Abstract
‘Toddy’ is an alcoholic beverage obtained by the natural fermentation of phloem sap. We isolated 27 yeast strains from coconut toddy in Sri Lanka. Sequences of the 26S rDNA gene D1/D2 region from 18 strains showed 100% identity to Saccharomyces cerevisiae, while eight strains showed 99% identity to Pichia manshurica and a single isolate showed 99% identity to Saccharomycodes ludwigii. Regarding salt tolerance, all isolated strains of S. cerevisiae were found to grow in the presence of 10.0% (w/v) NaCl in yeast extract-peptone-dextrose (YPD), one of the isolates (SLY-10) grew even with 13.0% (w/v) NaCl. All the isolates grew well at 40 °C, and the 18 S. cerevisiae strains showed significant growth even at 42 °C. All the 18 S. cerevisiae strains still showed growth better than reference strains did in a medium with 7.5% (w/v) NaCl at 40 °C. All S. cerevisiae isolates produced relatively high alcohol concentrations than reference strains did when growing on batch-fermented media with 100 g·L−1glucose in at 40 °C. The five strains (SLY-3, SLY-4, SLY-8, SLY-9 and SLY-10) produced significant amounts of alcohol even at 45 °C: alcohol productivity over 24 h at 40 °C and 45 °C was similar in two different glucose concentrations, and the amount of produced alcohol was higher with 160 g·L−1 glucose than with 100 g·L−1glucose, significantly at 45 °C.
Introduction
In Sri Lanka, the phloem sap from the coconut palm (Cocos nucifera) is collected from the tapped tender inflorescence and is mainly used to produce sweet syrup (coconut honey) or candy (coconut sugar and jaggery) by boiling the unfermented sap. Meanwhile, the milky white fermented sap is sold as an alcoholic beverage called ‘toddy’ or ‘palm wine’; it is referred as ‘tuba’ in the Philippines and ‘tuak’ in Indonesia [Citation1]. In Sri Lanka, the fermented toddy is consumed fresh or pasteurized. The unfermented phloem sap, known as ‘sweet toddy’, contains about 15–18% (w/v) sugars, mainly in the form of sucrose, which is fermented to ethanol by a complex mixture of airborne yeasts and bacteria [Citation2–4].
Fermenting toddy acquires about 3–4.5% (w/v) alcohol within 24 h with a considerable quantity of live microorganisms [Citation5,Citation6]. Toddy can also be distilled to produce a spirit known as ‘Arrack’. Double distilled Arrack is the basis for the local gin or rum, with the addition of the appropriate flavours [Citation7]. Yeasts are essential components of the microflora that are routinely used to produce many foods because of their abilities to produce ethanol and carbon dioxide from sugars. Among yeasts, Saccharomyces cerevisiae has drawn considerable attention due to its high ethanol yield. Many studies have been undertaken to improve ethanol productivity in S. cerevisiae by increasing its tolerance to stress, where high beginning concentrations of sugars result in high ending concentrations of ethanol [Citation8,Citation9].
Most previous investigations of yeasts isolated from fermenting coconut sap have been limited only to the identification of the microorganisms. Wijeyaratne [Citation10] reported two thermotolerant yeast strains isolated from coconut toddy in Sri Lanka; however, the detailed research on the physiological characterization of these toddy yeasts had not been completed previously. Since the toddy sample location was a coastal area in Sri Lanka and the country has a typical tropical climate throughout the year, we assume that isolated yeasts may have the ability to grow and ferment at high salinities and high temperatures. The present study aimed to isolate and identify the yeasts from coconut toddy and then to examine salt and temperature tolerances concerning the fermentation abilities of isolated yeast strains.
Materials and methods
Isolation, identification and characterization of yeasts
Coconut toddy samples were collected from a coastal area of Sri Lanka and transported in sterile bottles for laboratory analysis. Isolation of microorganisms was carried out by an enrichment technique. After inoculation, cultures were incubated for 48 h in a rotary shaker (200 rpm) at 30 °C. Enriched cultures were then streaked on yeast extract-peptone-dextrose (YPD) agar plates (1.0 g of yeast extract, 2.0 g of polypeptone, 2.0 g of glucose per 100 mL distilled water and 1.5 g of agar) and the inoculated plates were incubated at 30 °C for 48 h. The colonies with distinct morphological characteristics were selected and purified by repeatedly streaking on YPD agar medium. Isolated yeast cultures were maintained on YPD agar plates at 4 °C. Stock cultures of the isolated strains were prepared by mixing with 30% (v/v) sterile glycerol and were frozen at −80 °C until use.
PCR amplification of the 26S rDNA D1/D2 region
Genomic DNA was extracted from isolated yeast strains by mixing a minute amount of each colony with 10 µL PrepMan Ultra Sample Preparation Reagent (Applied BiosystemsTM, Thermo Fisher Scientific, USA). The samples were vortexed, boiled for 10 min in a Thermo-cycler T100 (BIO-RAD, Tokyo, Japan) and then allowed to cool for a few minutes. Samples were centrifuged at 14,000 rpm for 2 min and the clear supernatants, containing the extracted DNA, were used for polymerase chain reaction (PCR) analysis. Amplification of the 26S rDNA D1/D2 gene region was performed using the primers NL1: 5′-GCATATCAATAAGCGGAGGAAAAG-3′ and NL4: 5′-GCTCCGTGTTTCAAGACGG-3′ in a 100 µL reaction mixture [50 µL of GoTaq®Green Master Mix (Promega, Madison, WI), 1 µL of each primer (10 µM), 43 µL of Milli-Q water and 5 µL of DNA template] [Citation11]. The PCR was run with an initial denaturation at 95 °C for 5 min and then 30 cycles of denaturation at 95 °C for 1 min, annealing at 45 °C for 1 min and extension at 72 °C for 1 min, with a final extension at 72 °C for 5 min. The PCR products (4 µL) were analyzed by electrophoresis with a 1.6% (w/v) agarose gel. A 100-bp DNA ladder was used as a standard molecular weight marker. The gel was stained with ethidium bromide, visualized under UV light and photographed to document the bands present. The amplified D1/D2 regions of the 26S rDNA gene products were purified using the LaboPassTM PCR product purification kit/spin column method (Cosmo Genetech, Seoul, South Korea). The DNA concentrations (<20 ng/µL) of purified samples were measured using a Nano-drop spectrophotometer (Thermo Scientific™ ND2000USCAN, Thermo Fisher Scientific, USA) and sequenced with NL1 and NL4 primers. The obtained DNA sequences were analyzed using BLAST (NCBI).
Growth of isolated yeasts at different salinities and high temperatures
YPD agar and liquid media were used to inoculate the pre-cultured yeasts. Laboratory yeast strain S288C and Awamori yeast strain 101-18 were used as reference strains. The growth of yeast strains on YPD media supplemented with different salt concentrations (5.0, 7.5, 10.0, 11.0, 12.5, 13.0 and 13.5%) or incubated at high temperatures (30, 37, 40, 41, 42 and 43 °C) was measured by turbidity changes over time using McFarland units.
About 5 mL of pre-cultured yeast (1 × 107 CFU/mL or McFarland’s units = 16.6) were prepared in sterile YPD liquid media inoculated with each yeast strain and then incubated at 30 °C for 24 h while shaking at 200 rpm.
Salinity tolerance
Salt tolerance of all isolated yeast strains was examined on YPD agar and in liquid media (5 mL) prepared with different concentrations of NaCl: 5.0, 7.5 and 10.0% (w/v). Fifty microliter of each pre-cultured yeast strain was inoculated into YPD liquid media. Inoculated YPD agar plates and liquid media were incubated at 30 °C under static for 2 days and shaking at 200 rpm for 4–5 days respectively. Yeast strains that grew in the presence of 10.0% NaCl were further exposed to higher salinities with NaCl concentrations of 11.0, 12.5, 13.0 and 13.5%.
Temperature tolerance
All the isolated yeast strains were examined for thermotolerance under high incubation temperatures. Pre-cultured yeasts inoculated on YPD agar and in liquid media were incubated at 30, 37 or 40 °C while agar plates were kept under static incubation condition for 2 days and the liquid media were incubated with shaking at 200 rpm for 4–5 days. After ensuring that all strains grew on YPD at 40 °C, we examined yeast thermotolerance by inoculating in YPD liquid media and then incubating at temperatures above 40 °C while shaking at 200 rpm for 4–5 days.
Combined effects of salinity and temperature on yeast growth
All isolated strains of S. cerevisiae were used to examine the combined effects of salinity and high temperature on yeast growth. Because all the strains grew well at 30 °C in the presence of 7.5% NaCl, we examined the yeast tolerance in the presence of 7.5% NaCl at 37 °C or 40 °C. Five millilitres of YPD liquid medium supplemented with 7.5% NaCl (w/v) were inoculated with 50 µL of pre-culture and then incubated at 37 °C or 40 °C with shaking at 200 rpm for 4–5 days. The growth of all the strains was measured using the turbidity metre.
Fermentation ability
Alcohol production by all S. cerevisiae isolated strains was investigated on batch-fermented media containing 1.0 g of yeast extract, 2.0 g of polypeptone, 10.0 g of glucose per 100 mL distilled water under static conditions. Pre-cultured yeasts (5 mL) on YPD were centrifuged for 5 min at 3,000 rpm (4 °C), the supernatant was removed, a small portion of the medium was added to the harvested yeast cells and the whole cell suspension was transferred to the 100 mL of the medium.
Alcohol production was examined for all strains at different temperatures: 30, 37 or 40 °C. Yeast strains exhibiting the highest alcohol production at 40 °C were used to further examine their fermentation abilities at 41, 42, 43 and 45 °C; cultures were incubated under static conditions for 4–5 days. We also attempted to examine the fermentation ability of selected yeast strains using 160 g·L−1glucose at 30, 40 and 45 °C.
We collected 1 mL samples in 12-h intervals and then centrifuged the samples at 6,000 rpm for 5 min (4 °C) to obtain the supernatant. Alcohol quantification from collected supernatants was carried out using the enzyme alcohol dehydrogenase (ADH) extracted from membrane fraction of acetic acid bacteria [Citation12].
Alcohol quantification
Appropriately diluted samples (100 µL) were mixed with 900 µL of enzyme reaction buffer, composed of potassium ferricyanide (100 mmol L−1) and Mcllvaine buffer (pH 6.5), and then 5 µL of ADH enzyme was added. The reaction mixture was vortexed and incubated for 5 min at room temperature. Afterwards, 500 µL of dupanol reagent was added, vortexed and incubated for 20 min to stop the enzymatic reaction, which was confirmed when the golden-yellow colour was converted to a greenish-blue instantly. Then, 3.5 mL of distilled water was added, the samples were vortexed and the absorbance of each sample was determined at 660 nm. Finally, the alcohol concentrations (g·L−1) of samples were calculated along with prepared standard curves (0, 0.002, 0.004, 0.006, 0.008 and 0.01% ethanol).
Results and discussion
Identification and characterization of yeasts
Twenty-seven different yeast colonies were selected for isolation based on colony characteristics. Colony colour of all isolates ranged from a light-brown to a creamy-white. Light-brown colonies were observed as having smooth and shiny surfaces, with entire margins and convex or raised elevations. Creamy-white colonies were observed with rough and flattened surfaces, surrounded by undulate margins. Shapes of the cells were observed by microscopy (under magnification of ×400); the cells of light-brown colonies and shiny surfaces were noted as ovoid or lemon-shaped and creamy-white colonies with rough surfaces were observed as ellipsoidal-shaped. We temporary treated all colonies as distinguish strains, because of their different growth properties and tolerance to stresses (see below).
To identify yeast isolates, PCR amplification of the 26S rDNA D1/D2 gene region was carried out with the NL1 and NL4 primers. Based on obtained DNA sequence data (Supplemental Table S1), the first 18 yeast strains showed 100% identity to S. cerevisiae A44 (Gene bank Accession No: KM589478.1). Eight strains showed 99–100% identity to Pichia manshurica SHB_06 (Gene bank Accession No: KF268292.1): SLY-20, SLY-22, SLY-23, SLY-26 and SLY-27 showed 100% identity and SLY-19, SLY-21 and SLY-24 showed only one or two nucleotides differences (Supplemental Table S2). The SLY-25 strain also showed two nucleotides differences to Saccharomycodes ludwigii H4S5K10 (Gene bank Accession No: FM180540.1) (Supplemental Table S2). It is reported that P. manshurica was a naturally occurring yeast species found through several food fermentation industries and it was also identified from vinegar fermentation although in low abundance [Citation13]. S. ludwigii is commonly considered as a wine contaminant and it has a great potential of wine making because some strains possess ability of reducing alcohol degree and volatile acidity, as well as improving aromatic profile [Citation14]. Several reports are available on the microflora of fermenting palm toddy and their major group of yeasts belonged to the genus Saccharomyces, found in Sri Lanka [Citation15,Citation16] as well as in other countries [Citation17–19]. Thus, it appears that Saccharomyces is a common and vital yeast genus for the naturally occurring fermentation of palm saps.
Effect of salt stress on yeast growth
Since the location where the toddy sample was collected was a coastal area in Sri Lanka, we assume that isolated yeasts may have the ability to grow and ferment at high salinities. All 27 isolated yeast strains were examined for salt tolerance on YPD agar plates in the presence of 5.0%, 7.5% and 10.0% (w/v) NaCl. We found that all strains grew well in the presence of 5.0% NaCl (0.85 mol L−1), but some strains did not show any growth with 7.5% (1.3 mol L−1) or 10.0% NaCl (1.7 mol L−1) on agar plates (data not shown); therefore, we examined salt tolerance for all strains in the presence of 7.5% NaCl and higher in liquid media. All eight P. manshurica strains grew in the presence of 7.5% NaCl (w/v) in liquid culture as well as with no additional NaCl () and all strains of S. cerevisiae grew in the presence of both 7.5% and 10.0% NaCl (Figures S1(B,C), ). With the YPD media supplemented with 11.0% NaCl (w/v), six strains (SLY-3, SLY-4, SLY-10, SLY-14, SLY-15 and SLY-17) showed average growth and the Awamori yeast strain (101-18) showed comparatively good growth on 11% NaCl (Figure S1(D)). The laboratory yeast strain S288C showed poor growth with 11% NaCl (Figure S1(D), ). Similar results were reported by Abdel-Nasser and El-Moghaz [Citation20], where S. cerevisiae FY1679 and Pichia pastoris GS115 strains showed good tolerance to 1.5 mol L−1 NaCl. Tekarslan-Sahin et al. [Citation21] reported that the S. cerevisiae NaCl-resistant strain T8 could tolerate the highest concentration 8.5% NaCl (1.45 mol L−1). Here we found that several isolated Pichia strains displayed salt tolerance up to 10.0% NaCl (1.7 mol L−1) (), therefore, the Pichia strains isolated in this study were highly salt tolerant compared to the strains reported previously. Among all the isolates, only strain SLY-25 identified as S. ludwigii showed to be the least salt tolerant, growing in the presence of no more than 5.0% NaCl (0.85 mol L−1) (). Only the SLY-10 strain could grow in the presence of 12.5% NaCl (w/v) (2.1 mol L−1) in YPD. Its minimal inhibition concentration (MIC) to NaCl was found to be 13.5% (w/v) (2.3 mol L−1) in YPD at 30 °C. Increased salt concentrations caused ion toxicity and osmotic stress [Citation21,Citation22] and accumulation of Na+ levels lead to metabolic poisoning in yeast cells [Citation23]. Several studies reported that some yeast strains showed significant salt tolerance by activation of trehalose, glycerol [Citation24,Citation25] or glycogen [Citation21, Citation26] synthesis in cells to balance their intracellular osmotic pressure against the external environment [Citation25]. Because our isolated S. cerevisiae strains possessed high salt tolerance (), the mechanism of salt tolerance will be investigated in future studies. However, we found that all isolated strains of S. cerevisiae showed higher salt tolerance (≥ 10% NaCl) than the other strains of two genera identified from coconut toddy.
Table 1. Effects of salt content and incubation temperatures on isolate cell growth under aerobic conditions.
Yeast growth at high temperatures
Though the temperature routinely used for the growth of S. cerevisiae is 25–30 °C [Citation27], alcohol production at higher temperatures should be practiced for large-scale commercial ethanol production, which may reduce cooling costs, contaminations and overall production costs [Citation28]. In the current study, we found that all the isolates (S. cerevisiae, P. manshurica and S. ludwigii) grew well both on YPD agar and in liquid media at 37 °C as well as at 30 °C. At 40 °C, all the isolated strains still grew well, but the eight P. manshurica strains showed comparatively better growth than the other strains (). However, all isolated S. cerevisiae strains grew better at 40 °C than the two reference strains, the laboratory yeast strain S288C and the Awamori yeast strain (101-18), (Figure S2(C), ). Five strains (SLY-2, SLY-3, SLY-4, SLY-8 and SLY-9) showed better growth than the other strains at 41 °C (Figure S2(D)), compared to the two reference strains, which only slightly grew at 41 °C. Several studies reported that temperatures of 34 °C or higher significantly affected yeast metabolism and impaired its growth [Citation29,Citation30] and Banat et al. [Citation31] reported that growth of the S. cerevisiae IDY strain was severely inhibited at 40 °C. However, the S. cerevisiae strains among all the isolated strains showed high thermotolerance, growing well even at 42 °C under aerobic conditions (Figure S2(E)).
Yeast growth with both high salinity and high temperature
As mentioned above, in the presence of 7.5% (w/v) NaCl in YPD, all isolated S. cerevisiae strains grew as well as without additional NaCl at 30 °C (); therefore, we attempted to examine yeast growth in liquid culture while exposed to the simultaneously combined stresses of salt (7.5% NaCl) and high temperatures (37 and 40 °C).
At 37 °C, all strains of S. cerevisiae showed tolerance in the presence of 7.5% NaCl (w/v) in YPD. Awamori yeast strain (101-18) showed the best growth and five isolated yeast strains (SLY-3, SLY-4, SLY-8, SLY-9 and SLY-10) showed better growth than the other isolated strains, while the laboratory yeast strain S288C showed much-reduced growth (). Here we found that all the strains showed significant growth reduction while growing in the presence of 7.5% NaCl at 37 °C compared to growing at 37 °C without additional NaCl. However, all the strains showed approximately similar growth rates at 30 °C (Figure S3) and 37 °C (data not shown) in the presence of 7.5% NaCl in the YPD medium.
Figure 1. Growth of S. cerevisiae strains in the presence of 7.5% NaCl in YPD medium (w/v), incubated at 37 °C (A) and 40 °C (B) while shaking (reciprocally) at 200 rpm. Note: The comparison of the growth of all five strains was shown with two reference strains; laboratory yeast S288C and Awamori yeast 101-18 strain.
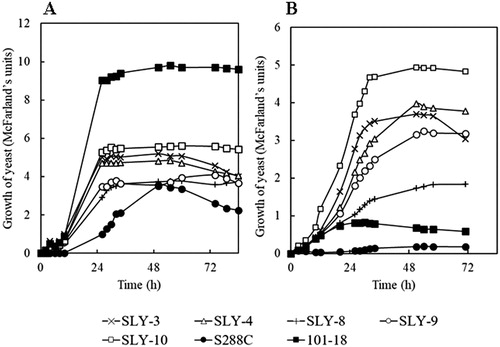
At 40 °C and supplemental 7.5% NaCl, all the isolated strains of S. cerevisiae still grew (Figure S3) and four strains (SLY-3, SLY-4, SLY-9 and SLY-10) showed comparatively good growth compared to the other strains did. The SLY-10 strain showed the best growth (). The two reference strains showed very little growth even without the addition of 7.5% NaCl. Interestingly, the addition of salt (7.5% NaCl) to the YPD medium at 40 °C, in contrast to salt addition at 30 °C or 37 °C, did not show so much growth inhibition of all strains. The exception of this is strain SLY-8, which grew less at 40 °C with additional of 7.5% NaCl (). Stressors can generate independent, cascading and/or synergistic adverse effects on microbial cells [Citation32,Citation33]; therefore, the four strains (SLY-3, SLY-4, SLY-9 and SLY-10) selected in this study with the highest tolerance for multiple stressors might be useful in the ethanol industry.
Fermentation at high temperatures
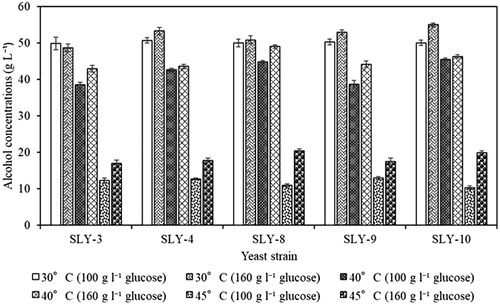
Alcohol production was examined in the 18 S. cerevisiae strains at different incubation temperatures. All strains produced alcohol approximately from 40 to 50 g·L−1 both at 30 and 37 °C after 72 h of fermentation in the presence of 100 g·L−1 of glucose (Figures S4(A,B)). When the incubation temperature was increased to 40 °C, most of the strains continued to produce relatively high alcohol concentrations in the range of 22.87 ± 0.49 to 45.53 ± 0.31 g·L−1, except for the SLY-12 strain, which like the Awamori yeast 101-18 strain produced lower alcohol concentrations (Figure S4C). Therefore, the nine yeast strains (SLY-2, SLY-3, SLY-4, SLY-6, SLY-7, SLY-8, SLY-9, SLY-10 and SLY-13) which produced high alcohol concentrations at 30, 37 and 40 °C were selected to examine their fermentation abilities at 41 °C and higher (Figures S4(D,E,F). Six strains (SLY-3, SLY-4, SLY-8, SLY-9, SLY-10 and SLY-13) produced substantial amounts of alcohol at 41 °C (27.77 ± 0.31 to 32.93 ± 0.42 g·L−1) and the five strains except SLY-13 produced a significant amount of alcohol even at 45 °C (). Krouwel and Barber [Citation34] stated that natural yeast fermentation ability could be lost in the temperature range from 35 to 45 °C. In our study we found that our S. cerevisiae strains produced alcohol with 100 g·L−1 glucose at 37 and 40 °C as well as at 30 °C (, S4(B,C)).
Figure 2. Comparison of alcohol production (over 72 h) by five strains of S. cerevisiae cultured on 100 ml batch-fermented media (YPD containing 100 g L−1glucose) at different incubation temperatures under static conditions. Note: Laboratory yeast (S288C) and Awamori yeast (101-18) strains were used as reference strains. The alcohol production of the two reference strains was not detected (n.d.) at temperatures; 42, 43 and 45 °C.
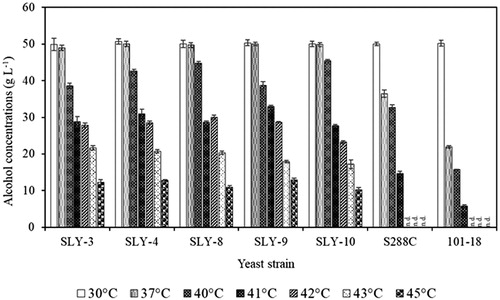
Fermentation by these five S. cerevisiae strains was also examined at 30, 40 and 45 °C with 160 g·L−1 of glucose in YPD media (). Alcohol productivity over 24 h for all five strains was between 0.460 and 0.592 g·L−1·h−1 at 30 °C with 160 g·L−1glucose and was significantly reduced (p < 0.05) compared to the productivity with 100 g·L−1glucose (1.282–2.005 g·L−1 h−1) (Figure S5(A-E)). In contrast, when fermentation was conducted at 40 and 45 °C, those strains did not show any significant differences (p < 0.05) in alcohol concentrations and productivity over 24 h when using 100 g·L−1 or 160 g·L−1 glucose (, S5(F-O)). However, the effects of the additional glucose (from 100 to 160 g·L−1) on alcohol production for all five strains showed significant increase after 24 h of incubation in the range of 16.96 ± 1.18 to 20.47 ± 0.59 g·L−1 at 45 °C (, S5(K-O)). Addition of more glucose to the medium enhanced the alcohol production significantly by all five strains at 45 °C (). Banat et al. [Citation31] reported that the S. cerevisiae IDY strain showed a maximum ethanol production with 160 g·L−1 glucose: 54.4 g·L−1 at 40 °C and the ethanol production was significantly reduced to 18.1 g·L−1 at 45 °C. All the five strains produced a higher amount of alcohol than that of the IDY strain and especially SLY-8 and SLY-10, which produced the highest amount of alcohol 20.47 ± 0.59 and 19.96 ± 1.02 g·L−1, respectively at 45 °C with 160 g·L−1 glucose (). These strains would be useful in bioethanol industry because of their robust properties and require to be analyzed further.
Conclusions
In this study, we obtained 27 different isolates, which were identified as S. cerevisiae (18 strains), P. manshurica (8 strains) and S. ludwigii (1 strain). All the isolated strains of S. cerevisiae showed the tolerance in the presence of 10% (w/v) NaCl and some of the strains showed higher tolerance up to 11% (w/v) NaCl. Interestingly, the only one strain among all isolates, SLY-10 strain showed the tolerance up to 12.5% (w/v) NaCl. All the isolates grew well at 40 °C and all the S. cerevisiae strains grew even at 42 °C. Moreover, we found that all the isolated strains of S. cerevisiae still grew at 40 °C in the presence of 7.5% (w/v) NaCl on YPD. All the strains of S. cerevisiae produced substantial amounts of alcohol with 100 g L−1 glucose at 40 °C and five strains (SLY-3, SLY-4, SLY-8, SLY-9 and SLY-10) showed the significant ethanol production with 160 g·L−1 glucose even at 45 °C. The strains obtained in this study would be useful in bioethanol industry because of their robust properties.
Supplemental Material
Download PDF (282.7 KB)Supplemental Material
Download PDF (315.2 KB)Supplemental Material
Download PDF (961.5 KB)Acknowledgements
We would like to thank Oriental Yeast Co. Ltd. (Tokyo, Japan) for providing the Yeast extract as a gift.
Disclosure statement
No potential conflicts of interest was reported by the authors.
References
- Hui Y, Evranuz E, eds. Handbook of plant-based fermented food and beverage technology. 2nd ed. New York: CRC Press; 2012.
- Vidanapathirana S, Atputharajah J, Samarajeewa U. Microbiology of coconut sap fermentation. Vidyodaya J Arts Sci Lett. 1983;11:35–39.
- Kapilan R, Kailayalingam R, Mahilrajan S. Determination of efficient fermentation inhibitor of sweet sap of Cocos Nucifera and optimization of concentration for quality outputs in Northern Sri Lanka. Int J Sci Res Agric Sci. 2015;2:166–174.
- Ghosh DK, Bandyopadhyay A, Das S, et al. Coconut sap (Neera) - untapped opportunity of spinoff gains in West Bengal, India. Int J Curr Microbiol Appl Sci. 2018;7:1883–1897.
- Perumpuli P, Watanabe T, Toyama H. Identification and characterisation of thermotolerant acetic acid bacteria strains isolated from coconut water vinegar in Sri Lanka. Biosci Biotechnol Biochem. 2014;78:37–41.
- Shetty P, D’Souza A, Poojari S, et al. Study of fermentation kinetics of palm sap from Cocos nucifera. Int J Appl Sci Biotechnol. 2017;5:375–381.
- Harries HC. Coconut - Milk bottle on the doorstep of mankind; Chapter II.E.2. In: Kiple KF, Ornelas KC, editors. The Cambridge world history of food. Cambridge (UK): Cambridge University Press; 2000. p. 388–397.
- Walker G, Stewart G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages. 2016;2:30.
- Azhar SHM, Abdulla R, Jambo SA, et al. Yeasts in sustainable bioethanol production: A review. Biochem Biophys Reports. 2017;10:52–61.
- Wijeyaratne SC. Temperature tolerance and other properties of two ethanol producing Saccharomyces cerevisiae strains isolated from coconut toddy. J Nat Sci Found Sri Lanka. 1998;26:77–91.
- Kurtzman CP, Robnett CJ. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5’ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol. 1997;35:1216–1223.
- Ameyama M. Enzymic microdetermination of D-glucose, D-fructose, D-gluconate, 2-keto-D-gluconate, aldehyde, and alcohol with membrane-bound dehydrogenase; carbohydrate metabolism. In: Wood WA, editor. Methods in enzymology; vol. 89. Michigan: Elsevier Academic Press; 1982. p. 20–29.
- Zhang Q, Huo N, Wang Y, et al. Aroma-enhancing role of Pichia manshurica isolated from Daqu in the brewing of Shanxi Aged Vinegar. Int J Food Prop. 2017;20:2169–2179.
- Vejarano R. Saccharomycodes ludwigii, Control and potential uses in winemaking processes. Fermentation. 2018;4:71.
- Jayathilake AN, Wijeyatatne SC. Biochemical and microbiological changes of Caryota urens (Kithul palm) phloem sap. Vidyodaya J Sci. 1999;8:91–108.
- Theivendirarajah K, Chrystopher RK. Microflora and microbial activity in palmyrah (Borassus flabellifer) palm wine in Sri Lanka. Mircen J. 1987;3:23–31.
- Ezemba CC, Archibong EJ. Comparative Studies of wine produced from coconut (Cocos nucifera) and mango fruit (Mangifera indica) using yeast isolated from palm wine. Int J Res Pharm Biosci. 2017;4:44–49.
- Olowonibi OO. Isolation and characterization of palm wine strains of Saccharomyces cerevisiae potentially useful as bakery yeasts. Eur J Exp Biol. 2017;07:1–13.
- Udomsaksakul N, Kodama K. Indigenous Saccharomyces cerevisiae strains from coconut inflorescence sap: characterization and use in coconut wine fermentation. CMU J Nat Sci. 2018;17:219–230.
- Abdel-Nasser A. El-Moghaz Comparative study of salt tolerance in Saccharomyces cerevisiae and Pichia pastoris yeast strains. Adv Bioresour. 2010;1:169–176.
- Tekarslan-Sahin SH, Alkim C, Sezgin T. Physiological and transcriptomic analysis of a salt-resistant Saccharomyces cerevisiae mutant obtained by evolutionary engineering. Bosn J Basic Med Sci. 2018;18:55–65.
- Mager W, Siderius M. Novel insights into the osmotic stress response of yeast. FEMS Yeast Res. 2002;2:251–257.
- Logothetis S, Walker GM, Nerantzis ET. Effect of salt hyperosmotic stress on yeast cell viability. Zb Mat Srp Prir Nauk. 2007;2007:271–284.
- Beney L, Martínez De Marañón I, Marechal PA, et al. Influence of thermal and osmotic stresses on the viability of the yeast Saccharomyces cerevisiae. Int J Food Microbiol. 2000;55:275–279.
- Babazadeh R, Lahtvee PJ, Adiels CB, et al. The yeast osmostress response is carbon source dependent. Sci Rep. 2017;7:1–11.
- Herdeiro RS, Pereira MD, Panek AD, et al. Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. Biochim Biophys Acta - Gen Subj. 2006;1760:340–346.
- Belloch C, Orlic S, Barrio E, et al. Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int J Food Microbiol. 2008;122:188–195.
- Techaparin A, Thanonkeo P, Klanrit P. High-temperature ethanol production using thermotolerant yeast newly isolated from Greater Mekong Subregion. Braz J Microbiol. 2017;48:461–475.
- Caspeta L, Nielsen J. Thermotolerant yeast strains adapted by laboratory evolution show trade-off at ancestral temperatures and preadaptation to other stresses. MBio. 2015;6:1–9.
- Charlebois DA, Hauser K, Marshall S, et al. Multiscale effects of heating and cooling on genes and gene networks. Proc Natl Acad Sci USA. 2018;115:E10797–E10806.
- Banat IM, Nigam P, Marchant R. Isolation of thermotolerant, fermentative yeasts growing at 52 °C and producing ethanol at 45 °C and 50 °C. World J Microbiol Biotechnol. 1992;8:259–263.
- Morano KA, Grant CM, Moye-Rowley WS. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics. 2012;190:1157–1195.
- Cunha JT, Romaní A, Costa CE. Molecular and physiological basis of Saccharomyces cerevisiae tolerance to adverse lignocellulose-based process conditions. Appl Microbiol Biotechnol. 2018;159–175.
- Krouwel PG, Braber L. Ethanol production by yeast at supraoptimal temperatures. Biotechnol Lett. 1979;1:403–408.