cells leading to variable levels of serum MMP-7 in patients with a different type of brain cancers.
Conclusions
The results of our study revealed several questions about the possible role of serum MMP-7 as a biomarker in brain diseases. The results suggest that serum MMP-7 levels might be useful in distinguishing malignant glioblastoma from benign brain tumours and brain metastases but cannot be applied as a reliable biomarker in the diagnosis of glioblastoma. Further studies with larger groups of patients with better selection, characterization of the tumour types and stages and with a longer follow-up are necessary in order to clarify the functions of MMP-7 in the pathogenesis, progression and prognosis of brain neoplasms and to explore its possible role as a biomarker.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lai JS, Jensen SE, Beaumont JL. Development of a symptom index for patients with primary brain tumors. Value Health. 2014;17:62–69.
- Swartling FJ, Hede SM, Weiss WA. What underlies the diversity of brain tumors? Cancer Metastasis Rev. 2013;32:5–24.
- Kahn K, Finkel A. It IS a tumor – current review of headache and brain tumor. Curr Pain Headache Rep. 2014;18:421.
- Gregg N, Arber A, Ashkan K, et al. Neurobehavioural changes in patients following brain tumour: patients and relatives perspective. Support Care Cancer. 2014;22:2965–2972.
- Richter A, Woernle CM, Krayenbuhl N, et al. Affective symptoms and white matter changes in brain tumor patients. World Neurosurg. 2015;84:927–932.
- Cahill J, LoBiondo-Wood G, Bergstrom N, et al. Brain tumor symptoms as antecedents to uncertainty: an integrative review. J Nurs Scholarsh. 2012;44:145–155.
- Zhao L, Jia K. Multiscale CNNs for brain tumor segmentation and diagnosis. Comput Math Methods Med. 2016; 8356294. doi:10.1155/2016/8356294. Epub 2016 March 16.
- Shiroishi MS, Castellazzi G, Boxerman JL, et al. Principles of T2-weighted dynamic susceptibility contrast MRI technique in brain tumor imaging. J Magn Reson Imaging. 2015;41:296–313.
- Gates M, Alsaidi M, Kalkanis S. Surgical treatment of solitary brain metastases. Prog Neurol Surg. 2012;25:74–81.
- Zanders ED, Svensson F, Bailey DS. Therapy for glioblastoma: is it working? Drug Discov Today. 2019;24:1193–1201.
- Fidler IJ. The biology of brain metastasis: challenges for therapy. Cancer J. 2015;21:284–293.
- Konnecke H, Bechmann I. The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin Dev Immunol. 2013;2013:914104.
- Szklarczyk A, Oyler G, McKay R, et al. Cleavage of neuronal synaptosomal-associated protein of 25 kDa by exogenous matrix metalloproteinase-7. J Neurochem. 2007;102:1256–1263.
- Apte SS, Parks WC. Metalloproteinases: a parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015;46:1–6.
- Kapoor C, Vaidya S, Wadhwan V, et al. Seesaw of matrix metalloproteinases (MMPs). J Cancer Res Ther. 2016;12:28–35.
- Wyganowska-Świątkowska M, Tarnowski M, Murtagh D, et al. Proteolysis is the most fundamental property of malignancy and its inhibition may be used therapeutically (Review). Int J Mol Med. 2019;43:15–25.
- Tacheva T, Dimov D, Anastasov A, et al. Association of the MMP7 -181A > G promoter polymorphism with early onset of chronic obstructive pulmonary disease. Balkan J Med Genet. 2017;20:59–66.
- Ke B, Fan C, Yang L, et al. Matrix metalloproteinases-7 and kidney fibrosis. Front Physiol. 2017;8:21.
- Han JC, Li XD, Du J, et al. Elevated matrix metalloproteinase-7 expression promotes metastasis in human lung carcinoma. World J Surg Oncol. 2015;13:5.
- Anastasov A, Vihinen P, Nikkola J, et al. Matrix metalloproteinases in development and progression of skin malignant melanoma. Sci Technol Med. 2011;1:234–241.
- Guilfoyle MR, Carpenter KL, Helmy A, et al. Matrix metalloproteinase expression in contusional traumatic brain injury: a paired microdialysis study. J Neurotrauma. 2015;32:1553–1559.
- Mu N, Gu J, Liu N, et al. PRL-3 is a potential glioblastoma prognostic marker and promotes glioblastoma progression by enhancing MMP7 through the ERK and JNK pathways. Theranostics. 2018;8:1527–1539.
- Xie H, Xue YX, Liu LB, et al. Expressions of matrix metalloproteinase-7 and matrix metalloproteinase-14 associated with the activation of extracellular signal-regulated kinase1/2 in human brain gliomas of different pathological grades. Med Oncol. 2011;28:433–438.
- Frye MA, Nassan M, Jenkins GD, et al. Feasibility of investigating differential proteomic expression in depression: implications for biomarker development in mood disorders. Transl Psychiat. 2015; December 8;5:e689. doi:10.1038/tp.2015.185.
- Szklarczyk A, Ewaleifoh O, Beique JC, et al. MMP-7 cleaves the NR1 NMDA receptor subunit and modifies NMDA receptor function. FASEB J. 2008;22:3757–3767.
- Buhler LA, Samara R, Guzman E, et al. Matrix metalloproteinase-7 facilitates immune access to the CNS in experimental autoimmune encephalomyelitis. BMC Neurosci. 2009;10:1471–2202.
- Tominaga N, Kosaka N, Ono M, et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716.
- Ragin AB, Wu Y, Ochs R, et al. Serum matrix metalloproteinase levels correlate with brain injury in human immunodeficiency virus infection. J Neurovirol. 2009;15:275–281.
- Gaide Chevronnay HP, Selvais C, Emonard H, et al. Regulation of matrix metalloproteinases activity studied in human endometrium as a paradigm of cyclic tissue breakdown and regeneration. Biochim Biophys Acta. 2012;2012:146–156.
- McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol. 2003;162:1831–1843.
- Sizemore ST, Sizemore GM, Booth CN, et al. Hypomethylation of the MMP7 promoter and increased expression of MMP7 distinguishes the basal-like breast cancer subtype from other triple-negative tumors. Breast Cancer Res Treat. 2014;146:25–40.
- Yokoyama Y, Grunebach F, Schmidt SM, et al. Matrilysin (MMP-7) is a novel broadly expressed tumor antigen recognized by antigen-specific T cells. Clin Cancer Res. 2008;14:5503–5511.
- Raghu H, Nalla AK, Gondi CS, et al. uPA and uPAR shRNA inhibit angiogenesis via enhanced secretion of SVEGFR1 independent of GM-CSF but dependent on TIMP-1 in endothelial and glioblastoma cells. Mol Oncol. 2012;6:33–47.
- Rome C, Arsaut J, Taris C, et al. MMP-7 (matrilysin) expression in human brain tumors. Mol Carcinog. 2007;46:446–452.
- Tie X, Han S, Meng L, et al. NFAT1 is highly expressed in, and regulates the invasion of, glioblastoma multiforme cells. PLoS One. 2013;8:e66008.
- Xu SL, Liu S, Cui W, et al. Aldehyde dehydrogenase 1A1 circumscribes high invasive glioma cells and predicts poor prognosis. Am J Cancer Res. 2015;5:1471–1483.
- Shi Y, Chen C, Zhang X, et al. Primate-specific miR-663 functions as a tumor suppressor by targeting PIK3CD and predicts the prognosis of human glioblastoma. Clin Cancer Res. 2014;20:1803–1813.
- Li H, Yu L, Liu J, et al. miR-320a functions as a suppressor for gliomas by targeting SND1 and β-catenin, and predicts the prognosis of patients. Oncotarget. 2017;8:19723–19737.
- Jormsjo S, Whatling C, Walter DH, et al. Allele-specific regulation of matrix metalloproteinase-7 promoter activity is associated with coronary artery luminal dimensions among hypercholesterolemic patients. ATVB. 2001;21:1834–1839.
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839.
- Kouwenhoven M, Ozenci V, Gomes A, et al. Multiple sclerosis: elevated expression of matrix metalloproteinases in blood monocytes. J Autoimmun. 2001;16:463–470.
- Newby AC. Metalloproteinase production from macrophages – a perfect storm leading to atherosclerotic plaque rupture and myocardial infarction. Exp Physiol. 2016;101:1327–1337.
- Harter PN, Bernatz S, Scholz A, et al. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget. 2015;6:40836–40849.
