Abstract
RNA interference (RNAi) mediated by microRNA (miRNA) is widely utilized to induce apoptosis and further inhibit the proliferation of cancer cells. MiroRNA-34a (miR-34a) is one of the most potent apoptosis inducers by targeting survivin, which is associated with cancer initiation and progression. To further induce apoptosis and inhibit proliferation in Hela cells, the cytokine E4orf4 is included. One of the advantages of E4orf4 is that it can induce apoptosis independent of p53, which is especially useful for p53-mutant cancers. In our study, we successfully constructed a vector co-expressing miR-34a and E4orf4. The vector was transfected into Hela cells and the proliferation, metastasis and apoptosis of Hela cells were evaluated. We further compared the combined use of miR-34a and E4orf4 with pri-miR-34a in the potency of apoptosis induction. It is concluded that miR-34a and E4orf4 have a synergistic impact on Hela cell proliferation, metastasis and apoptosis, providing an efficient tool for further cervical cancer gene therapy.
Keywords:
Introduction
Apoptosis plays an essential role in initiation and progression of cancer. With the intensive study of the underlying mechanisms of apoptosis, gene therapy targeting apoptosis has become a promising strategy for cancer therapy [Citation1,Citation2]. It is widely accepted that apoptosis is regulated by multiple genes. Survivin, as a member of the IAP (apoptosis inhibiting protein) family overexpressed in various tumours, is one of the strongest apoptosis inducers [Citation3]. Proliferation of cancer cells is promoted by survivin through direct inhibition of the activity of caspase-3 and/or caspase-7 or through prevention of mitochondria releasing cytochrome C, which could in turn inactivate the caspase. Clinically, survivin is related to poor prognosis, radiotherapy tolerance and chemotherapy tolerance and tumour recurrence [Citation4].
MicroRNAs (miRNA) are a class of potent regulators involved in various cellular activities and are emerging as a potential means of gene therapy. It is demonstrated that miR-34a plays an important role in the occurrence and metastasis of cancer through downregulation of the expression of target genes such as survivin [Citation5,Citation6]. Ectopic expression of miRNA-34a suppresses the proliferation and metastasis of head and neck squamous cell carcinoma cells by survivin inhibition. The negative regulatory role of miRNA-34a in survivin expression has also been confirmed in gastric cancer [Citation7].
Another potent apoptosis inducer is adenovirus E4 open-reading-frame 4protein (E4orf4), which is a cytokine composed of 114 amino acids. E4orf4 can inhibit the activity of heterotrimeric protein phosphatase 2A (PP2A) by binding to its regulatory B subunits, and induces cell death in a dose-dependent manner. E4orf4 can specifically induce apoptosis in cancer cells independent of p53 [Citation8,Citation9], which is especially useful for cancers with p53 mutation leading to resistance to radiotherapy or chemotherapy. In the present study, we constructed a vector co-expressing miR-34a and E4orf4 (pGFP-E4orf4-miR-34a) to explore their synergistic effects on apoptosis in Hela cells. The efficiency of apoptosis induction by pGFP-E4orf4-miR-34a and pri-miR-34a is compared.
Materials and methods
Cell culture and transfection
Hela cells (CCTCC, China) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% foetal bovine serum (Hyclone, USA) and 1% penicillin/streptomycin (Hyclone, USA). The cells were incubated in 5% CO2 at 37 °C. Transfection by lipofectamine 2000 (Invitrogen, USA) was performed following the manufacturer’s instructions.
Molecular cloning
E4orf4 (NC_017825.1) was amplified using the following primer pair: E4orf4-forward, 5′-ctagctagcTCACAGCGAGGTAAGGTGGC-3′; E4orf4-reverse, 5′-ccgctcgagacATGGTTCTTCCTGTTCTTCCCTC-3′. The lowercase portions denote restriction sites (XhoI and NheI, respectively). The polymerase chain reaction (PCR) products were inserted into pEGFP-C3 plasmid by the restriction/ligation method to construct pEGFP-E4orf4. pEGFP-E4orf4-miR-34a and pri-miR-34a was constructed into the backbone of pEGFP-E4orf4 in a similar manner, respectively. Ligation products were transformed into E. coli competent cells and plasmids were extracted. The construct was further verified by sequencing (BGI Tech, China).
Cell proliferation analysis
MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay was performed 24 h, 48 h, 72 h and 96 h after transfection. In brief, cells were inoculated into a 96-well plate and incubated for 4 h. Subsequently, the cell viability was analyzed by MTS (Promega, USA). The absorbance at 450 nm was analyzed by a microplate reader. The proliferation rate was calculated as increasing folds of absorbance at 450 nm compared with that of the blank group (without any treatment) at 24 h.
Reverse transcription quantitative PCR (RT-qPCR)
Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer’s instructions and was reverse transcribed to cDNA. The primer pair specific to survivin was as follows: forward, 5′-AAATGACTTGGCTCGATGCT-3′; reverse, 5′-TCCATCATCTTACGCCAGACT-3′. The expected length of the targeted products was 180 bp. rRNA (18 s) was used as a reference gene and the primer pair was as follows: forward, 5′-CCTGGATACCGCAGCTAGGA-3′; reverse, 5′-GCGGCGCAATACGAATGCCCC-3′. The length of the targeted products was expected to be 112 bp. The PCR protocol was set as: 95 °C for 5 min; (95 °C for 15 s, 60 °C for 15 s and 72 °C for 32 s) for 40 cycles; 60 °C for 7 min using StepOnePlus (Applied Biosystems, Foster City, CA).
Western blotting
Cells were lysed and proteins were quantified and loaded for sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). The electrophoresis was run at 80 V for 50 min in 5% gel. The proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane. The antibody specific to survivin was from Southern Biotech (USA). The second antibody was from Wuhan Boster Bio-Technology (China). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was used as a reference.
Flow cytometry
Cells were harvested 48 h after transfection and washed twice by pre-cooled phosphate buffered saline (PBS) and fixed in 70% pre-cooled ethyl alcohol overnight. Annexin V-FITC (1.25 μL), 10 μL propidium iodide and 0.2% triton X-100 were mixed with the cells. After incubation for 30 min at 4 °C in the dark, the suspension was analyzed by flow cytometry (FACS Calibur, BD). The data were analyzed by ModFit software.
Transwell assay
Hela cells (24 h after gene transfection) were harvested and resuspended with DMEM-F12 and MEM medium. The cell suspension (1 × 105 cells) was added to the upper chamber and incubated for 24 h and 48 h. Cells in the lower chamber were fixed by 4% paraformaldehyde for 15 min and washed by PBS, stained with 0.1% crystal violet for 10 min. The number of Hela cells in the lower chamber was counted under a microscope.
Tunel assay
TUNEL assay was performed using a cell apoptosis detection kit (Promega, USA). In brief, cells were collected and washed using PBS. The cells were then fixed with 4% paraformaldehyde for 25 min and incubated with 0.2% Triton X-100 for 5 min at room temperature. TUNEL reaction mixture was added to the cells, then the cells were kept at 37 °C in a humidified incubator for 1 h. All cells were dyed with haematoxylin.
Statistical analysis
The experimental data are shown as mean values with standard error of the means (±SEM). Each experiment was performed independently three times. One-way analysis of variance (ANOVA) was used to indicate the difference. p < 0.05 was regarded as statistically significant.
Results and discussion
MiR-34a and E4orf4 can synergistically inhibit the cell growth
MTS assay was utilized to determine the inhibitory effect on the growth of Hela cells exerted by miR-34a, E4orf4 and the combination of miR-34a and E4orf4, respectively. The cell viability was measured and compared 24 h, 48 h, 72 h and 96 h after transfection. As shown in , miR-34a could inhibit the growth of Hela cells about 72 h after transfection with a proliferation rate of 407.08%. E4orf4 inhibited cell proliferation with a proliferation rate of 321.4%. For the cells transfected by the vector co-expressing miR-34a and E4orf4, the proliferation decreased significantly (233.98%) at 96 h compared with other groups. Untreated cells, lipo2000 treated cells and pEGFP-transfected cells were set as negative control. There was no significant difference in the absorbance at 450 nm among the negative groups at 96 h.
Figure 1. (A) Synergistic effect of survivin knockdown and E4orf4 overexpression in suppressing cell growth. The absorbance at 450 nm in each group was measured 24 h, 48 h, 72 h and 96 h after transfection. Untreated cells (blank control), cells treated with lipo2000 and cells transfected with the plasmid expressing EGFP (pEGFP) were set as the negative controls. (B) The impact of recombinant vectors on the expression of survivin by RT-qPCR at 36 h after transfection. Expression of survivin mRNA in HeLa cells was determined by using GAPDH as a reference. (C) Inhibition of survivin expression by E4orf4, miR-34a and miR-34a/E4orf4 were analyzed and compared by western blotting at 48 h after transfection. Blank group and lipo2000 group were used as negative controls. GAPDH was used as a reference. (D) Cell apoptosis rate was analyzed by flow cytometric analysis at 48 h after transfection. Annexin V FITC and PI-FL displays the fluorescence intensity of FITC-annexin V and PI, respectively. n = 3, *p < 0.05 versus blank group, lipo2000 group and pEGFP group. #p < 0.05 versus pEGFP-E4orf4 group and pEGFP-miR-34a group.
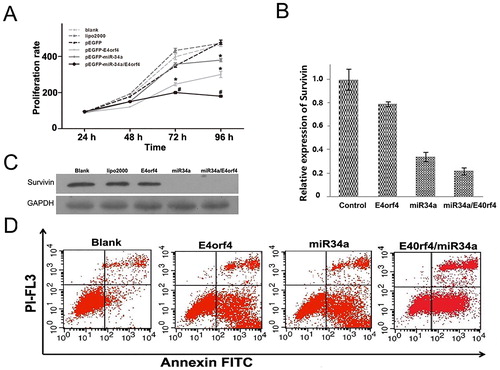
Apoptosis is induced by miR-34a targeting survivin
RNA interference, which is usually mediated by siRNA, shRNA or microRNA, is an efficient means for gene silencing which has attracted much attention. Zhong et al. [Citation10] inhibited survivin expression using antisense RNA, and Hela cell proliferation and cell cycle were successfully suppressed [Citation10]. In our study, we further investigated Hela cell apoptosis induction. However, as indicated in our study, the therapeutic effect of miR-34a was limited. It is reasonable that the combined use of apoptosis inducers can play a synergistic effect on the cancer cell inhibition because of the complicated mechanism underlying tumour initiation, progression, metastasis and recurrence [Citation11]. The cytokine E4orf4 can serve as an ideal candidate, which has gained widespread application in cancer therapy [Citation12]. One of the advantages of E4orf4 is that it can exert therapeutic effect in a p53 independent manner [Citation8]. In this study, we combined miR-34a and E4orf4 to promote cancer cell apoptosis. Hela cells were transiently transfected by pEGFP, pEGFP-miR-34a and pEGFP-E4orf4-miR-34a, respectively. On the mRNA level, the expression of survivin was significantly inhibited by pEGFP-miR-34a (by 66%) and pEGFP-E4orf4-miR-34a (by 78%) compared with the negative control (). There was no significant difference between pEGFP-miR-34a and pEGFP-E4orf4-miR-34a, indicative of the primary role of miR-34a in survivin inhibition. It was further verified by western blot that survivin was significantly suppressed in the miR-34a group and the miR-34a/E4orf4 group (). Flow cytometry showed that the apoptosis rate (53.63 ± 1.67%) by the combination of E4orf4 and miR-34a was higher than that by E4orf4 (48.14 ± 0.12%) alone or miR-34a (47.73 ± 0.67%) alone ().
Comparison of the impacts of the combined miR-34a/E4orf4 and pri-miR-34a on cell proliferation, metastasis and apoptosis
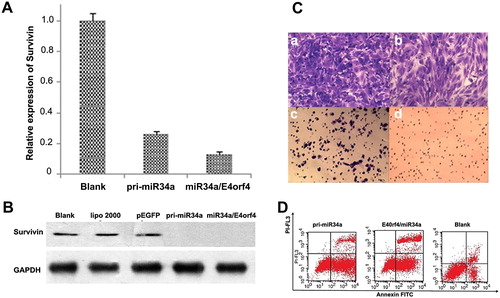
The expression of survivin was examined by RT-qPCR 36 h after transfection. The inhibition rate by miR-34a/E4orf4 and pri-miR-34a was 73.9% and 86.9%, respectively (). On the protein level, survivin expression was efficiently inhibited by both miR-34a/E4orf4 and pri-miR-34a according to the grey scale analysis (). GAPDH was used as an internal reference. It was shown by transwell assay that the invasion ability of Hela cells could be efficiently inhibited by miR-34a/E4orf4 and pri-miR-34a (). PEGFP-E4orf4-miR-34a could efficiently induce apoptosis of Hela cells (54%), which was stronger than pri-miR-34a (62.08%) (). Overall, the combined application of miR-34a and E4orf4 produced a much stronger effect than pri-miR-34a in terms of Hela cell proliferation inhibition, metastasis inhibition and apoptosis induction.
Figure 2. (A) Downregulation of survivin mRNA by miR-34a/E4orf4 and pri-miRNA-34a in Hela cells 36 h after transfection. The expression of survivin mRNA in HeLa cells was determined by RT-qPCR analysis, with GAPDH as a reference. (B) Inhibition of survivin expression by pri-miRNA-34a and miR34/E4orf4 were analyzed and compared by western blotting at 48 h after transfection. Blank group, lipo2000 group and pEGFP group were used as negative controls. (C) Evaluation of invasion of Hela cells induced by pEGFP (b), pri-miR-34a (c), survivin-miRNA (d) and the untreated cells were set as the control (a). (D) Analysis of apoptosis induced by pri-miR-34a and miR-34a/E40rf4 by flow cytometry. n = 3, *p < 0.05.
With the increasing insight into the mechanism of apoptosis, gene therapy based on apoptosis induction has been widely explored and shows great promise in the treatment of cancer [Citation13]. It is imperative to develop a specific and efficient tool to induce cancer cell apoptosis to inhibit tumour growth. MicroRNAs provide a cost-effective, fast and efficient means for gene therapy [Citation14,Citation15]. Currently, the strategy for downregulating survivin to induce apoptosis shows great promise in cancer gene therapy [Citation16]. Future endeavours should be directed to the development of a delivery system such as non-viral vectors [Citation17,Citation18] that could efficiently and safely introduce genes into cancer cells.
Conclusions
In this study, we successfully constructed a vector co-expressing miR-34a and E4orf4, which is proved to be more efficient in apoptosis induction. Also, we demonstrated that the induction of miRNA-34a could significantly inhibit survivin expression in Hela cells. The combination of miR-34a/E4orf4 was superior to pre-miR-34a in terms of Hela cell proliferation inhibition, metastasis inhibition and apoptosis induction.
Declaration of interest
The authors have no conflict of interest.
Funding
This work was supported by the Natural Science Foundation of Guangdong Province (Nos. 2018A030313678, 2016A030311054), PhD research start-up foundation of the Third Affiliated Hospital of Guangzhou Medical University (No. 2018B04), National Natural Science Foundation of China (No. 81671707, No. 81971621, No. 81371572), Young Scientists Fund of the National Natural Science Foundation of China (No. 81901764), Medical Scientific Research Foundation of Guangdong Province (No. B2019136), Research projects of Guangzhou Science Technology and Innovation Commission (No. 201607010201), Research fund of National Education Steering Committee for Graduates in Medical Degree (B3-20170302-06) , Research fund for Lin He’s Academician Workstation of New Medicine and Clinical Translation the Higher Education Colleges, and Youth Foundation of Scientific Research of The Third Affiliated Hospital of Guangzhou Medical University (No. 2018Q03, No. 2018Q28).
Author contributions
Conceived and designed the experiments: H. Z., Z. Y. C. and F. R. Performed the experiments: H. Z., Y. W. and Y. L. Analyzed the data: H. Z. Contributed reagents/materials/analysis tools: Z. Y. C. Wrote the paper: H. Z.
References
- Mohamed MS, Mai KB, Almutairi FM, et al. Inhibitors of apoptosis: clinical implications in cancer. Apoptosis. 2017;22(12):1487–1423.
- El-Shemi AG, Ashshi AM, Oh E, et al. Efficacy of combining ING4 and TRAIL genes in cancer-targeting gene virotherapy strategy: first evidence in preclinical hepatocellular carcinoma. Gene Ther. 2017;25(1):54–65.
- Huang Q, Zeng Y, Lin H, et al. Transfection with livin and survivin shRNA inhibits the growth and proliferation of non-small cell lung cancer cells. Mol Med Rep. 2017;16(5):7086–7091.
- Khan Z, Khan AA, Yadav H, et al. Survivin, a molecular target for therapeutic interventions in squamous cell carcinoma. Cell Mol Biol Lett. 2017; 22(1):8.
- Yoshimasa S, Toshiaki N, Hidetsugu S. microRNA-34a as a therapeutic agent against human cancer. J Clin Med. 2015;4(11):1951–1959.
- Liu K, Huang J, Xie M, et al. MIR34A regulates autophagy and apoptosis by targeting HMGB1 in the retinoblastoma cell. Autophagy. 2014;10(3):442–452.
- Cao W, Yang W, Fan R, et al. miR-34a regulates cisplatin-induce gastric cancer cell death by modulating PI3K/AKT/survivin pathway. Tumor Biol. 2014;35(2):1287–1295.
- Kleinberger T. Induction of cancer-specific cell death by the adenovirus E4orf4 protein. Adv Exp Med Biol. 2014;818:61–97.
- Kleinberger T. Mechanisms of cancer cell killing by the adenovirus E4orf4 protein. Viruses. 2015;7(5):2334–2357.
- Zhong MZ, Yan D, Zhi S. Effects to HeLa cells of the inhibition of survivin by antisense RNA. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 2001;33(5):547–551.
- Kemp V, Dautzenberg IJC, Cramer SJ, et al. Characterization of a replicating expanded tropism oncolytic reovirus carrying the adenovirus E4orf4 gene. Gene Ther. 2018;25(5):331–344.
- Rhee JK, Park O, Lee A, et al. Glycol chitosan-based fluorescent theranostic nanoagents for cancer therapy. Mar Drugs. 2014;12(12):6038–6057.
- Wen S, Zhang J, Zhou P, et al. The anti-tumour effect of a DNA vaccine carrying a fusion gene of human VEGFR2 and IL-12. Biotechnol Biotechnol Equip. 2016;30(5):956–962.
- Ji W, Sun B, Su C. Targeting microRNAs in cancer gene therapy. Genes 2017;8(1):21.
- Hosseinahli N, Aghapour M, Duijf PHG, et al. Treating cancer with microRNA replacement therapy: a literature review. J Cell Physiol. 2018;233(8):5574–5588.
- Gupta SK, Gandham RK, Sahoo AP, et al. Viral genes as oncolytic agents for cancer therapy. Cell Mol Life Sci. 2015; 72(6):1073–1094.
- Zhang H, Tu JW, Liao YY, et al. Chitosan-conjugated lipid microbubble combined with ultrasound for efficient gene transfection. Biotechnol Biotechnol Equip. 2018;32(4):982–987.
- Zhang H, Chen Z, Du M, et al. Enhanced gene transfection efficiency by low-dose 25 kDa polyethylenimine by the assistance of 1.8 kDa polyethylenimine. Drug Deliv. 2018;25(1):1740–1745.
