?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This paper reports the prebiotic efficacy of soluble crude polysaccharides (SCP) from palm kernel cake on the growth rates and acidifying property of probiotic lactic acid bacteria (LAB), Lactobacillus plantarum ATCC 8014 and Lactobacillus rhamnosus ATCC 53103, to investigate into the potential SCP of an agriculture by-product as a novel prebiotic. The highest extraction yield of SCP (2.90%) was obtained using NaOH. The extracted polysaccharides were highly soluble (>97%). The highest percentage of protein (0.63 ± 0.09%) and the total carbohydrate content (55.36 ± 0.33%) were observed in NaOH-extracted SCP (SCPN) and citric acid-extracted SCP (SCPCA). Water-extracted SCP (SCPW) contained the highest percentage of glucose, whereas SCPCA demonstrated the presence of two major monosaccharides, galactose and mannose. Structural analysis of SCP showed peaks in the range of 1000–1077 cm−1, an indication of the presence of polysaccharides and alpha (α)- or beta (β)-glycosidic linkages. The degree of hydrolysis increased with the increase of pH from 5 to 8 following 4 h incubation. SCPW and SCPCA were more resistant (95%) to α-amylase as compared to fructooligosaccharides (FOS). As in the case of FOS (∼98%), SCPW (∼99%) and SCPCA (∼98%) were resistant to artificial human gastric juice and their degree of hydrolysis had negative correlation with the pH. The product was found to markedly stimulate the proliferation of the tested probiotics and acid production. All the above findings are supportive of the fact that polysaccharides extracted from CKC have a vast potential to be exploited as novel prebiotic.
Introduction
Present-day consumers are very conscious of the close association between lifestyle, diet and good health, which is the rationale for the demand of products capable of enhancing health beyond mere provision of basic nutrition. Considerable interest has been focused on the development of probiotic in combination with prebiotic (symbiotic), which provide beneficial effects on the consumer’s health. This combination could lead to obvious enhancement of growth for both existing beneficial bacterial strains in the colon as well as the newly added probiotics contributing significant advantages to both consumers and food manufacturers [Citation1].
Of late, considerable emphasis has been given to the probiotic potential of lactic acid bacteria (LAB). The health-promoting properties of certain LAB populating the human gasterointestinal tracts (GIT) is the basis for the food industry to develop new functional food products containing probiotics [Citation2]. Probiotics are generally defined as live microorganisms which confer health benefits in the host when administered in sufficient amounts [Citation3]. On the other hand, prebiotics are non-digestible substances mainly oligosaccharides, polysaccharides, protein hydrolyzates, short-chain fatty acids, plant and herbal extracts [Citation4]. They exert positive effects on probiotics, which results in general improvement of host health. Although numerous food polysaccharides, oligosaccharides and dietary fibres have been reported to possess prebiotic activity [Citation5], these dietary carbohydrates do not possess prebiotic properties. Hence, there is a need to establish specific criteria to identify that certain food ingredients are potential prebiotics. These potential prebiotics must be neither hydrolysed, nor absorbed in the upper part of the gastrointestinal tract (GIT); they should stimulate the growth of beneficial bacteria in the colon, become metabolically activated and able to change the colonic microbiota to a more healthier composition [Citation6, Citation7].
There are numerous challenges in the production of prebiotics at industrial scale, which include the use of novel techniques, economical and abundant sources and low cost production. In Malaysia, in terms of economic importance, coconut stands fourth amongst agricultural products after palm oil, rubber and paddy. About 63% of the coconuts are used for domestics consumption, while 37% in the form of desiccated and activated carbon are exported and processed for industrial application [Citation8]. Coconut kernel cake (CKC) is a by-product following the extraction of milk and oil from the kernel (). It is reported that the dried CKC contains 60% fats, 20% carbohydrate, 9% proteins, 8% natural sugars and 7% moisture [Citation9]. These values are largely dependent on the sources of the coconut kernel and other factors such as oil extraction method [Citation10]. The 20% carbohydrate in CKC is mainly comprised of 61% mannan polymers, whereas the remaining components include cellulose, galacto-glucomannan, glucomannan, arabinomannogalactan and arabinoxylogalactan [Citation11]. All these characteristics along with its low cost, availability and accessibility in Malaysia make it an alternative source for the search of a prebiotic.
This study was directed towards extracting the CKC’s soluble polysaccharides using water, citric acid and NaOH, analyzing their chemical composition and functional properties and evaluating their digestibility using artificial human gastric juice. Growth stimulation and acid production by two probiotic LAB namely Lactobacillus plantarum ATCC 8014 and Lactobacillus rhamnosus ATCC 53103, were evaluated when cultured on the CKC polysaccharides in vitro.
Materials and methods
Sample source and preparation
Mature coconuts were purchased from a local market in Serdang, Selangor, Malaysia. Following removal of the kernel from its shell, the former was finely ground using a blender and the milk extracted through squeezing manually. Water was added to the residue to extract any remaining milk. The residues were then dried in an oven at 50 °C for 48 h and again ground using a blender and sieved through a number 50 sieve [Citation11, Citation12].
Chemicals and bacterial strains
Fructooligosaccharides (FOS, purity ≥ 90%), trifluoroacetic acid (TFA), Bradford reagent, phenol, peptone water, alpha (α)-amylase from human saliva (Type IX-A), inositol (C6H12O6), pyridine (C5H5N), acetic anhydride (C4H6O3) and beta(β)-glucan from barley were purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). de Man, Rogosa and Sharpe (MRS) broth and agar, anaerobic gas pack, glucose, galactose, mannose, arabinose, rhamnose and xylose were sourced from Merck (Darmstadt, Germany). Ethanol (C2H6O), hydroxyl ammonium chloride (HONH2.HCl), petroleum ether (C6H14), sulfuric acid (H2SO4), citric acid (C6H8O7), sodium hydroxide (NaOH) and 3,5-dinitrosalicylic acid (DNS) were supplied by SAFC (St. Louis, MO, USA). All chemicals used in this study were of analytical grade. Two LAB strains used in this study were L. plantarum ATCC8014 and L. rhamnosus ATCC 53103 from the American Type Culture Collection (ATCC, Rockville, MD, USA).
Chemical compositions of CKC
The method of the Association of Official Analytical Chemists [Citation13] was used to analyze the chemical compositions of the CKC. Total protein content was estimated by the micro-Kjeldahl method [Citation14]. An automated fat (AFD) system (FOSS Soxtec™ 2050, Hilleroed, Denmark) was used to determine the crude fat content and it was calculated using Equation (1):
(1)
(1)
where W1 is the weight of sample; W2 is the weight of the extraction cup; W3 is the weight of the extraction cup plus the weight of the residue .
Total carbohydrate content was calculated using Equation (2):
(2)
(2)
Extraction of CKC soluble polysaccharides using water, sodium hydroxide and citric acid
The extraction procedure for CKC polysaccharides was as described in a previous study by Bello et al. [Citation15]. The samples were initially defatted with 95% (v/v) petroleum ether (C6H14) at a ratio of 1:5 at 60 °C for 5 h and dried at 27 °C. This was followed by a treatment of the dried sample with 80% (v/v) ethanol (C2H6O), re-dried and used for extraction procedures using water, 1% sodium hydroxide (pH, 12) and 1% citric acid (pH, 2.3). The soluble polysaccharides obtained were centrifuged at 12,857 ×g for 10 min at 4 °C and the pellet was washed twice with distilled water, dried at 50 °C and kept at 28 °C for subsequent analyses. The percentage yield of soluble polysaccharide was determined using Equation (3):
(3)
(3)
where W1 is the raw sample weight and W2 is the polysaccharide weight after extraction.
Total carbohydrate and protein contents and solubility rate of CKC crude polysaccharides
The total carbohydrate content of CKC crude polysaccharides was evaluated using the phenol sulfuric acid method [Citation16]. The total protein concentration was determined as described by Abbasiliasi et al. [Citation17], whereas the solubility rate of CKC crude polysaccharides was evaluated as explained by Azmi et al. [Citation18] and calculated using Equation (4). Details of all of the above procedures are as reported in a previous study by Bello et al. [Citation15].
(4)
(4)
where W1 is the filter paper weight before drying and W2 is the filter paper weight after drying.
Monosaccharide composition of soluble polysaccharides of CKC
The method of Qiao et al. [Citation19] with some modifications was used to prepare the sample for evaluation of monosaccharide composition. Samples were analyzed using the gas chromatography (GC-6890N, Agilent, California, US) with a flame ionization detector and a HP-5 capillary column (30 m × 0.32 mm × 0.25 m) (Agilent, California, US). Operational conditions of the GC were as described in a previous study by Bello et al. [Citation15].
Structural properties of CKC crude soluble polysaccharides
Analysis of the extracted soluble crude polysaccharides of CKC was carried out using the Fourier transform infrared (FTIR) spectroscopy. Infrared spectra were recorded with a FTIR spectrometer (Nicolt, USA) in the range of 4000–280 cm−1 using KBr disk method. β-Glucan from barley was used as the standard reference.
Thermal analysis of CKC crude soluble polysaccharides
Thermal properties of CKC crude soluble polysaccharides was performed as described by Jia et al. [Citation20] using a scanning calorimeter (DSC, Mettler Toledo Star System, Columbus, USA). Five mg of the sample was weighed onto aluminum pans sealed hermetically and heated at a temperature range of 0–350 °C at the rate of 10 °C/min in a nitrogen atmosphere. The parameters of onset (To), peak (Tp), end set (Te) and enthalpy change (ΔH) were recorded.
Determination of prebiotic potential of CKC crude soluble polysaccharides
Prebiotic potential of CKC crude soluble polysaccharides were determined through (a) the effect of artificial human gastric juice and α-amylase on hydrolysis of CKC crude soluble polysaccharides and (b) the growth and proliferation of probiotics on CKC crude soluble polysaccharides and their acidifying activity. The detailed procedures for the above were performed as described in our previous study by Bello et al. [Citation15].
Statistical analysis
Data obtained were analyzed using SAS (version 10) to compare the means of all calculated parameters. One-way analysis of variance (ANOVA) with post-hoc Tukeys’s honestly significant different (HSD) test was carried out to identify significant differences (P < 0.05) between data sets.
Results and discussion
Chemical composition of CKC
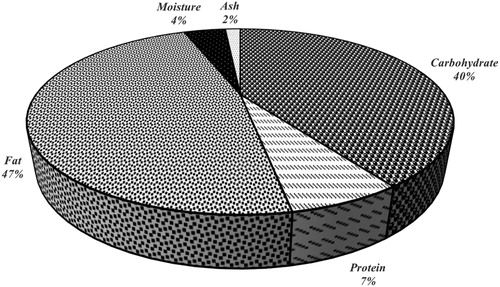
The chemical composition of CKC is shown in . Fat (47.4%) was the major component in CKC followed by carbohydrates (40.54%). The carbohydrate content of CKC was less than that of fruits and vegetables (∼>72%). The carbohydrates content of plants and vegetables indicates their potentials as dietary fibers and it was reported that the most carbohydrate-rich plants have less than 20% fat [Citation15]. The carbohydrate content could be influenced by the oil extraction method and insoluble fats. This water insoluble fat could be bound to the cell wall and become a limiting factor for obtaining greater carbohydrates content from a sample [Citation21]. During milk extraction from the kernal almost all the water soluble materials are extracted along with the milk, leaving the insoluble substances attached to the cell wall of the residue, which could be the rationale for the high fat content in the CKC. It has been reported that the fat content of CKC was higher than that of other fruits and vegetables (0.5–10.9%) [Citation22]. The crude fat content of CKC in this study was in agreement with those of previously reported by Yalegama et al. [Citation23], and Manikantan et al. [Citation24]. However, Khuwijitjaru et al. [Citation11] reported a lower percentage, which might be associated with the extraction method of coconut milk prior to obtaining the residue.
Protein contents of different plants varied greatly based on the total nitrogen and amino acid composition [Citation25]. Protein content of CKC in this study (∼7%) is higher than those of 1.6%, 4.2% and 0.83% as reported by Mohd Nor et al. [Citation16], Yalegama et al. [Citation23] and Khuwijitjaruet al. [Citation11]. Moisture content of CKC is ∼4%, which is higher than that reported by Yalegama et al. [Citation23]. However in previous studies by Ng et al. [Citation12] and Mohd Nor et al. [Citation16] moisture content was higher than 50%. Moisture content in plants varies even within a single species, which is related to water holding capacity of plants, water retention and swelling capacity [Citation26]. The ash content of plant indicates its mineral content after incineration process at high temperature. Ash content of CKC in this study (∼2%) was higher compared to those of Yalegama et al. [Citation23] and Mohd Nor et al. [Citation16]. Overall observation in the differences of proximate composition between the samples could have resulted from plants types, geographical, seasonal and climatic conditions.
Yield of CKC soluble crude polysaccharides after extraction
The process of polysaccharide extraction is important from the perspective of its subsequent use in industry or further research and development. Defatting of samples using polar and nonpolar solvents can limit the release of polysaccharides form the cell wall of the plants [Citation18, Citation27]. Therefore in this study CKC were first defatted with both ethanol and petroleum ether before extraction. The yield of CKC soluble crude polysaccharides following extraction is as shown in . The highest extraction yield of SCP (2.90 ± 0.36%) was obtained using NaOH and there was no significant difference (P < 0.05) between the extraction yields using water and citric acid. The percentage of yield from defatted crude polysaccharide of coconut residue in a previous study by Mohd Nor et al. [Citation16] was 0.73 ± 0.04%, which was lower compared to the yields obtained in the present study. It was reported that the extraction yield could be influenced by the solvent used, the ratio of water to raw material, extraction temperature and time [Citation28].
Table 1. Extraction yield percentage of CKC soluble crude polysaccharide.
Water extraction is a mild and stable procedure, which is easy to control and does not destroy the polysaccharide molecules. However, it is associated with long extraction time and high temperature [Citation29]. The higher yield in alkaline pH is relevant to the structure of polysaccharide and isoelectric point of protein. At low acidic pH, the high concentration of hydrogen ions in the solvent promotes the dissolution of protein [Citation30]. The results from this study concur with those of a previous study by Bello et al. [Citation15], who reported that higher soluble polysaccharides could be achieved with NaOH. However some influencing factors such as the extracting solvent, the ratio of solvent to raw material, the temperature, the experimental procedure and the plant types affect the efficacy of polysaccharides extraction [Citation31, Citation32]. All these factors need optimization for reduced energy consumption and less degradation of polysaccharides, to obtain maximum yield.
Total carbohydrate and protein contents and solubility rates of CKC crude polysaccharides
The total carbohydrate and protein contents and solubility rates of CKC crude polysaccharides are shown in . The soluble crude polysaccharides of the citric acid extract (SCPCA) had the highest (55.36 ± 0.33%) total carbohydrate content. The total carbohydrate content obtained in this study was higher compared to that reported by Mohd Nor et al. [Citation16] and Yalegama and Chavan [Citation33]. It was reported that the lowest content of total carbohydrate is related to the highest yield of crude polysaccharides [Citation34].
Table 2. Total carbohydrate and protein contents and solubility rate of CKC crude soluble polysaccharides.
The protein content in all soluble polysaccharide extracts was low (<1); however, these levels were higher compared to that of a previous report (0.009%) by Mohd Nor et al. [Citation16]. The soluble crude polysaccharides of the NaOH extract (SCPN) showed the highest percentage of protein (0.63 ± 0.09%). Low protein yield could be related to the extraction conditions such as temperature, ratio of sample to extractant, extraction duration, number of extraction steps and centrifugation speed [Citation35].
CKC polysaccharides in the present study exhibited high solubility rates (>95%). Polysaccharides contain many hydroxyl groups from hydrogen bonds, which interact strongly with water [Citation36]. It was reported that inter- and intra-molecular hydrogen bond could affect to the solubility rates of polysaccharides in water [Citation37].
Monosaccharide composition of CKC soluble crude polysaccharides
The percentage of monosaccharides composition of CKC from the soluble polysaccharides of the three solvents are as presented in . Glucose showed the highest percentage in the soluble crude polysaccharides of the water extract (SCPW) while SCPCA contained two major monosaccharides-galactose and mannose. Polysaccharides of CKC, as reported in other studies, are comprised mainly of mannose, glucose and galactose, arabinose with small amount of rhamnose and xylose [Citation11, Citation38]. It was reported that geographical area and seasonal harvest of the plant sources could contribute to the variation in the monosaccharide composition [Citation12, Citation39]. Total yield of monosaccharides for SCPN (∼23%) was lower compared to those of SCPW and SCPCA (∼94%). This could be attributed to the pH of the solvent, the ratio of solvent to polysaccharides, extraction time and their interaction, which need to be optimized in the extraction of CKC polysaccharides.
Table 3. Monosaccharide composition of CKC crude soluble polysaccharides.
Structural properties of CKC soluble crude polysaccharides
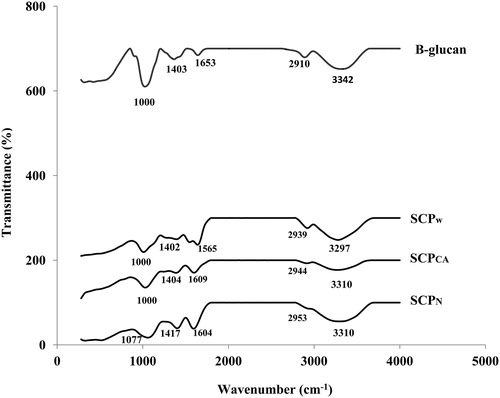
FTIR was used to identify the structural properties of CKC crude polysaccharides and the results are in . The major chemical groups in polysaccharides to be identified through the position and intensity of the bands are specific for each polysaccharide [Citation40, Citation41]. There are similarities in the absorption pattern and structural characteristics of the extracted polysaccharide of three different solvents with that of the β-glucan as the positive control. Peaks in the range of ∼1000–1077cm−1 indicated the presence of polysaccharides. Absorption at ∼1200–1000 cm−1 is characteristic of the C–OH group, which indicates the presence of oligosaccharides [Citation42] and/or corresponded to α- or β-glycosidic linkages [Citation41, Citation43]. The peak in the region of ∼1400 cm−1 is assigned to COO-symmetric stretch of carboxyl group [Citation44]. Absorption at ∼1600 cm−1 is due to the stretching vibration of the carbonyl group. The band observed in the range of ∼2910–2953 cm−1 corresponded to C–H stretching vibration, which is an indication of the presence of polysaccharides [Citation40]. Strong absorption was observed at ∼3297–3342 cm−1 attributed to the existence of hydroxyl vibration modes of O–H stretching band, which contribute to intra- and inter- molecular interactions between the polysaccharide chains [Citation20].
Thermal properties of CKC soluble crude polysaccharides
Thermal properties of CKC crude polysaccharides are as presented in . Different extracted CKC polysaccharides showed different onset temperature or the initial endothermic phase. The initial endothermic phase was related to the presence of impurities in the sample and the vaporization of water, which occurs over a range of temperature. Similarly, endothermic peaks with differences in the enthalpy changes observed for these three extracts of CKC. The endothermic peak could be attributed to the disruption of hydrogen bonded network of water and polymer chains in polysaccharides [Citation45]. SCPN showed the highest enthalpy values (136.38 J/g). It was reported that the high enthalpy value could be associated with the samples crystalline nature, high mannose content and low galactose content [Citation46].
Table 4. Thermal properties of CKC soluble crude polysaccharides.
Assessment of prebiotic potential of CKC soluble crude polysaccharides
Evaluation of acid resistance CKC soluble crude polysaccharides through their hydrolysis by artificial human gastric juice
The ability of polysaccharides to resist the gastric acidity is one of the requirements that potential prebiotics need to meet. It underlies their ability to reach the colon to effectively stimulate probiotic bacteria. Hence, we evaluated the degree of hydrolysis of selected polysaccharides of CKC after exposure to artificial human gastric juice following 6-h incubation at 37 °C (). The percentage of hydrolysis decreased with the increase of the pH value of the artificial human gastric juice from 1 to 5. Low pH showed higher hydrolysis degree due to the more easily ruptured glycosidic bond as compared to that at higher pH. Similar to FOS (∼98%), SCPW (∼99%) and SCPCA (∼98%) were also resistant to acidity and their hydrolysis degree had negative correlation with the pH value.
Figure 4. Hydrolysis of FOS, SCPCA and SCPW after treatment with artificial human gastric juice.
Note: The error bars represent the range of sample variation between three replicates and the standard deviation.
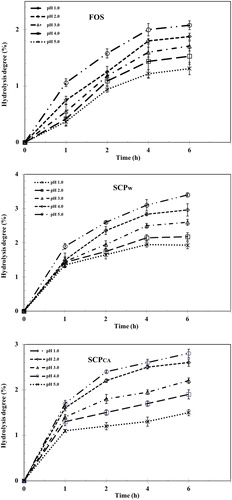
After 6 h of incubation at pH 1.0 the percentage of hydrolysis for FOS, SCPW and SCPCA were 2.07%, 2.14% and 1.50%, respectively. These results concur with those of other extracted polysaccharides, which showed high resistance (>95%) to artificial human gastric juice [Citation18, Citation47]. It was reported that hydrolysis of polysaccharides when subjected to human gastric juice influenced by monosaccharide composition, rings and linkages [Citation4]. Moreover, high amount of mannose and manno-oligosaccharides have been reported to be resistant to digestive enzymes [Citation48]. The high amount of mannose and galactose in SCPCA could be associated with its high non-digestibility and high resistance in gastric juice.
It was also observed that the degree of hydrolysis increased with the duration of incubation in all tested pH. These results suggested that polysaccharides of CKC, linked to β-linkages and stable in acidic conditions are able to pass through the stomach in unchanged form to reach the colon and stimulated the growth of beneficial bacteria. Differences in hydrolysis degree in different pH conditions may result from the structural composition of extracted polysaccharides and their possible connections to other compounds.
Degree of hydrolysis of SCPw and SCPCA after treatment with α-amylase
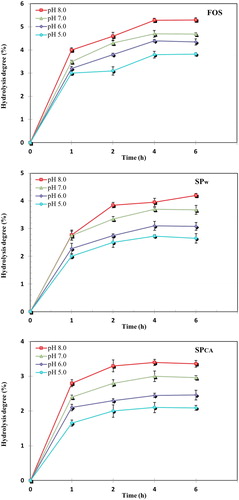
α-Amylase is known to hydrolytically break the glycosidic bonds of the starch molecule and its derivatives [Citation49]. Resistance of polysaccharides to α-amylase are among the prerequisite of prebiotics criteria and important for the food industry. The breakdown of resistance products only produces a small amount of energy for the organism. This energy supply is related solely to the oxidative breakdown of absorbed short-chain fatty acids from the large intestine. These short-chain fatty acids are end products of bacterial fermentation of carbohydrates in the colon [Citation50].
The degree of hydrolysis of SCPW and SCPCA after treatment with α-amylase at pH 5, 6, 7 and 8 for 1, 2, 4 and 6 h at 37 °C are as shown in . The degree of hydrolysis increased with the increase of pH from 5 to 8, which indicated that pH had influence on hydrolysis. The results showed that the digestibility of SCPCA and SCPw at different pH values followed the order: pH 8 > pH 7 > pH 6 > pH 5. Furthermore, the degree of hydrolysis of SCPW and SCPCA increased with the increase in incubation time up to 4 h and there was no significant difference (P < 0.05) between these hydrolysis at 4 to 6 h. The maximum hydrolysis of SCPW, SCPCA and FOS after 6 h of incubation was 4.13, 3.36 and 5.28%, respectively, which indicated that SCPW and SCPCA were more resistant to α-amylase compared to FOS (∼95%) as a reference prebiotic. The high non-digestibility rate of both soluble polysaccharides of CKC might be related to its structural features, which were linked together by β-glycosidic linkages similar to that of the well-known commercial prebiotic, FOS [Citation51].
Proliferative effect of soluble crude polysaccharides on probiotic LAB strains
Results from the proliferative effect of different types and concentration of CKC soluble crude polysaccharides on two probiotic LAB strains, L. plantarum ATCC 8014 and L. rhamnosus ATCC 53103, after 48 h incubation are as shown in . Both tested strains showed higher CFU as compared to those of blank samples in the different concentration of soluble crude polysaccharides. Proliferation increased with the increase of concentration of soluble crude polysaccharides. Proliferation of L. plantarum was higher in media supplemented with SCPCA as compared to that of SCPW. The proliferation results showed that the CKC soluble polysaccharides could be utilized by tested probiotics, which could be related to the high solubility rate (>99%) of the CKC polysaccharides in water. Good water-soluble carbohydrates could be utilized more easily, readily, rapidly and completely, which promotes the bacterial proliferation as stated by Wang et al. [Citation52]. Other than that, molecular weight of polysaccharides could also influence its absorption by probiotic bacteria [Citation4, Citation32]. Growths of probiotics are mostly affected by the monosaccharaides composition, glycosidic linkages and degree of polymerization of a given carbohydrates compound. It was reported that fructose, glucose, galactose and xylose are often found in the composition of potential prebiotic [Citation53]. The carbohydrate composition of soluble polysaccharides of CKC comprised of galactose, glucose and xylose.
Table 5. Proliferation of L. plantarum ATCC 8014 and L. rhamnosus ATCC 53103 on SCPW and SCPCA in vitro.
Among all the tested soluble polysaccharides, growth of L. plantarum was higher in both samples compared to that of L. rhamnosus. It was reported that different probiotic species or even strains tend to vary in the utilization of carbon sources, which affect their growth [Citation54]. The fermentation pattern of prebiotics depends on their types, which are soluble or insoluble. Soluble polysaccharides are more effective for bacterial fermentation and as such it becomes more beneficial to the gut health [Citation55, Citation56].
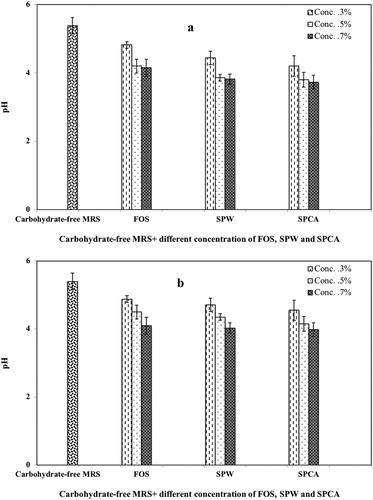
Acidifying activity of probiotic LAB strains on soluble crude polysaccharides
The acidifying activity of L. plantarum ATCC 8014 and L. rahmnosus ATCC 53103 during growth in fermentation media supplemented with SCPW, SCPCA and FOS after 48 h incubation are shown in . There was a significant difference (P < 0.05) between the carbohydrate free MRS with those of supplemented with polysaccharides. Acid production by probiotic bacteria increased with the increased prebiotic concentrations up to 5%. The acidifying activity was influenced by the types of media, where the minimum pH was achieved with 5% SCPCA for L. plantarum ATCC 8014. Reduction in pH reflected the production of organic acids due to the consumption of the carbohydrates by the strain. From this perspective, bacteria can metabolize the polysaccharides to produce short-chain fatty acids and other organic acids, which resulted in the drop of pH of the media. pH changes of the medium is associated with the total number of bacteria in cultivation media and supplementation with different polysaccharides, where the increase in the total number of bacteria is followed by a reduction in pH. In the medium, in the absence of polysaccharide the lowest total number of all tested strains following by higher pH were recorded. Similar results on growth and acidifying performance of different probiotics such as Lactobacillus acidophilus, Bifidobacteria strains and Mangifera pajang were reported using other soluble polysaccharides after 24–48 h of incubation [Citation4, Citation47, Citation57]. However the prebiotic effect of polysaccharides is dependent upon their molecular weight, monosaccharides composition and chain conformation.
Conclusions
Our findings indicated that CKC polysaccharides constitute an effective growth substrate for enhancement of L. plantarum ATCC 8014 and L. rhamnosus ATCC 53103 proliferation. Furthermore, the polysaccharides demonstrated stronger activity such as better resistance to human gastric juice and α-amylase as compared to that of commercial prebiotic-FOS, which ensures their reaching the colon to effectively stimulate the growth of probiotic bacteria. In view of the fact that food is usually retained in the human stomach, where gastric juice is present, approximately ∼98% of the polysaccharides is expected to reach the intestine upon consumption. Reduction of pH through the consumption of carbohydrate and formation of short-chain fatty acids and other organic acids could result in growth inhibition of pathogenic colon bacteria, which in this respect should be studied. Apart from the above, techno-functional benefits of CKC soluble polysaccharides for the future development of dairy industry based functional fermented products could be considered from the perspective of their influence on flavour, texture and sensory properties. However, if CKC is to be used as dietary fiber, further treatment to remove fat needs to be carried out from the perspective of increasing its potential for such purpose. Moreover, further extensive studies are required to develop food supplements or nutraceuticals, which constitute a growth substrate for probiotics from CKC. Analytical methods to obtain the optimal process parameters and their interactions for extraction of CKC polysaccharides by response surface methods to provide a reference for their use in the industry should also be carried out.
Disclosure statement
There is no conflict of interest as declared by authors.
Additional information
Funding
References
- Schrezenmeir J, De Vrese M. Probiotics, prebiotics, and symbiotic: approaching a definition. Am J Clin Nutr. 2001;73:361–364.
- Dardir HA. In vitro evaluation of probiotic activities of lactic acid bacteria strains isolated from novel probiotic dairy products. Glob Vet. 2012;8:190–196.
- Food and Agriculture Organization of the United Nations/World Health Organization working group on drafting for the evaluation of probiotics in food. Guidelines for the evaluation of probiotics in food. London, Ontario, Canada: FAO/WHO; 2002, p. 30.
- Wang X, Huang M, Yang F, et al. Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro. Carbohydr Polym. 2015;125:232–240.
- Gibson GR, Probert HM, Loo JV, et al. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275.
- Anal AK, Singh H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci Tech. 2007;18:240–251.
- Nowak R, Nowacka-Jechalke N, Juda M, et al. The preliminary study of prebiotic potential of Polish wild mushroom polysaccharides: the stimulation effect on Lactobacillus strains growth. Eur J Nutr. 2018;57:1511–1521.
- Saif A, Abdallah A, Muhamad S, et al. Study of antioxidant activity and physicochemical properties of coconut milk (Pati santan) in Malaysia. J Chem Pharm Res. 2015;7:967–973.
- Bawalan DD, Chapman KR. Virgin coconut oil: production manual for micro-and village-scale processing. FAO Regional office for Asia and the Pacific. Bangkok, Thailand: Thammada Press Co. Ltd. 2006;2006:1–112.
- DebMandal M, Manda S. Coconut (Cocos nucifera L.: Arecaceae): in health promotion and disease prevention. Asian Pac J Trop Med. 2011;4:241–247.
- Khuwijitjaru P, Pokpong A, Klinchongkon K, et al. Production of oligosaccharides from coconut meal by subcritical water treatment. Int J Food Sci Technol. 2014;49:1946–1952.
- Ng SP, Tan CP, Lai OM, et al. Extraction and characterization of dietary fiber from coconut residue. J Food Agric Environ. 2010;8:172–177.
- Horwitz W, Latimer GW. Official methods of analysis of AOAC international. 18th ed. Maryland: Association of Official Analytical Chemistry International; 2005.
- Havilah ED, Morris WR, Woolnough J. A microcolorimetric method for determination of ammonia in Kjeldahl digests with a manual spectrophotometer. Lab Pract. 1977;26:545–547.
- Bello B, Mustafa S, Tan JS, et al. Evaluation of the effect of soluble polysaccharides of palm kernel cake as a potential prebiotic on the growth of probiotics. 3 Biotech. 2018;8:346–360.
- Mohd Nor NAN, Abbasiliasi S, Marika MN, et al. Defatted coconut residue crude polysaccharides as potential prebiotics: study of their effects on proliferation and acidifying activity of probiotics in vitro. J Food Sci Technol. 2016;54:1–10.
- Abbasiliasi S, Tan JS, Tengku ITA, et al. Primary recovery of a bacteriocin-like inhibitory substance derived from Pediococcus acidilactici Kp10 by an aqueous two-phase system. Food Chem. 2014;151:93–100.
- Azmi A, Mustafa S, Hashim DM, et al. Prebiotic activity of polysaccharides extracted from Gigantochloa levis (Buluh beting) shoots. Molecules 2012;17:1635–1651.
- Qiao D, Liu J, Ke C, et al. Structural characterization of polysaccharides from Hyriopsis cumingii. Carbohydr Polym. 2010;82:1184–1190.
- Jia X, Zhang C, Qiu J, et al. Purification, structural characterization and anticancer activity of the novel polysaccharides from Rhynchosia minima root. Carbohydr Polym. 2015;132:67–71.
- Slavin JL. Carbohydrates, dietary fiber, and resistant starch in white vegetables: links to health outcomes. Adv Nutr. 2013;4:351–355.
- Grigelmo-Miguel N, Martin-Belloso O. Characterization of dietary fiber from orange juice extraction. Food Res Int. 1998;35:355–361.
- Yalegama LLWC, Nedra Karunaratne D, Sivakanesan R, et al. Chemical and functional properties of fiber concentrates obtained from byproducts of coconut kernel. Food Chem. 2013;141:124–130.
- Manikantan MR, Arivalagan M, Mathew AC, et al. Effect of processing parameters on recovery of hot process virgin coconut oil and co-products utilization. J Plant Crops. 2015;43:21–27.
- Andini R, Yoshida S, Ohsawa R. Variation in protein content and amino acids in the leaves of grain, vegetable and weedy types of Amaranths. Agronomy. 2013;3:391–403.
- Adepoju OT. Proximate composition and micronutrient potentials of three locally available wild fruits in Nigeria. Afr J Agric Res. 2009;4:887–892.
- Ballesteros LF, Cerqueira MA, Teixeira JA, et al. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr Polym. 2015;127:347–354.
- Cai W, Gu X, Tang J. Extraction, purification and characterization of the polysaccharides from Opuntiamilpaalta. Carbohydr Polym. 2008;71:403–410.
- Gu C, Pan S. The comparison and analysis of three extraction methods for polysaccharides in purslane. J Food Nutr Res. 2014;2:401–405.
- Tan S, Xu Q, Luo Z, et al. Inquiry of water-soluble polysaccharide extraction conditions from grapefruit skin. Engineering 2011;3:1090–1094.
- Huang SQ, Li JW, Wang Z, et al. Optimization of alkaline extraction of polysaccharides from Ganoderma lucidum and their effect on immune function in mice. Molecules 2010;15:3694–3708.
- Wichienchot S, Jatupornpipat M, Rastall RA. Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem. 2010;120:850–857.
- Yalegama LLWC, Chavan JK. Studies on utilization of coconut flour as a source of cell wall polysaccharides. Trop Agric Res. 2006;18:126–134.
- Thetsrimuang C, Khammuang S, Chiablaem K, et al. Antioxidant properties and cytotoxicity of crude polysaccharides from Lentinus polychrous Lév. Food Chem. 2011;128:634–639.
- Kain RJ, Chen Z, Sonda TS, et al. Study on the effect of control variables on the extraction of peanut protein isolates from peanut meal (Arachis hypogaea L.). Am J Food Technol. 2009;4:47–55.
- Yanhua W, Fuhua W, Zhaohan G, et al. Optimization of extraction process for polysaccharide in Salvia miltiorrhiza bunge using response surface methodology. Open Biomed Eng J. 2014; 8:153–159.
- Huang Z, Zhang L. Chemical structures of water-soluble polysaccharides from Rhizoma Panacis Japonici. Carbohydr Res. 2009;344:1136–1140.
- Thongsook T, Chaijamrus S. Modification of physiochemical properties of copra meal by dilute acid hydrolysis. Int J Food Sci Technol. 2014;49:1461–1469.
- Wu Y, Cui SW, Tang J, et al. Preparation, partial characterization and bioactivity of water-soluble polysaccharides from boat-fruited sterculia seeds. Carbohydr Polym. 2007;70:437–443.
- Abbasiliasi S, Joo Shun T, Tengku Ibrahim TA, et al. Use of sodium alginate in the preparation of gelatin based hard capsule shells and their evaluation in vitro. RSC Adv.. 2019; 9:16147–16157.
- Synytsya A, Míčková K, Synytsya A, et al. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: structure and potential prebiotic activity. Carbohydr Polym. 2009;76:548–556.
- Baseri MK, Baker S. Identification of cellular components of medical plants using FTIR. Rom J Biophys. 2011;21:277–284.
- Capek P, Sasinkova V, Wellner N, et al. FT–IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr Polym. 2002;43:195–203.
- Gannasin SP, Adzahan NM, Hamzah MY, et al. Physicochemical properties of tamarillo (Solanum betaceum Cav.) hydrocolloid fractions. Food Chem. 2015;182:292–301.
- Bothara SB, Singh S. Thermal studies on natural polysaccharide. Asian Pac J Trop Biomed. 2012;2:1031–1035.
- Cerqueira MA, Souza BWS, Simões J, et al. Structural and thermal characterization of galactomannans from non-conventional sources. Carbohydr Polym. 2011;83:179–185.
- Al-Sheraji SH, Ismail A, Manap MY, et al. Fermentation and non-digestibility of Mangifera pajang fibrous pulp and its polysaccharides. J Funct Foods. 2012;4:933–940.
- Asano I, Hamaguchi K, Fujii S, et al. In vitro digestibility and fermentation of mannooligosaccharides from coffee mannan. Food Sci Technol Res 2003;9:62–66.
- Li Y-P, Yao L-H, Wu G-J, et al. Antioxidant activities of novel small-molecule polysaccharide fractions purified from Portulaca oleracea L. Food Sci Biotechnol. 2014;23:2045–2052.
- Bik E, Ugalde J, Cousins J, et al. Microbial biotransformations in the human distal gut. Br J Pharmacol. 2018;175:4404–4414.
- Mussatto SI, Mancilha IM. Non-digestible oligosaccharides: a review. Carbohydr Polym. 2007;68:587–597.
- Wang Y. Prebiotics: present and future in food science and technology. Food Res Int. 2009;42:8–12.
- Manning TS, Gibson GR. Microbial-gut interactions in health and disease. Prebiotics. Best Pract Res Clin Gastroenterol. 2004;18:287–298.
- Rada V, Bartonová J, Vlková E. Specific growth rate of bifidobacteria cultured on different sugars. Folia Microbiol. 2002;47:477–480.
- Brownawell AM, Caers W, Gibson GR, et al. Prebiotics and the health benefits of fiber: current regulatory status, future research, and goals. J Nutr. 2012;142:962–974.
- Howlett JF, Betteridge VA, Champ M, et al. The definition of dietary fiber- discussions at the ninth vahouny fiber symposium: building scientific agreement. Food Nutr Res. 2010;54:5750.
- Mumcu AS, Temiz A. Effect of prebiotics on growth and acidifying activity of probiotic bacteria. GIDA 2014;39:71–77.