Abstract
The increasing antibiotic resistance among pathogenic bacteria is a challenge that drives the development of new antibacterial substances. Marine inhabitants are excellent sources of antimicrobial proteins and considered as promising candidates for the treatment of microbial infections. In the present study, we obtained haemocyanin from Eriphia verrucosa and studied its potential to suppress the growth of some pathogenic bacteria and yeasts. The putative antibacterial molecules were isolated from the haemolymph by chromatography appropriate for producing the native haemocyanin (EvH) and its five structural units (SUs). The results showed that EvH had no antimicrobial activity unlike its glycosylated SUs. All haemocyanin SUs exhibited differential antibacterial activity depending on their grade of glycosylation. The strongest antimicrobial activity of SU1 (with highest carbohydrate content) was against Escherichia coli and Bacillus subtilis. The least glycosylated SU3 and SU4 exhibited the lowest antimicrobial activity against all strains. The fraction SU1 has the potential to be applied as a substitute for some commonly used antibiotics. It was demonstrated that the grade of haemocyanin glycosylation plays an important role in its functional antibacterial properties.
Introduction
Marine inhabitants have been investigated as a valuable source of functional and chemotherapeutic compounds during the last decade [Citation1]. As crustaceans living in an aquatic environment, they have confronted a broad variety of challenges and have developed effective strategies for detecting and eliminating invasive pathogens [Citation2,Citation3]. Marine invertebrates lack a highly specific adaptive immune system and they use their innate and non-adaptive immune system to resist pathogen invasions [Citation4–6]. Cellular immunity predominantly involves the phagocytic activity of haemocytes, whereas humoral immunity requires the release of antimicrobial factors [Citation7]. Humoral defences are related to the haemolymph and include receptor proteins, clotting proteins and antimicrobial peptides [Citation8]. Among the humoral components, antibacterial peptides/proteins are predominant and constitute the first line of defence [Citation9,Citation10]. They involve haemocyanin, which has been found to possess promising antibacterial activity in the host immunity when infection occurs [Citation11–14]. Haemocyanin is a copper-based glycoprotein that serves as an oxygen carrier and is composed of a different number of subunits that assemble into an extremely large macro-molecular entity that exhibits a complex allosteric behaviour during oxygen binding. This multifunctional molecule can convert into a phenoloxidase-like enzyme under certain conditions [Citation15], which would allow it to act as an antiviral agent or generate reactive oxygen species (ROS) as an antimicrobial strategy [Citation16]. Furthermore, haemocyanin acts as non-specific immune protein [Citation17] and acquires stronger antibacterial activity when treated with sodium dodecyl sulphate [Citation18] or after activation by microbial proteases [Citation19]. Haemocyanin from Litopenaeus vannamei interacts with human IgG and IgA as an antigen [Citation20] and acts as a related immune-enhancing protein [Citation21].
Recent studies suggest that an important modification of haemocyanin, which enables it to function as a molecule involved in the immune process is its glycosylation. Moreover, the glycosylation of haemocyanin subunits has been reported to be important for its antiviral effects [Citation22,Citation23] and various anti-tumour properties [Citation24,Citation25]. The glycosylation of arthropod haemocyanin has been identified in Carcinus aestuarii and Haliotis tuberculate [Citation26,Citation27]. The diversity in glycan content and type of glycosylation of haemocyanin contribute to its functional properties and diversity [Citation28]. These modifications are also involved in pathogen recognition and bacterial invasion [Citation29]. Furthermore, the degree of glycosylation of haemocyanin subunits is affected by and depends on the presence of pathogens [Citation30]. In a previous study, our working group showed that three (EvH1, EvH2 and EvH3) out of four purified haemocyanin (EvH) subunits from marine crab Eriphia verrucosa haemolymph were glycosylated and several putative O-linkage sites were found in the identified amino acid sequence of EvH [Citation31].
However, little is currently known about the antibacterial effect of the haemocyanin isoforms as a consequence of the different glycosylation types in arthropods. Therefore, the aim of the present study was to investigate whether haemocyanin from E. verrucosa haemolymph and/or its glycosylated structural units have antimicrobial effect against different clinical pathogens and whether its isoforms have potential for biotechnological applications as antibiotic substitutes.
Materials and methods
Collection of haemolymph
The haemolymph (3–5 mL per crab) was extracted from 10 female E. verrucosa crabs collected from the polysaprobic area near to Kamchia region of the Black Sea. The haemolymph was homogenized in 0.01 mol/L Tris-buffer (Sigma-Aldrich, Steinheim, Germany) and centrifuged at 15,000g and 4 °C for 30 min. The haemocyanin-containing supernatant was collected and stored at 4 °C.
Isolation and purification of haemocyanin and its subunits
A total volume of 45 mL of the crude haemocyanin serum was separated on a 100 kDa molecular weight cut-off membrane in a special ultrafiltration unit (Millipore, Germany) under vacuum conditions. The native haemocyanin (EvH) was purified from the concentrated sample by gel filtration in a Sephacryl S300 column (Amersham Pharmacia Biotech Inc., Piscataway, USA). Briefly, 6 mL of serum was loaded onto the column and was eluted with 0.05 mmol/L Tris-buffer, pH 8 with a flow rate of 5 mL/min. The eluted fraction (EvH) was concentrated using Millipore filter (100 kDa). The haemocyanin concentration was determined spectrophotometrically [Citation32].
To dissociate the aggregates, part of the haemocyanin concentrate (25 mL) was dialysed versus 0.05 mol/L Tris-buffer, pH 9.4, containing 0.01 mol/L ethylenediaminetetraacetic acid (EDTA) for 48 h at 4 °C. The dissociated sample was chromatographed in an anion exchange Fast Flow Sepharose Q column (Amersham Pharmacia Biotech Inc.) equilibrated with buffer A (0.05 mol/L Tris-buffer/0.01 mol/L еDTA), рн 9.4. The subunits were eluted with buffer B (0.05 mol/L Tris-buffer/0.01 mol/L еDTA/1 mol/L NaCl), рн 9.4, with a flow rate of 2 mL/min. The following programme was used: 0% в for 10 min, 0–100% B for 67 min and a final step of 100–0% for 5 min. The collected fractions were desalted (1:1, dH2O) and concentrated in ultrafiltration tubes with a 10-kDa membrane for 15 min at 5000g, 4 °С. Then, 10% SDS polyacrylamide gel electrophoresis was carried out to analyse the purity of the isolated subunits. The subunits were further purified by HPLC in a Nucleosil 100 RP C18 column (250 × 10 mm; Machery–Nagel, Duren, Germany) using the following conditions: eluent A, 0.1% trifluoroacetic acid; eluent B, 80% acetonitrile in A; gradient program: 15% B for 5 min followed by 15–100% B in 55 min at a flow rate of 1 mL/min.
MALDI-TOF mass spectrometry
MALDI analyses were performed using a MALDI-TOF Ultraflex II instrument (Bruker Daltonics, Bremen, Germany) operating in linear positive ion mode (nitrogen laser λ = 337 nm). Five microliters of each sample (structural subunits) were mixed with 5 μL of sinapinic acid matrix solution (saturated solution in H2O/acetonitrile (50/50; v/v) containing 0.1% trifluoracetic acid). About 2-μL spots of this mixture were dropped on the sample holder. External mass calibrations were done using the Protein Calibration Standard II programme (Bruker Daltonics), based on the average values of [M + H]+ of trypsinogen, protein A, bovine albumin (BSA) and the average values of [M + 2H]2+ of the same proteins at mass/charge (m/z) 23,982, 44,613, 66,500, 22,307 and 33,300, respectively.
Carbohydrate analysis
Isolated structural units of haemocyanin were analyzed by orcinol-sulphuric test to determine the carbohydrate content. About 2–4 μL of the purified dissociated samples were applied to the thin layer plate and air-dried. The plate was sprayed with orcinol/H2SO4 and heated for 20 min at 100 °C. The orcinol/H2SO4 solution contained: 0.02 g of orcinol, 20% H2SO4 and H2O to a total volume of 10 mL. Mannose was used as a standard at concentrations: 0.1, 0.25, 0.5, 1 and 2 mg/mL.
Microbial strains, media and culture conditions
The antimicrobial activities of the purified haemocyanin and its subunits were determined against the following bacterial strains: Escherichia coli NBIMCC 3397, Salmonella enterica subsp. enterica ser. Enteritis NBIMCC 8691, Pseudomonas aeruginosa ATCC 27853, Bacillus subtilis NBIMCC 3562, Staphylococcus epidermidis NBIMCC1093, Staphylococcus aureus ATCC 6538P and two fungal strains: Candida albicans ATCC 10231 and Cryptococcus neoformans. The bacterial strains were maintained on Muller Hinton Agar (MHA), while fungal strains were grown on MHA supplemented with 2% glucose.
Antimicrobial assays
The initial screening for antibacterial activities was conducted by the agar-well diffusion method [Citation33]. Erythromycin (50 µg/mL) and fluconazole (50 µg/mL) were used as positive controls. Dimethyl sulphoxide (DMSO) was used as a negative control. Briefly, 50 μL of each haemocyanin fraction (50 µg/mL protein) was loaded into the wells of MHA agar medium, containing 100 μl/mL microbial inoculum (cell density 0.5 McF, 108 CFU/mL). The plates were incubated at 37 °C for 24 h. The antibacterial activity was expressed as the diameter of the zone of inhibition measured in millimetres (mm). The results were expressed as mean values with standard error of the means (M ± SEM).
Determination of MIC and MBC
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the purified haemocyanin units were determined by the standardized protocol CLSI M07-A9 and CLSI M26-A according to CLSI [Citation34]. The colony suspension method was used for bacterial inoculum preparation. Direct saline suspensions were prepared of isolated colonies from 18 to 24 h agar plates and were adjusted to a turbidity equivalent of 0.5 McF (1 × 108 CFU/mL). Then, the 0.5 McF suspensions were diluted 1:20 to yield 1 × 106 CFU/mL. Serial twofold dilutions from 0.87 to 50 µg/mL of each purified isoform in sterile water were made. A mixture of 100 µL of Muller Hinton broth, 100 µL of inoculum suspension (5 × 104 CFU/well) and 100 µL of series concentrations for each sample was placed in sterile 96-well plates and incubated at 37 °C for 18 h. The absorbance at 600 nm was recorded with a microplate reader (BMG Labtech, Offenburg, Germany). The MIC was defined as the lowest concentration of the tested sample that completely prevented bacterial growth. The MBC was determined from the broth dilution of MIC tests by subculturing 100 µL of each dilution on MHA agar plates without the test agent. The agar-well diffusion method was also applied for confirmation of the MICs of the haemocyanin fractions. Briefly, 50 µL of bacterial suspensions (108 CFU/mL) were spread on MHA Petri dishes, and then 50 µL of serial twofold dilutions of samples were loaded into the wells of the agar medium. After incubation for 18–24 h at 37 °C, the diameter of the inhibition zones was measured.
Results and discussion
E. verrucosa is among the aquatic predators of mollusks and sea urchins found in intertidal habitats of Mediterranean Sea, Black Sea as well as Eastern Atlantic from Brittany to Mauritania and the Azores. It is usually encountered in stony coastal zones and occupies infralittoral crevices at shadow zones with a well-developed algal coverage. It migrates during springtime for reproduction towards shallower waters with different saprobic characteristics [Citation35]. In this study, the haemolymph of the warty crab E. verrucosa (Forskål) was used as a model to investigate the effect of a highly saprobic area on the antimicrobial response of haemocyanin.
Preparation of haemocyanin and its subunits
The crude haemocyanin was obtained from collected haemolymph from female E. verrucosa crabs and was separated by means of an ultrafiltration system. The concentrated sample was used to produce purified native haemocyanin (EvH) and its dissociate subunits. The native haemocyanin was purified by gel filtration chromatography on a Sephacryl S300 column. One main peak was eluted from the column by 0.05 mol/L Tris-buffer. The sample (protein concentration of 3.86 ± 0.45 mg/mL) developed a haemocyanin-specific blue colour with bathocuproine sulphonic acid staining. The dissociation of the haemocyanin aggregates was performed by dialysis versus 0.05 mol/L Tris-buffer in alkaline conditions (pH 9.4). Five well-separated fractions (SU1, SU2, SU3, SU4 and SU5) were eluted on a Fast Flow Sepharose Q column with a non-linear gradient in 0.05 mol/L Tris-buffer/0.01 mol/L еDTA/1 mol/L NaCl), рн 9.4 (). The obtained fractions were desalted and concentrated by ultrafiltration and their protein content was determined: 2.53 mg/mL SU1, 1.30 mg/mL SU2, 0.76 mg/mL SU3, 0.93 mg/mL SU4 and 0.39 mg/mL SU5. Electrophoretic analysis confirmed a high-grade separation of haemocyanin structural subunits (, insert). The five haemocyanin isomers were purified separately on a Nucleosil 100 RP C18 column, where one major peak was eluted from the column for each fraction (). Then, to prove that the isolated fractions were the haemocyanin isoforms, the molecular masses of the subunits from E. verrucosa haemocyanin were determined by MALDI-TOF (). The molecular mass of the subunits was in the range of about 74–75 kDa: 75,098 Da (SU1); 74,488 Da (SU2); 75,753 Da (SU3); 74,513 Da (SU4) and 74,713 Da (SU5).
Figure 1. Fractionation and purification of haemocyanin. (A) Fractionation of E. verrucosa dissociated haemocyanin chromatographed on a Fast Flow Sepharose Q column. Insert: Electrophoretic patterns of isolated fractions on a 10% SDS PAA gel electrophoresis (peaks 2, 3, 4 and 5). (B) HPLC purification of structural subunit 5 (SU5) in a Nucleosil 100 RP C18 column. (C) MALDI spectrum of structural subunit 5 (SU5) from E. verrucosa haemocyanin isolated on an anion exchange Fast Flow Sepharose Q column. The sample was measured by MALDI-TOF Ultraflex II.
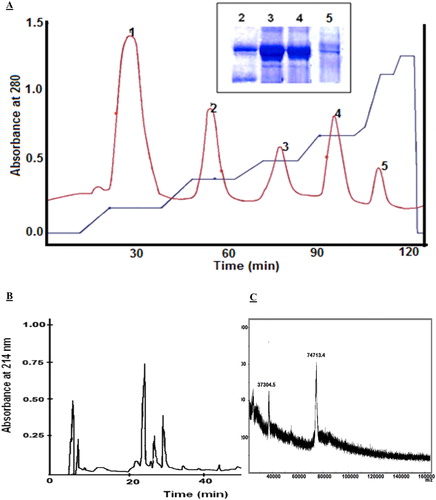
In our previous study [Citation31], we purified and characterized four structural subunits (Mw 75–76 kDa) from the haemocyanin of E. verrucosa. The elution fraction number 5 was considered as non-dissociated haemocyanin. The different chromatographic strategy applied in the present study produced five fractions containing dissociated haemocyanin subunits. Similarly, horse crab Portunus trituberculatus and mangrove crab Scylla serrata reportedly have five types of monomeric arthropod haemocyanin subunits [Citation36,Citation37]. Some crustaceans express distinct haemocyanin subunits during certain developmental stages, or synthesise such subunits in response to certain physiological conditions [Citation38]. Many researchers have reported that the expression levels of hemocyanin genes are affected by bacterial or viral infection [Citation30,Citation38]. These data could explain the presence of a different number of glycosylated haemocyanin subunits in the haemolymph of E. verrucosa.
Glycosylation of structural subunits
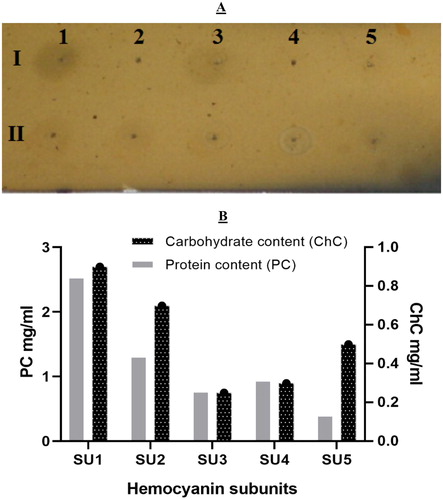
In the present study, the degree of glycosylation of the haemocyanin subunits was of particular interest since haemocyanin samples were obtained from crabs inhabiting a microbially overloaded marine environment. The carbohydrate content of the five dissociated haemocyanin fractions (SU1–SU5) determined by the orcinol-sulphuric test is shown in . In this study, all structural units of E. verrucosa haemocyanin were glycosylated. The highest carbohydrate content was found in fraction SU1 (0.90 mg/mL), followed by SU2 (0.70 mg/mL), SU5 (0.5 mg/mL), SU4 (0.30 mg/mL), and the lowest carbohydrate content was in fraction SU3 (0.25 mg/mL). The protein content of the fractions did not always correlate with the carbohydrate content. For example, fraction SU5 was more glycosylated than fractions SU3 and SU4, which had higher protein content (). Our previous study [Citation31] showed that only three out of four isolated E. verrucosa haemocyanin subunits were glycosylated. No glycans were identified in subunit 4. In that investigation, the haemolymph was obtained from crabs inhabiting a different region (the mesosaprobic area of the Kaliakra region) from those in the present study (the polysaprobic area of the Kamchia region). We speculate that the glycosylation diversity contributed to the functional diversity of haemocyanin and represented environmental adaptation to each habitat transition.
Figure 2. Carbohydrate analysis of haemocyanin subunits. (A) Orcinol–sulphuric acid test of E. verrucosa haemocyanin subunits eluted by HPLC and applied on a silica-gel plate. The spots on lane I are: 1 (2 mg/mL mannose); 2 (1 mg/mL mannose); 3 (0.5 mg/mL mannose); 4 (0.25 mg/mL mannose); 5 (0.1 mg/mL mannose). The spots on lane II are: 1 – SU1; 2 – SU2; 3 – SU3; 4 – SU4; 5 – SU5. (B) Carbohydrate and protein content of haemocyanin structural units of E. verrucosa haemolymph.
Antimicrobial assay
The antibacterial activities of the isolated native haemocyanin as well as its dissociated fractions (SU1–SU5) were evaluated and compared to the positive controls, erythromycin and fluconazole. Investigations were performed against a range of different clinical pathogens: three Gram-positive bacteria: S. epidermidis, S. aureus and B. subtilis; three Gram-negative bacteria: E. coli, S. enterica ser. Enteritis and P. aeruginosa; and two yeasts strains: C. albicans and C. neoformans. In the present study, the subunits (SU1–SU5) of E. verrucosa haemocyanin showed varying antibacterial activity against the tested bacterial pathogens (). Surprisingly, the native molecule of EvH had no antimicrobial activity against any of the tested microorganisms. Similarly, the five SUs did not show any effect against the yeast strains C. albicans and C. neoformans. The maximum antibacterial activity was recorded for SU1 against all the tested clinical pathogens (). The structural unit SU1 caused maximal bactericidal (100%) effect on E. coli and B. subtilis, and a very rapid reduction in bacterial density within 24 h for S. epidermidis (80%), S. enterica (77%), S. aureus (71%) and P. aeruginosa (56%). All haemocyanin subunits showed a strong inhibitory effect against E. coli, more than 42% stronger than the positive control. Thus, subunits SU1, SU2 and SU5 show potential to become a substitute for some commonly used antibiotics to which bacterial resistance has developed.
Figure 3. Antibacterial activity of native haemocyanin and its fractions. (A) Antibacterial activity of native haemocyanin (EvH) and its five fractions (numbers 1–5 represent subunits SU1–SU5, respectively) against different pathogenic bacteria: A/Bacillus subtilis; B/Escherichia coli; C/Salmonella enterica; D/Staphylococcus epidermidis. (B) MIC of subunit 1 (SU1) on test microorganism Staphylococcus epidermidis: 1 – 50 µg/mL; 2 – 25 µg/mL; 3 – 12.5 µg/mL; 4 – 6.25 µg/mL; 5 – 3.125 µg/mL; 6 – 1.562 µg/mL.
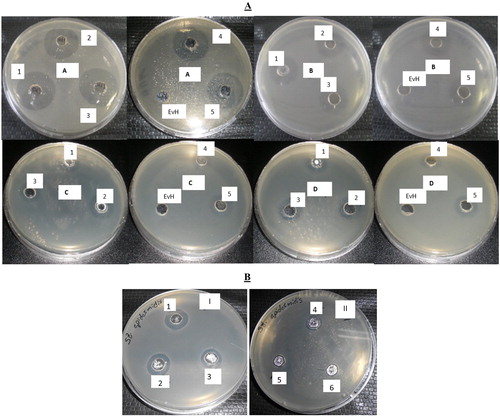
Table 1. Antibacterial activity of E. verrucosa haemocyanin and its subunits based on zones of inhibition (mm).
The antimicrobial activities of the haemocyanin isoforms were quantitatively assessed by determining the MIC and MBC values (, ). The smallest values of MIC (3.12 µg/mL) and MBC (6.25 μg/mL) were obtained against B. subtilis and E. coli for the five tested fractions. The highest values of MIC (25.0 µg/mL) and MBC (50 μg/mL) were measured for S. aureus and P. aeruginosa for the antibacterial activity of fractions SU3 and SU4, respectively. The MBC values revealed that the E. verrucosa haemocyanin subunits were bacteriostatic at lower concentrations and bactericidal at higher concentrations. These different inhibitory effects of the SUs could not be related to the type of cell wall of pathogenic strains and were based on the grade of glycosylation. SU1 is the fraction with the highest carbohydrate content and highest antibacterial activity. In particular, the least glycosylated SU3 and SU4 exhibited the lowest antimicrobial activity against all strains. These results indicate that the extent of haemocyanin glycosylation changed with pathogenic bacterial infection, supporting the suggestion that glycosylation of haemocyanin has been important for the recognition of pathogens [Citation30]. Our results demonstrated the significance of haemocyanin glycosylation as an antibacterial response feature in environmental conditions, based on comparing the carbohydrate content of the subunits and their antibacterial activity.
Table 2. Minimum inhibitory concentration MIC (µg/mL) and minimum bactericidal concentration MBC (μg/mL) of haemocyanin structural units on test microorganisms.
In this study, we determined that non-dissociated E. verrucosa haemocyanin had no antimicrobial activity. The lack of inhibitory effect of the major protein of the haemolymph was not an expected result, as many studies have confirmed the inhibitory activity of crude haemolymph. These results suggest that some antimicrobial peptides/proteins, different from haemocyanin, could perform the antimicrobial activity of the haemolymph. Similarly, the crude haemolymph of S. serrata, induced by the injection of E. coli, has significant antibacterial activity. Contrastingly, the non-induced haemolymph has no antimicrobial activity [Citation37]. The native haemocyanin must be induced to acquire antibacterial activity. According to Zhang et al. [Citation30], the level of haemocyanin glycosylation increased after Vibrio alginolyticus and Vibrio fluvialis infection, especially between 24 and 72 hpi (hours post infection), only decreasing after 96 hpi.
Conclusions
In this study, we report for the first time data about the antibacterial activity of E. verrucosa haemocyanin isoforms. The obtained results indicated that the degree of glycosylation of haemocyanin isoforms is likely determined by microbial contamination in the environment and strongly suggested that some subunits could be used as a platform for the discovery of novel antibacterial drugs. Future studies of the active domains of the haemocyanin subunits and direct induction of their glycosylation will allow the production of recombinant proteins with strong specific binding to clinical pathogens that have acquired resistance to conventional antibiotics.
Acknowledgements
The study was supported by the Operational Programme “Science and Education for Smart Growth” 2014-2020, co-financed by the European Union through the European Structural and Investment Funds, [Grant number BG05M2OP001-2.009-0019-С01 dated 02.06.2017]; and by from the Bulgarian Ministry of Education and Science [Grant number L01-217/30.11.2018] under the National Scientific Program "Innovative Low-Toxic Biologically Active Means for Precision Medicine" (BioActiveMed).
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Giri A, Ohshima T. Bioactive marine peptides: nutraceutical value and novel approaches. Adv Food Nutr Res. 2012;65:73–105.
- Jiravanichpaisal P, Lee BL, Soderhall K. Cell-mediated immunity in arthropods: hematopoiesis, coagulation, melanization and opsonization. Immunobiology. 2006;211:213–236.
- Gil-Turnes MS, Hay ME, Fenical W. Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science. 1989;246:116–118.
- Zasloff M. Antibiotic peptides as mediators of innate immunity. Curr Opin Immunol. 1992;4:3–7.
- Bulet P, Hetru C, Dimarcq JL. Antimicrobial peptides in insects: structure and function. Dev Comp Immunol. 1999;23:329–344.
- Ravichandran S, Sivasubramaninan K, Anbuchezhian RM. Antimicrobial activity from the haemolymph of the crab Ocypode macrocera (H. Milne-Edwards 1852). World Appl Sci J. 2010;11:578–581.
- Ellis R, Parry H, Spicer J, et al. Immunological function in marine invertebrates: responses to environmental perturbation. Fish Shellfish Immunol. 2011;30:1209–1222.
- Soderhall K, Cerenius L. Crustacean immunity. Annu Rev Fish Dis. 1992;2:3–23.
- Smith VJ, Chisholm J. Antimicrobial proteins in crustaceans. In: Beck G, Sugumaran M, Cooper EL, editors. Advances in experimental medicine and biology. Vol. 484, Phylogenetic perspectives on the vertebrate immune system. Boston (MA): Springer; 2001.) p. 95–112.
- Velayutham M, Kamanuri SK, Saravanan K, et al. Cation metals specific hemocyanin exhibits differential antibacterial property in mud crab, Scylla serrata. Biologia. 2016;71/2:176–183.
- Coates CJ, Decker H. Immunological properties of oxygen-transport proteins: hemoglobin, hemocyanin and hemerythrin. Cell Mol Life Sci. 2017;74:293–317.
- Qin Z, Babu VS, Wan Q, et al. Antibacterial activity of hemocyanin from red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. 2018;75:391–399.
- Yan F, Zhang YL, Jiang RP, et al. Identification and agglutination properties of hemocyanin from the mud crab (Scylla serrata). Fish Shellfish Immunol. 2011;30:354–360.
- Zhang Y, Yan F, Hu Z, et al. Hemocyanin from shrimp Litopenaeus vannamei shows hemolytic activity. Fish Shellfish Immunol. 2009;27:330–335.
- Coates CJ, Nairn J. Diverse immune functions of hemocyanins. Dev Comp Immunol. 2014;45:43–55.
- Jiang N, Tan NS, Ho B, et al. Respiratory protein-generated reactive oxygen species as an antimicrobial strategy. Nat Immunol. 2007;8:1114–1122.
- Terwilliger NB. Hemocyanins and the immune response: defense against the dark arts. Integr Comp Biol. 2007;47:662–665.
- Manubens A, Salazar F, Haussmann D, et al. Concholepas hemocyanin biosynthesis takes place in the hepatopancreas, with hemocytes being involved in its metabolism. Cell Tissue Res. 2010;342:423–435.
- Coates CJ, Nairn J. Hemocyanin-derived phenoloxidase activity: a contributing factor to hyperpigmentation in Nephrops norvegicus. Food Chem. 2013;140:361–369.
- Zhang YL, Wang SY, Xu AL, et al. Affinity proteomic approach for identification of an IgA-like protein in Litopenaeus vannamei and study on its agglutination characterization. J Proteome Res. 2006;5:815–821.
- Qiao J, Du ZH, Zhang YL, et al. Proteomic identification of the related immune-enhancing proteins in shrimp Litopenaeus vannamei stimulated with vitamin C and Chinese herbs. Fish Shellfish Immunol. 2011;31:736–745.
- Dolashka-Angelova P, Lieb B, Velkova L, et al. Identification of glycosylated sites in Rapana hemocyanin by mass spectrometry and gene sequence, and their antiviral effect. Bioconjugate Chem. 2009;20:1315–1322.
- Dolashka P, Velkova L, Shishkov S, et al. Glycan structures and antiviral effect of the structural subunit RvH2 of Rapana hemocyanin. Carbohydr Res. 2010;345:2361–2367.
- Arancibia S, Espinoza C, Salazar F, et al. A novel immunomodulatory hemocyanin from the limpet Fissurella latimarginata promotes potent anti-tumor activity in melanoma. PLoS One. 2014;9:e87240.
- Salama WM, Mona MM. In vitro anti-tumor effects of hemocyanin isolated from Atergatis roseus and Eriphia verrucosa crabs. J CBR. 2018;1:28–36.
- Dolashka-Angelova P, Dolashki A, Savvides SN, et al. Structure of hemocyanin subunit CaeSS2 of the crustacean Mediterranean crab Carcinus aestuarii. J Biochem. 2005;138:303–312.
- Velkova L, Dolashka P, Lieb B, et al. Glycan structures of the structural subunit (HtH1) of Haliotis tuberculata hemocyanin. Glycoconj J. 2011;28:385–395.
- Zhang YL, Xing LG, Yan F, et al. Comparative analyses of five hemocyanin isomers from shrimp Litopenaeus vannamei. Chin J Biochem Mol Biol. 2009;25:655–661. in Chinese.
- Valguarnera E, Kinsella RL, Feldman MF. Sugar and spice make bacteria not nice: protein glycosylation and its influence in pathogenesis. J Mol Biol. 2016;428:3206–3220.
- Zhang Z, Wang F, Chen C, et al. Glycosylation of hemocyanin in Litopenaeus vannamei is an antibacterial response feature. Immunol Lett. 2017;192:42–47.
- Dolashki A, Radkova M, Todorovska E, et al. Structure and characterization of Eriphia verrucosa hemocyanin. Mar Biotechnol. 2015;17:743–752.
- Johnson BA, Bonaventura C, Bonaventura J. Allosteric modulation of Callinectes sapidus hemocyanin by binding of L-lactate. Biochemistry. 1984;23:872–878.
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71–79.
- Cockerill FR, Wikler MA, Alder J, et al. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. 9th ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2012.
- Jouili S, Arculeo M, Mansour L, et al. Biological characteristics of three Brachyuran crab species in the Lagoon of Elbibane, South-Eastern Tunisia. Cah Biol. 2016;57:217–226.
- Herskovits TT. Recent aspects of the subunit organization and dissociation of hemocyanins. Comp Biochem Physiol, B. 1988;91:597–611.
- Hoq MI, Seraj MU, Chowdhury S. Isolation and characterization of antibacterial peptides from the mud crab, Scylla serrata. Pak J Biol Sci.. 2003;6:1345–1353.
- Wang J, Zhang F, Song W, et al. Characterization of hemocyanin from the mud crab Scylla paramamosain and its expression analysis in different tissues, at various stages, and under Vibrio parahaemolyticus infection. Genet Mol Res. 2015;14:16639–16651.
