Abstract
The aim of this study was to investigate the expression of miRNA-222 in intervertebral disc degeneration (IDD), and explore its regulatory mechanism. Totally 30 patients with IDD and 36 normal control subjects were included. Quantitative real-time polymerase chain reaction (PCR), Western blot analysis, and enzyme-linked immunosorbent assay (ELISA) were preformed to detect the mRNA and protein expression levels of MMP1, and the miRNA-222 expression levels, in the nucleus pulposus (NP) tissues and blood samples. Bioinformatics analysis was performed, and dual-luciferase reporter assay was conducted for confirmation. HNPC cells were cultured in vitro, and the effects of agomiRNA-222 transfection were analyzed. The results showed that MMP1 expression was significantly up-regulated in the NP tissues and blood samples of patients with IDD, and the miRNA-222 expression was significantly down-regulated in both specimens. Bioinformatics analysis showed that MMP1 was the direct target for miRNA-222, which was confirmed by the dual-luciferase reporter assay. Over-expression of miRNA-222 significantly decreased the expression levels of MMP1 in HNPC cells in vitro, and the expression levels of type II collagen were significantly up-regulated. These results suggest that MMP1 expression is up-regulated in the NP and blood of patients with IDD, which may be related to the down-regulation of miRNA-222. The miRNA-222 may regulate the expression of related proteins in intervertebral disc nucleus cells through MMP1, thus affecting the disease pathogenesis and development.
Introduction
Intervertebral disc degeneration (IDD) is one of the main causes of low back pain and lumbar disc herniation, which is also one of the most common diseases in the world. IDD causes enormous direct costs and indirect losses, and even loss of labour [Citation1,Citation2]. Matrix metalloproteinases (MMPs) are a group of endopeptidases that have great structural homology and are capable of degrading extracellular matrix proteins. They are named after the metal protein ions zinc and calcium [Citation3]. Usually, the normal intervertebral disc is between the upper and lower cartilage endplates, composed of the outer annulus fibrosus (AF) and the inner layer of jelly-like, proteoglycan-rich nucleus pulposus (NP) [Citation4]. Normal intervertebral discs consist of extracellular matrix (ECM) and a small number of cells in it (which account for only 1% of the disc volume). The NP cells synthesize type II collagen, and the AF cells synthesize types II and I collagen [Citation5]. Tsarouhas et al. [Citation6] have found that MMPs are closely related to the degeneration of the intervertebral disc. Among them, MMP-1, also known as fibroblast type I collagenase, is a key enzyme that degrades human skin collagen [Citation7], which plays an important role in the matrix degradation.
As the study of genes enters the microRNA (miRNA) stage, some scholars have found that up-regulation of miRNAs can inhibit the expression of MMPs [Citation8]. MicroRNA can regulate the differentiation and maturation of immune cells [Citation9], as well as the osteogenic differentiation of stromal stem cells [Citation10]. Among them, miRNA-222 is one of the upstream regulatory genes for MMP1, which plays an important role in the formation of pathological scars [Citation11]. So far, there are limited studies concerning the expression of miRNA-222 in the degenerative tissue of the intervertebral disc and its regulating role on MMP1.
In this study, the relationship between the miRNA-222 and MMP1 expressions in the development and progression of IDD was investigated. Quantitative real-time PCR, Western blot analysis, ELISA, cell transfection, and gene bioinformatics prediction were used to detect the mRNA and protein expressions in the degenerative disc tissue.
Subjects and methods
Subjects
A total of 30 patients with IDD, 18 males and 12 females, aged 20–58 years old (with a median age of 41.9 years), diagnosed in the Rui Jin Hospital from December 2015 to August 2017, were enrolled in this study. Meanwhile, 36 subjects with traumatic disc injuries, 20 males and 16 females, aged 20–60 years old (median age of 41.6 years), during the same period were included as a control group. NP tissue and blood samples were collected from these subjects. None of these subjects had history of hormonal therapy, Chinese medicine, or radiotherapy and chemotherapy.
Ethics statement
Prior written and informed consent forms were obtained from every patient, and the study was approved by the Ethics Review Board of our hospital.
Sample collection and preparation
The peripheral blood serum samples were collected with the density gradient centrifugation combined with adherent separation method. Briefly, 10–15 mL peripheral venous blood was collected from each subject and placed at 4 °C for 1–2 h. Then the upper level of serum was harvested and subjected to centrifugation at 400 × g at 4 °C for 10 min. The serum sample was dispensed into 100-μL EP tubes and frozen at −70 °C.
The IDD lesion and normal tissues were collected and stored in liquid nitrogen. The tissue was ground into powder by grinding in liquid nitrogen, and was then collected into 1.5-mL EP tissues for the further extraction of mRNA and proteins.
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was extracted with Trizol. The cDNA template was obtained by reverse transcription with the TIANScript II cDNA kit (KR107; Tiangen, Beijing, China). Quantitative real-time PCR was performed with the SYBR Green SuperReal PreMix (FP204; Tiangen) by the miRcute miRNA quantitative real-time PCR kit (FP401; Tiangen) on the PCR-iQ5 machine (Bio-Rad, Hercules, CA, USA). The following primer sequences were used: MMP1, forward 5′-ATT CTA CTG ATA TCG GGG CTT TGA-3′ and reverse 5′-ATG TCC TTG GGG TAT CCG TGT AG-3′; and GAPDH, forward 5′-TCC TGT GGC ATC CAC GAA ACT-3′ and reverse 5′-GAA GCA TTT GCG GTG GAC GAT-3′. The 20-μL PCR system consisted of 10 μL qRT-PCR-Mix, 0.5 μL primer each, 2 μL cDNA, and 7 μL ddH2O. The reaction conditions were set as follows: 95 °C for 3 min; 94 °C for 20 s, 58 °C for 20 s, 72 °C for 20 s, for 39 cycles; 72 °C for another 10 min. The expression levels of target genes were calculated using the 2-ΔΔCt method [Citation12]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal reference.
For the detection of miRNA-222 expression in the tissue samples, the following primer sequences were used: U6, forward 5′-CGC TTC GGC AGC ACA TAT AC-3′ and reverse 5′-CAG GGG CCA TGC TAA TCT T-3′; and miRNA-222, forward 5′-CGC AGC TAC ATC TGG CTA CTG-3′ and reverse 5′-GTG CAG GGT CCG AGG T-3′. The reaction conditions were as follows: 95 °C for 5 min; 95 °C for 30 s, 60 °C for 35 s, 72 °C for 20 s, for a total of 40 cycles. U6 was used as internal reference.
Western blot analysis
The tissue was lysed with lysis solution. Protein concentration was determined by the BCA method (RTP7102; Real-times, Beijing, China). Totally 20 μL protein was separated by 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), which was electronically transferred onto the PVDF (polyvinylidene difluoride) membrane. After blocking with 5% non-fat milk at room temperature for 1 h, the membrane was incubated with rabbit anti-human anti-MMP1 primary antibody (1:500 dilution; ab38929; Abcam, Cambridge, MA, USA) and rabbit anti-human anti-β-actin (1:5000 dilution; ab129348; Abcam), respectively, at 4 °C overnight. Then the membrane was incubated with goat anti-rabbit secondary antibody (1:3000 dilution; ab6721; Abcam) at room temperature for 1 h. Colour development was performed with the ECL method (ab65623; Abcam), and the protein bands were captured and analyzed with the Image lab software (MA, USA). Beta-actin was used as internal reference.
Enzyme-linked immunosorbent assay (ELISA)
The separated serum samples were subjected to the Human MMP1 ELISA Kit (ab100603; Abcam). Briefly, standard samples at different concentrations were added into the standard wells. On the other hand, 10 μL sample and 40 μL dilution solution were added into the wells. Except for the blank control well, 100 μL horseradish peroxidase (HRP)-conjugated detection antibody was added into the standard and sample wells. The microplate was sealed with a membrane, and incubated for 1 h. After washing 5 times, 50 μL of substrates A and B each were added to each well, and the plate was incubated at 37 °C for 15 min. Then 50 μL stop solution was added into each well, and the optical density value at 450 nm (OD450) was measured over 15 min on a microplate reader.
Bioinformatics analysis
The upstream regulating miRNAs of MMP1 was predicted with the bioinformatics analysis. Based on the existing references concerning the upstream miRNAs of MMP1, the following software was used to search for the possible regulating miRNAs for MMP1: miRanda (http://www.microrna.org/microrna/home.do), TargetSean (www.targetscan.org), PiTa (http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html), RNA hybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/), and PICTA (http://pictar.mdc-berlin.de/) software.
Cell transfection
Cell transfection was performed with lipo2000. Totally 3 × 105 cells were plated onto the 24-well plate, and cultured with the F12/DMEM medium containing 10% foetal bovine serum (FBS) without antibiotics. Transfection was performed when 70% confluence was reached. The plasmid/siRNA/agomiR, together with 1 μL lipo2000, was separately added into the EP tube containing 50 μL Opti Memi. After 5 min at room temperature, the separate solution was mixed and placed at room temperature for 20 min, which was used to culture the cells. After co-culture for 6 h, the culture medium was replaced with fresh F12/DMEM culture medium containing 10% FBS. After 48 h, the cells were collected, and the mRNA and protein expression levels were detected in the samples.
Dual-luciferase reporter assay
The wild-type and mutant seed sequences for miRNA-222 in the 3′-UTR of MMP1 were chemically synthesized by Sangon, Shanghai, China, with the Spe-1 and HindIII restriction sites added to the ends. Two DNA fragments were cloned into the pMIR-REPORT luciferase reporter plasmid (Ambion, Austin, TX, USA), with the mutant 3′-UTR seed region as control. The wild-type and mutant 3′-UTR DNA sequences (0.8 μg) were transfected into the 293 T cells with liposomes, followed by the transfection of antagomiRNA-222 (100 nmol/L) for 24. The cells were lysed, and luciferase was detected with the GloMax 20/20 luminometer. Renilla was used as internal reference.
Statistical analysis
Data are expressed as mean values with standard deviation (± SD). SPSS 18.0 software was used for statistical analysis. One-way analysis of variance (ANOVA) was used for multiple group comparison, and the t-test was performed for the pair-wise comparison. p < 0.05 was considered as statistically significant.
Results and discussion
Changes in mRNA and protein expression of MMP1 in nucleus pulposus and serum in IDD
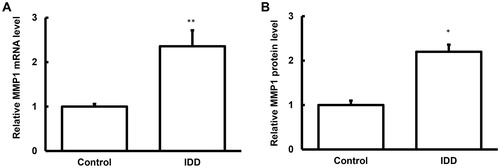
To investigate the mRNA and protein expression levels of MMP1 in the NP tissues, the quantitative real-time PCR and Western blot analysis were performed. Our results showed that both the mRNA and protein expression levels of MMP1 in the NP tissues were significantly up-regulated (p < 0.05) compared with the control group (). Moreover, similar results were observed for the MMP1 levels (mRNA and protein) in the serum samples, as detected with the quantitative real-time PCR and ELISA. Compared with the control subjects, both the mRNA and protein levels of MMP1 in the serum samples of IDD patients were significantly up-regulated (p < 0.05) (). Taken together, these results suggest that MMP1 may play a regulatory role in the pathogenesis and development of IDD.
Figure 1. MMP1 expression levels in the nucleus pulposus tissues of IDD.
Note: The mRNA (A) and protein (B) expression levels of MMP1 in the nucleus pulposus tissues of IDD patients and normal control subjects were detected with quantitative real-time PCR and Western blot analysis, respectively. Compared with the control group, *p < 0.05, **p < 0.01.
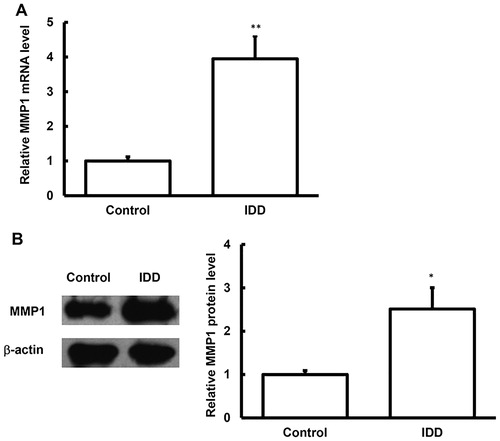
Figure 2. MMP1 expression levels in the blood samples of IDD.
Note: The mRNA (A) and protein (B) expression levels of MMP1 in the blood samples of IDD patients and normal control subjects were detected with quantitative real-time PCR and ELISA, respectively. Compared with the control group, *p < 0.05, **p < 0.01.
Changes in miRNA-222 expression in IDD
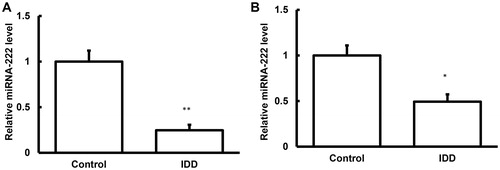
To detect the expression levels of miRNA-222 in these samples, quantitative real-time PCR was performed. Our results showed that, compared with the control group, the expression levels of miRNA-222 were significantly decreased (p < 0.05) in the samples from the IDD patients (). Together with the bioinformatics, which indicated a relationship between MMP1 and miRNA-222 (), these results suggest that miRNA-222 might play a regulatory role in the pathogenesis and development of IDD, and probably influence the MMP1 protein expression levels by negatively regulating the transcriptional levels of the target genes.
Figure 3. Expression levels of miRNA-222 in the nucleus pulposus tissues and blood samples of IDD.
Note: The expression levels of miRNA-222 in the nucleus pulposus tissues (A) and blood samples (B) of IDD patients and normal control subjects were detected with quantitative real-time PCR. Compared with the control group, *p < 0.05, **p < 0.01.
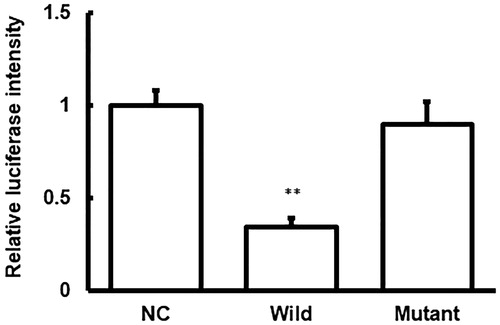
Dual-luciferase reporter assay
To further confirm the reaction between miRNA-222 and MMP1, the dual-luciferase reporter assay was performed. Our results showed that co-transfection of agomiRNA-222 and pMIR-REPORT luciferase reporter plasmids led to significantly declined luciferase value (p < 0.05), whereas no significant differences (p > 0.05) were observed in the luciferase value for the mutant group (). Based on these results, we speculate that miRNA-222 could combine with MMP1 to regulate its expression.
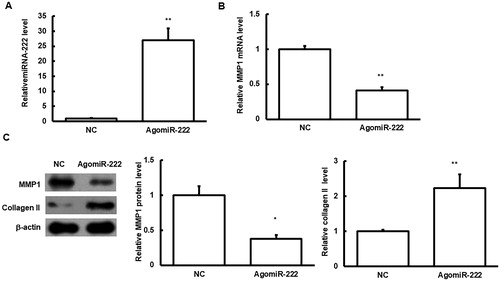
Effects of agomiRNA-222 transfection on HNPC cells
The effects of transfection of agomiRNA-222 on the HNPC cells were then investigated. Our results showed that, after transfection of agomiRNA-222, the protein expression levels of MMP1 were significantly declined in the HNPC cells, and the expression levels of type II collagen were significantly increased (p < 0.05) (). These results suggest that the elevated miRNA-222 level could influence the expression of type II collagen through regulating MMP1.
Figure 6. Effects of agomiRNA-222 transfection on HNPC cells.
Note: HNPC cells were transfected with agomiRNA-222. (A) The expression levels of miRNA-222 were detected. (B) The mRNA expression levels of MMP1 in these cells were detected with quantitative real-time PCR. (C) The protein expression levels of MMP1 and type II collagen in these cells were detected with Western blot analysis. Compared with the NC group, *p < 0.05, **p < 0.01.
In this study, the expression of MMP1 in the NP of IDD was investigated, as well as the expression of its upstream gene miRNA-222. Moreover, the biological functions of miRNA-222 and MMP1 were explored, and the molecular mechanism through which miRNA-222 regulated the MMP1 expression to affect the pathogenesis and development of IDD.
The main components of the extranuclear matrix of the NP include water, type II collagen (20%), and proteoglycan (50%, mainly aggrecan), and the minor components include types III, IV, IX, and XI collagen, as well as small proteoglycan [Citation9]. Therefore, the intervertebral disc is mainly composed of the extracellular matrix, containing various collagens. After 10 years of age, the spinal cord cells in the NP of human beings would basically disappear. Adult NP cells represent a kind of cartilage-like cells, mainly characterized by the expression of type II collagen [Citation13]. Type II collagen is one of the most important collagen components in the intervertebral disc, also known as cartilage collagen, which is mainly found in cartilage and intervertebral disc nucleus [Citation14]. Sive et al. [Citation15] have confirmed by in situ hybridization that the expression of type II collagen in the NP cells of the intervertebral disc gradually decreases with the progression of degeneration [Citation15].
MMPs participate in various physiological and pathological processes, such as embryo formation, neovascularization, wound healing, cardiovascular disease, and cancer development under physiological conditions [Citation16]. MMP1 plays a very important role in the pathogenesis and development of IDD, and MMP1 is always significantly up-regulated in the serum of patients with lumbar disc degeneration [Citation17]. Moreover, it has been shown that in the AF tissue of IDD, the MMP1 expression levels are significantly up-regulated, while the expression levels of type II collagen are significantly down-regulated [Citation18]. In the NP of IDD, MMP1 degrades type II collagen, contributing to the disease progression [Citation19]. We mainly investigated the NP tissue and blood sample in patients with IDD. In line with previous findings, our results showed that the expression levels of MMP1 in the NP and blood samples were significantly up-regulated, indicating that MMP1 may play an important role in the disease pathogenesis and development.
The regulation of mRNA transcription and expression is a complex process involving multiple factors, and the regulatory factors for MMP1 expression have not yet been fully elucidated. The miRNAs discovered in recent years can achieve negative feedback regulation by cleaving MMP1 mRNA and inhibiting its translation, which is very important in physiological and pathological processes. Moreover, more and more miRNAs have been recognized as biomarkers for various diseases [Citation20,Citation21]. It has been shown that miRNA-222 can target MMP1 to regulate the biological function of tongue squamous tumour cells [Citation22], and miRNA-222 itself is one of the key factors of many diseases. MiRNA-222 can regulate the MEK and TRAIL pathways in the thyroid follicle epithelial cells [Citation23], and affect the progression of gastric cancer by regulating its target gene p27kip1 [Citation24]. Abnormal expression of miRNA-222 can also be found in mouse models of gastric cancers [Citation25]. In ovarian cancer cell-associated macrophages, miR-222 also has its unique regulatory role [Citation26]. For the selection of MMP1 upstream microRNA, in addition to the references in the literature, we also used a series of databases. Our results showed that miRNA-222 did target and cleave MMP1. In the experiments, our results showed that miRNA-222 had low expression in the NP in IDD, which was significantly different from the normal control group. We speculate that miRNA-222 affects the MMP1 expression in the NP tissues. Results from the dual-luciferase reporter assay showed that MMP1 was a direct target gene for miRNA-222. By culturing the human NP HNPC cell lines, and up-regulating the expression levels of miRNA-222 in the cells by using agomiRNA-222, we found that the expression levels of MMP1 in the cells were significantly down-regulated, and, correspondingly, the expression levels of type II collagen were significantly decreased in the cells. These results further confirmed that miRNA-222 affected the expression levels of type II collagen in the cells through negatively regulating MMP1, causing lesions in the intervertebral disc. Based on our results, the mRNA expression level of MMP1 in the serum might serve as a potential biomarker for the disease. Of course, further in-depth studies are still needed to address these issues in the future.
Conclusions
The results from this study showed that there was a negative regulation relationship between miRNA-222 and MMP1. After the expression level of miRNA-222 was decreased in the NP tissue of patients with IDD, the expression levels of MMP1 were also down-regulated, which changed the expression levels of type II collagen, exerting its biological function in the disease development.
Acknowledgement
We thank Director Peng Cao, Department of Orthopaedics, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, for the kind assistance.
Disclosure statement
All authors declare no financial competing interests and no non-financial competing interests.
References
- Masuda K, Oegema TR, Jr., An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29(23):2757–2769. PubMed PMID:15564925.
- Takae R, Matsunaga S, Origuchi N, et al. Immunolocalization of bone morphogenetic protein and its receptors in degeneration of intervertebral disc. Spine. 1999;24(14):1397–1401. PubMed PMID:10423782.
- Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. PubMed PMID: 25415508; PubMed Central PMCID: PMCPMC4316204.
- Roughley PJ, Geng Y, Mort JS. The non-aggregated aggrecan in the human intervertebral disc can arise by a non-proteolytic mechanism. Eur Cell Mater. 2014;28:129–136. discussion 136. PubMed PMID: 25214019.
- Wang SZ, Rui YF, Lu J, et al. Cell and molecular biology of intervertebral disc degeneration: current understanding and implications for potential therapeutic strategies. Cell Prolif. 2014;47(5):381–390. PubMed PMID: 25112472.
- Tsarouhas A, Soufla G, Katonis P, et al. Transcript levels of major MMPs and ADAMTS-4 in relation to the clinicopathological profile of patients with lumbar disc herniation. Eur Spine J. 2011;20(5):781–790. PubMed PMID: 20857147; PubMed Central PMCID: PMCPMC3082673.
- D'Armiento J. Matrix metalloproteinase disruption of the extracellular matrix and cardiac dysfunction. Trends Cardiovasc Med. 2002;12(3):97–101. PubMed PMID: 12007733.
- Kim KH, Jung JY, Son ED, et al. miR-526b targets 3' UTR of MMP1 mRNA. Exp Mol Med. 2015;47:e178. PubMed PMID: 26292968; PubMed Central PMCID: PMCPMC4558487.
- Eleme K, Taner SB, Onfelt B, et al. Cell surface organization of stress-inducible proteins ULBP and MICA that stimulate human NK cells and T cells via NKG2D. J Exp Med. 2004;199(7):1005–1010. PubMed PMID: 15051759; PubMed Central PMCID: PMCPMC2211882.
- Iliopoulos D, Kavousanaki M, Ioannou M, et al. The negative costimulatory molecule PD-1 modulates the balance between immunity and tolerance via miR-21. Eur J Immunol. 2011;41(6):1754–1763. PubMed PMID: 21469086.
- Zhang Y, Lin X, Zhang L, et al. MicroRNA-222 regulates the viability of fibroblasts in hypertrophic scars via matrix metalloproteinase 1. Exp Ther Med. 2018;15(2):1803–1808. PubMed PMID: 29434768; PubMed Central PMCID: PMCPMC5776557.
- Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. BioTechniques. 2005;39(1):75–85. PubMed PMID: 16060372.
- Specchia N, Pagnotta A, Toesca A, et al. Cytokines and growth factors in the protruded intervertebral disc of the lumbar spine. Eur Spine J. 2002;11(2):145–151. PubMed PMID: 11956921; PubMed Central PMCID: PMCPMC3610507.
- Cauci S, Vigano M, de Girolamo L, et al. High levels of circulating type II collagen degradation marker (CTx-II) are associated with specific VDR polymorphisms in patients with adult vertebral osteochondrosis. Int J Mol Sci. 2017;18(10):2073. PubMed PMID: 28961166; PubMed Central PMCID: PMCPMC5666755.
- Sive JI, Baird P, Jeziorsk M, et al. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55(2):91–97. PubMed PMID: 11950957; PubMed Central PMCID: PMCPMC1187156.
- Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol. 2012;181(6):1895–1899. PubMed PMID: 23063657; PubMed Central PMCID: PMCPMC3506216.
- Deng B, Ren JZ, Meng XQ, et al. Expression profiles of MMP-1 and TIMP-1 in lumbar intervertebral disc degeneration. Genet Mol Res. 2015;14(4):19080–19086. PubMed PMID: 26782559.
- Cho H, Seth A, Warmbold J, et al. Aging affects response to cyclic tensile stretch: paradigm for intervertebral disc degeneration. Eur Cell Mater. 2011;22:137–145. discussion 145-6. PubMed PMID: 21932191.
- Cho H, Park SH, Lee S, et al. Snapshot of degenerative aging of porcine intervertebral disc: a model to unravel the molecular mechanisms. Exp Mol Med. 2011;43(6):334–340. PubMed PMID: 21499012; PubMed Central PMCID: PMCPMC3128911.
- Li X, Yu Z, Li Y, et al. The tumor suppressor miR-124 inhibits cell proliferation by targeting STAT3 and functions as a prognostic marker for postoperative NSCLC patients. Int J Oncol. 2015;46(2):798–808. PubMed PMID: 25531908.
- Lv ZC, Fan YS, Chen HB, et al. Investigation of microRNA-155 as a serum diagnostic and prognostic biomarker for colorectal cancer. Tumor Biol. 2015;36(3):1619–1625. PubMed PMID: 25528214.
- Liu X, Yu J, Jiang L, et al. MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. Cancer Genom Proteom. 2009;6(3):131–139. PubMed PMID: 19487542; PubMed Central PMCID: PMCPMC2890246.
- Aherne ST, Smyth P, Freeley M, et al. Altered expression of mir-222 and mir-25 influences diverse gene expression changes in transformed normal and anaplastic thyroid cells, and impacts on MEK and TRAIL protein expression. Int J Mol Med. 2016;38(2):433–445.
- Lloyd KA, Moore AR, Parsons BN, et al. Gastrin-induced miR-222 promotes gastric tumor development by suppressing p27kip1. Oncotarget. 2016;7(29):45462–45478. PubMed PMID: 27323780; PubMed Central PMCID: PMCPMC5216734.
- Choi B, Yu J, Han TS, et al. Gastric carcinogenesis in the miR-222/221 transgenic mouse model. Cancer Res Treat. 2017;49(1):150–160. PubMed PMID: 27338035; PubMed Central PMCID: PMCPMC5266385.
- Ying X, Wu Q, Wu X, et al. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget 2016;7(28):43076–43087. PubMed PMID: 27172798; PubMed Central PMCID: PMCPMC5190009.