Abstract
Tanshinone IIA (Tan IIA) is a natural product that has been identified to have anti-proliferative properties against cervical cancer. The study was designed to investigate the anti-tumor effects of Tan IIA at different concentrations on the proliferation and apoptosis of human cervical carcinoma C4-1 cells. Human cervical carcinoma C4-1 cells were treated with different doses of Tan IIA, and MTT assay was performed to determine effects on cell viability. Flow cytometry was used to detect apoptosis and Western blot analysis was carried out to determine alterations in the expression of key proteins. Tan IIA treatment (0.5, 1.0, 2.0, and 5.0 mg/mL) significantly inhibited the growth of C4-1 cells in a time- and dose-dependent manner. Significant differences were observed in the growth inhibition rate between Tan IIA group and the control group (0.5-1.0 mg/L Group: p < 0.05; 2.0–5.0 mg/L Group: p < 0.01). Flow cytometry showed that C4-1 cells became apoptotic after treatment with Tan IIA. In the control group, the apoptosis rate of C4-1 cells was 2.56 ± 0.21% whilst in the Tan IIA-treated groups, different concentrations markedly increased the rate of apoptosis (1.0 mg/L group, 7.9 ± 0.43% [p < 0.05]; 2.0 g/L group, 10.2 ± 0.42%; 5.0 mg/L, 20.44 ± 1.24% [p < 0.01]; except 0.5 mg/L group, 5.8 ± 0.32% [p > 0.05]). Western blot assays indicated that the Tan IIA decreased Bcl-2, HPV 16 E6 and E7 protein levels, but elevated Bax and cleaved-Caspase-3 expression in cells. Tan IIA may inhibit the proliferation and induce the apoptosis of C4-1 cells in a concentration-dependent manner.
Introduction
Tanshinone IIA (Tan IIA) is an ingredient extracted from Salvia miltiorrhiza, a Chinese herb that has been used as a therapy for cardiovascular diseases [Citation1]. Tan IIA may protect myocytes against oxidative stress and inflammation, and has been extensively applied in the therapy of coronary heart disease and angina [Citation2,Citation3]. Historically, Traditional Chinese Medicine (TCM) combined with conventional treatment of cancer plays an important role in protecting patients against treatment complications and improving quality of life after treatment. Tan IIA is a potential pharmaco-therapeutic that may also be used as a cancer therapy [Citation4]. Recent studies revealed that Tan IIA may reverse the malignant phenotype of cancer cells by compromising migration and invasion properties [Citation5]. Evidence also shows that Tan IIA may inhibit the proliferation of cancer cells including breast, lung, liver, ovarian, osteosarcoma and leukemia [Citation6].
The World Health Organization reports that cancer is a major cause of death [Citation7]. In recent years, the development of genomic and proteomic approaches is driving cancer therapy into a new era of precision medicine, giving the most appropriate to the correct patient at the optimum time. In this context, different methods are required to diagnose and treat cancers based on their individual biological characteristics. Generally, anti-tumor effects of drugs result from the induction of apoptosis of cancer cells. Thus, it is important to determine the influence of traditional drugs on the apoptosis in cancer cells [Citation8,Citation9].
Epidemiological and clinical findings have shown that cervical cancer is closely related to the infection by high risk human papillomavirus (especially HPV16 and HPV18) [Citation10,Citation11]. In developing countries, cervical cancer is the second most common female malignancy and significantly threatens the health and life of women. Mortality resulting from cervical and other life-threatening cancers in women is higher than 85%. There is a critical unmet need for the development of novel therapies which could potentially be derived from unexplored sources of drugs such as Chinese herbs [Citation12].
Previous studies have indicated that Tan IIA could inhibit the proliferation of human cervical cancer lines including HeLa, CaSki, SiHa and C33a cells infected by HPV18 or HPV16, and induce their apoptosis [Citation13]. Additionally, it has been demonstrated that Tan IIA inhibits cervical cancer growth through suppressed expression of HPV E6 and E7 genes, and modulates related proteins including E6AP and E2FI [Citation14]. Our studies have shown that a lotion for external usage containing mainly extracts of Salvia miltiorrhiza could inhibit HPV mRNA expression [Citation15]. However, each tumor cell line is a unique system due to tumor heterogeneity, so the influence of Tan IIA on the HPV18 positive cervical cancer cell line C4-1 is still unclear and yet to be reported. This study aimed to investigate the effect of Tan IIA on the proliferation and apoptosis of HPV18 positive cervical cancer C4-1 cells. Our findings may provide theoretic evidence directly supporting Tan IIA as a potential therapeutic in cervical cancer.
Materials and methods
Materials
Tan IIA (purity with 99.2% HPLC) was acquired from the Chinese Food and Drug Administration. Dimethylsulfoxide (DMSO), L-glutamine and antibiotics were obtained from Sigma-Aldrich Co (St Louis, MO, USA). All reagents were of analytical grade. Tan IIA was diluted with DMSO to a concentration of 10.0 mg/L and preserved at −20 °C for further use. The indicated concentrations were 0.5, 1.0, 2.0 and 5.0 mg/L and the control group used 0.9% saline solution according to the previous reports [Citation16].
Cell culture and treatments
C4-1 cells lines were purchased from the Cell Culture Centre, the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. The cells were cultured in DMEM containing 10% fetal bovine serum (FBS) (Invitrogen, USA) at 37 °C in a humidified environment with 5% CO2. The culture medium was refreshed once every 2-3 days and cells were passaged at a ratio of 1:3 whereby C4-1 cells in the logarithmic growth phase were harvested and re-suspended at a density of 1.0 × 107/L. The cultured cells were divided randomly into two groups: a control group (blank control), a treatment group with additional Tan IIA at different concentrations as described above. Cell viability and proliferation curve was determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Cell counts were carried out at days 2, 3, 4, 5, 6 and seven after seeding. Briefly, cells were added to 96-well plates (100 μL/well) and allowed to adhere. The culture medium was removed and the cells were treated with Tan IIA at different concentrations for 24 h. Cell growth was observed under an inverted microscope (Olympus, IX71) and the cells were photographed. Then, 20 μL of 5 mg/mL MTT (Funakoshi Co., Tokyo, Japan) was added to each well, followed by incubation in the dark at 37 °C for 4 h. The supernatant was then removed and 150 μL of DMSO was added to each well. After further incubation for 10 min with constant shaking, the absorbance (A) at 450 nm was measured with an Infinite F50 microplate reader (Tacan, Mannedorf, Switzerland) and the cell proliferation inhibition rate (CPIR) was calculated according to the following formula: CPIR = 1−(A treated−Ablank)/(Acontrol−Ablank), where Atreated, Acontrol and Ablank are the cell absorbance in the treated group, control group (cells cultured with no drugs) and blank group (no cells plated), respectively. A total of seven replicate wells were measured in each group. Three independent repeats were performed for all experiments.
Detection of cell apoptosis by flow cytometry
After Tan IIA treatment for 12 h, cells in the different groups were harvested (1 × 106/group) and then fixed with 70% cold ethanol overnight at 4 °C. After centrifugation and staining with Annexin V-FITC (fluorescein isothiocyanate), and propidium iodide (PI) (Invitrogen Life Technology, USA), cells were incubated in the dark for 30 min and then analyzed using the flow cytometry (Becton Dickinson, San Joe, CA, USA). PI was used for cell nucleus staining and FITC for cytomembrane staining. Data were analyzed using WinMDI software (Version 2. 8).
Detection of protein expression by Western blot assay
Cells were treated with the indicated concentrations of Tan IIA for 12 h and harvested by centrifugation. Total protein was extracted from the cells using RIPA cell lysis buffer (Invitrogen Life Techonology, USA) on ice, followed by centrifugation (4730 g, 5 min). The cell lysates were collected and protein concentration was determined using the BCA protein method kit (Beyotime Institute of Biotechnology, Shanghai, China). Equal amounts of protein (50 μg) were boiled and subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (10 μL/sample) at 200 V for 1.5 h. Proteins were then transferred onto a polyvinylidene fluoride (PVDF) membrane (EMDMillipore, Billerica, MA, USA) at 200 mA for 2 h. Then, the membrane was blocked in 5% non-fat milk at 37 °C for 2 h. After washing in PBST (phosphate buffered saline containing 0.05% Tween20) three times (5 min in each), the membrane was incubated with a 1:1000 dilution of goat anti-human monoclonal BCL-2 (cat. no. sc-56015), anti-Bax (cat. no. sc-4239), anti-cleaved-Caspase-3 (cat. no. sc-4239) and anti-HPV E6/E7 (Santa Cruz Biotechnology Inc., Danvers, MA, USA) in addition to a GAPDH (glyceraldehyde 3-phosphate dehydrogenase) antibody (Cell signaling Technology Inc., Danvers, MA, USA), at 4 °C overnight. GAPDH was utilized as the internal loading control. Following washing three times in PBST (5 min in each), the membrane was treated with horseradish peroxidase (HRP)-conjugated mouse anti-goat secondary antibodies at room temperature for 2 h. After washing in PBST three times (5 min in each), the membrane was visualized with ECL substrate (Thermo Fisher Scientific, Waltham, MA, USA) and protein bands were scanned using an ECL Imager. The protein expressions of target genes were normalized to that of GAPDH control and protein expression compared between the Tan IIA treated and control groups. Quantitative analysis of the relative levels of target proteins was performed using the NIH ImageJ software (version 4. 0; National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed with SPSS version 10.0. Data are expressed as values with standard deviation (±SD). Qualitative and quantitative data were comparable by chi-square test and t-test. Analysis of variance (one-way ANOVA) and post hoc Bonferroni multiple comparisons test was utilized to compare differences between groups. A value of p < 0.05 was considered as statistically significant.
Results and discussion
We determined the effect of Tan IIA on the proliferation and growth of C4-1 cells through 7 days by MTT assay. After treatment with Tan IIA at different concentrations for 24 h, the MTT assay was performed and the absorbance was measured in the different groups at different times to draw a curve of drug dose versus cell proliferation inhibition rate. After Tan IIA treatment, the cell proliferation decreased significantly and the growth inhibition increased in a dose dependent manner (). A significant difference in growth inhibition was observed between the Tan IIA and the control group (0.5–1.0 mg/L Group: p < 0.05; 2.0–5.0 mg/L Group: p < 0.01), suggesting that Tan IIA may significantly inhibit the proliferation of cervical cancer cells in a dose dependent manner (.
Figure 1. Effect of different concentrations of Tan IIA on proliferation and morphology of C4-1 cells. Cell growth inhibition (A) and morphology (B) of C4-1 cells.
Note: C4-1 cells were grown in DMEM with or without the indicated concentrations of Tan IIA for 5 days and were plated in 96-well plates. (A) Cell proliferation inhibition rate (CPIR) was calculated on day 5 based on MTT assay results. Data are expressed as mean values from three independent experiments. #P < 0.05, *P < 0.01 vs. the control, determined by ANOVA and post hoc Bonferroni multiple comparisons test. (B) Morphology of C4-1 cells with or without Tan IIA on day 5 was observed using inverted microscopy (magnification 50×).
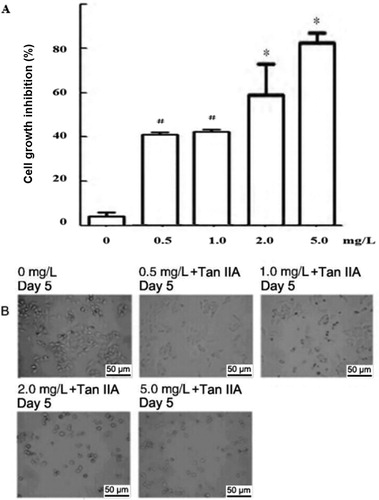
Table 1. Effect of Tan IIA treatment on proliferation of C4-1 cells.
Following treatment with Tan IIA at different concentrations, cell morphology was observed under an inverted microscope. As shown in , distinct differences in shape were observed between Tan IIA-treated cells and the control group. In the Tan IIA-treated groups with different concentrations at different time points, the total volume of cells decreased. Also, the intercellular junctions disappeared, the cells became detached from each other and the cell growth was reduced compared to the control group. These data illustrated that Tan IIA induced cell jury in a dose-and time-dependent manner.
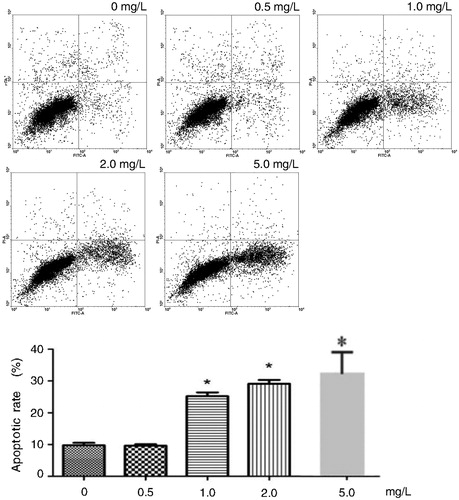
The results of the flow cytometry analysis showed that the rate of apoptosis increased markedly compared to the control group after treatment with Tan IIA at different concentrations for 12 h. There was a significant difference between the Tan IIA-treated and the control group. In the control group, the rate of apoptosis in C4-1 cells was 2.56 ± 0.21%, the apoptosis rate in the Tan IIA-treated groups at different concentrations increased markedly (1.0 mg/L group, 7.9 ± 0.43% [p < 0.05]; 2.0 mg/L group, 10.2 ± 0.42%; 5.0 mg/L, 20.44 ± 1.24% [p < 0.01], except 0.5 mg/L group, 5.8 ± 0.32% [p > 0.05]) ().
Figure 2. Effects of different concentrations of Tan IIA on apoptosis of C4-1 cells.
Note: Cell apoptosis was assayed by flow cytometry using PI and FITC staining.
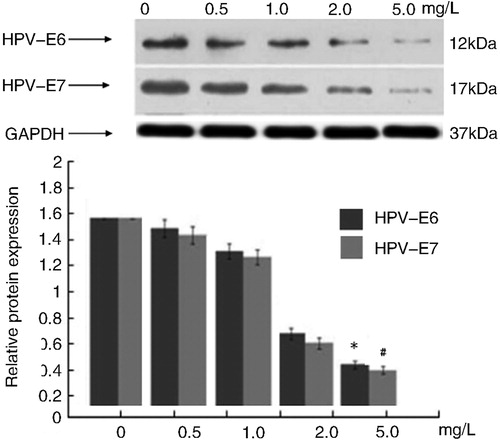
To further characterize the molecular mechanisms involved in the response to Tan IIA, the expressions of five proteins were determined by Western blot. Quantitative analysis of results indicated that after treatment with Tan IIA at different concentrations for 12 h, the protein expression of Bcl-2 decreased significantly (p < 0.05 vs. the control); however, the protein expression of Bax and cleaved-caspase-3 increased dramatically compared with the control group (p < 0.01). These data indicated that Tan IIA could increase cleaved caspase-3 induced cell apoptosis (). Additionally, the effect of Tan IIA treatment on HPV16 E6 and E7 oncogenes in C4-1 cells indicated that the drug caused significant decreases in HPV16 E6 and E7 protein levels ().
Figure 3. Effects of different concentrations of Tan IIA on expression of Bcl-2, Bax and Caspase-3 proteins in C4-1 cells.
Note: Protein expression levels were detected by Western blotting. GAPDH was used as internal control and normalized to 100%. The results were similar in three separate experiments. Quantitative analysis of the relative levels of target proteins was done using the NIH ImageJ software. #P < 0.05, *P < 0.01 vs. the control.
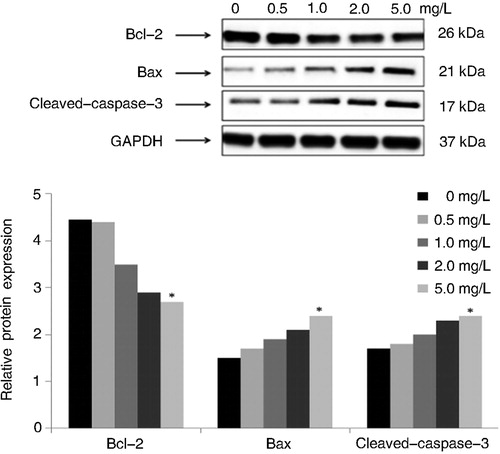
Figure 4. Effects of different concentrations of Tan IIA on protein expression of HPV E6/E7 in C4-1 cells.
Note: Protein expression levels were detected by Western blotting. GAPDH was used as internal control and normalized to 100%. The results were similar in three independent experiments. Quantitative analysis of the relative levels of target proteins was done using the NIH ImageJ software. P < 0.01 vs. the control.
S. miltiorrhiza is one of the most common Chinese herbs used in clinical practice which is extracted from the root of Salvia. Salvia has different subtypes, and their ingredients are also diverse [Citation16]. Tanshinone (Tan) is a diethyl ether or ethanol extract of Salvia root and the major effective ingredient of S. miltiorrhiza. Tan can be further divided into more than 15 types including TanI (I), Tan IIA (II), Tan IIB (III), cryptotanshinone (IV), isotanshinone I(V), isotanshinone IIA (IV), isocryptotanshinone (VII), hydroxytanshinone IIA (VIII), methyl tanshinonate (IX), miltirone(X), Salviol (XI), dihydrotanshinone I (XII), neotanshinone A (XIII), neotanshinone B (XIV) and neotanshinone C (XV) [Citation17]. Out of all these extracts, Tan IIA has natural anti-oxidant activity. In clinical cardiovascular pharmacology, Tan IIA has anti-atherosclerotic activity and is able to reduce myocardial infarction, decrease myocardial oxygen consumption and inhibit thrombosis and platelet aggregation [Citation18]. Moreover, there is evidence showing that Tan is a promising anticancer treatment. Studies have confirmed that Tan IIA induces apoptosis in cervical cancer cells including HL-60 cells and K562 [Citation19,Citation20]. Tan IIA possesses anti-tumor effects by inducing differentiation and apoptosis. The inhibitory effects were tested by in vitro cell proliferation experiments with several cell lines. Breast cancer, lung cancer, kidney cancer and cervical cancer were included among the most common cancers. Additionally, induction of autophagic cell death by Tan IIA also contributed to its anticancer activity. However, the exact mechanism remains to be fully elucidated [Citation21–23].
In the vast majority of cervical cancer biopsies and in many cell lines derived from these tumors, the DNA of specific types of HPV16 and HPV18 has been found in the majority of human genital carcinomas, such as HPV-16 DNA in the cell lines CaSki and SiHa, HPV-18 DNA in HeLa and C4-1. Since tissue culture systems susceptible to transformation by HPV are not presently available, these cell lines provide unique systems to study the expression of HPV16 and HPV18 genes in cells derived from human cervical carcinoma.
In the present study, we used an in vitro C4-1 cervical carcinoma cells model to evaluate the anticancer efficacy of Tan IIA in human cervial cancer C4-1 cells. We observed that Tan IIA inhibited the growth of C4-1 cells and caused apoptosis. Observation of cell morphology showed that Tan IIA significantly changed the morphology of C4-1 cells, and the MTT assay revealed that Tan IIA markedly inhibited the growth of C4-1 cells after treatment for 72 h. The responses were both time- and dose-dependent. Apoptosis plays a critical role in the survival, growth and pathogenesis of cancers. Apoptosis is mediated by at least three endogenous and exogenous pathways. Activation of the apoptotic pathway is a key factor by which cytotoxic herbs can kill tumor cells [Citation24]. The endogenous pathway is important for triggering apoptosis and its activation is related to the imbalance amongst Bcl-2 family members. When Bax phosphorylation is inhibited, the un-phosphorylated Bax may bind to BCL-XL or Bcl-2 to form heterodimers which have anti-apoptotic activity [Citation25]. This may trigger the imbalance amongst Bcl-2 family members. Then, holes form in the outer mitochondrial membrane and cytochrome C is released from the mitochondrial membrane gap. Finally, a caspase cascade is induced which is pivotal for the initiation of apoptosis [Citation26,Citation27].
The Bcl-2 family consists of appromixately 20 homologes of anti- and pro- apoptotic molecules. Many reports have shown that high expression levels of Bcl-2 and Bcl-XL proteins in cancer cells are resistant to drug-induced apoptosis. The caspases are a family of intracellular cysteine protease with specificity for aspartic acid residues. Caspases-3 is one of the most important executioner caspases which is capable of cleaving cellular substrates. Additionally, caspase-3, which mediates cell death, plays an important role in pathogenesis and response to therapy [Citation28,Citation29]. We noted that Tan IIA at different concentrations significantly increased the rate of early apoptosis in C4-1 cells. Further investigations showed Tan IIA reduced Bcl-2 expression and enhanced Bax expression, resulting in caspase-3 activation and leading to cell apoptosis. Our findings are in agreement with the previous report of Munagala et al. [Citation13], who observed the expression of Bcl-2 and survivin to be down-regulated whilst the expression of Bax to be up-regulated concurrently in CaSki, SiHa and C33a human cervical cancer cell lines. Generally, the expression levels of HPV-specific oncoproteins E6 and E7 are essential in maintaining the growth of cervical cancer. Previous studies have determined the inhibitory growth effect of Tan IIA on cervical cancer cells and provided some insights into targets related to apoptosis [Citation30,Citation31]. The similar patterns of HPV18 transcription in the different cervical carcinoma cell lines suggest a functional role of HPV18 early genes for the malignant phenotype of these cells [Citation32]. In this context, our findings showed that Tan IIA downregulated the expression of E6 and E7 oncogenes in the HPV-positive cell lines.
HPV E6 protein binds to p53 and stimulates its degradation through an ubiquitin dependent protease system [Citation33]. HPV E6 protein causes destabilization and disruption of Rb or the E2f repressor complexes. Our findings demonstrated that Tan IIA treatment caused suppression of HPV E6 and E7 oncogene expression. These results were in agreement with previous reports, indicating that Tan IIA exhibited strong inhibitory growth effects in C4-1 cells similar to CaSki cervical cancer cells via a different molecular axis involving p39 and JNK signaling [Citation34].
There are some limitations to the present study, although the significant growth inhibition of HPV-C4-cells was confirmed in vitro, changes in p53, pRb or E2f protein levels were not observed. Further studies are required to investigate the mechanisms underlying the anticancer effects of Tan IIA in C4-1 cells through a certain cell signaling pathway.
Conclusions
Our findings indicated that Tan IIA treatment significantly inhibited the growth of C4-1 cells in a time-and dose-dependent manner. Western blot assays indicated that the Tan IIA decreased Bcl-2, HPV 16 E6 and E7 protein levels, but elevated Bax and Cleaved-caspase-3 expression in cells. Tan IIA may induce apoptosis in human cervical cancer C4-1 cells and inhibited their growth. Caspase-3 activation may be one of the most important mechanisms underlying the Tan IIA induced apoptosis of C4-1 cells responsible for modifying the balance between proliferation and apoptosis.
Acknowledgements
This study was a part of National Natural Science Foundation of China project (81402979), and received financial support from the Health Developing Program in Jilin Province, China (20132099; 20180101142JC) and Science and Technology Research Projects of the Department of Education of Jilin Province, China (Contract No. 2011-132; JJKH 20190662KJ).
Disclosure statement
No potential conflict of interest was reported by the author(s). The authors alone are responsible for the content and writing of the paper.
Additional information
Notes on contributors
Ruowen Zhang
Ruowen Zhang designed the present study. Mingcheng Li performed the assay, and analyzed and interpreted the data. Siqi Duan wrote the draft manuscript. Jiayu Chen and Gang Wang revised the manuscript.
References
- Chen SJ. Drug-target networks for Tanshinone IIA identified by data mining. Chin J Nat Med. 2015;13(10):751–759.
- Yang R, Liu A, Ma X. Sodium tanshinone IIA sulfonate protects cardiomyocytes against oxidative stress-mediated apoptosisi through inhibiting JNK activation. J Cardiovasc Pharmacol. 2008;51(4):396–401.
- Liu X, Guo CY, Ma XJ, et al. Anti-inflammatory effects of tanshinone IIA on atherosclerostic vessels of ovariectomized ApoE mice are mediated by estrogen receptor activation and through the ERK signaling pathway. Cell Physiol Biochem. 2015; 35(5):1744–1755.
- Xu H, Hao YL, Xu LN, et al. Tanshinone sensitized the antitumor effects of irradiation on laryngeal cancer via JNK pathway. Cancer Med. 2018; 7(10):5187–5193.
- Johnson SB, Park HS, Gross CP, et al. Complementary medicine, refusal of conventional cancer therapy, and survival among patients with curable cancers. JAMA Oncol 2018;4:1375–1381.
- Su CC. Tanshinone IIA inhibits human gastric carcinoma AGS cell growth by decresing BiP, TCTP, Mc11 and BclxL and increasing Bax and CHOP protein expression. Int J Mol Med. 2014;34(6):1661–1668.
- Wang MZ, Feng RM, Wang S, et al. Clinical performance of Human Papillomavirus testing and visual inspection with acetic acid in primary, combination, and sequential cervical cancer screening in China. Sex Transm Dis. 2019;46(8):540–547.
- He L, Gu K. Tanshinone IIA regulates colorectal cancer apoptosis via attenuation of Parkin‑mediated mitophagy by suppressing AMPK/Skp2 pathways. Mol Med Rep 2018; 18(2):1692–1703.
- Qin J, Shi H, Xu Y, et al. Tanshinone IIA inhibits cervix carcinoma stem cells migration and invasion via inhibiting YAP transcriptional activity. Biomed Pharmaco Ther. 2018; 105:758–765.
- Gullett NP, Ruhul Amin ARM, Bayraktar S, et al. Cancer prevention with natural compounds. Semin Oncol. 2010;37(3):258–281.
- Kim DH, Shin EA, Kim B, et al. Reactive oxygen species-mediated phosphorylation of p38 signaling is critically involved in apoptotic effect of Tanshinone I in colon cancer cells. Phytother Res. 2018; 32(10):1975–1982.
- Zhang Y, Li S, He H, et al. Influence of Tanshinone IIA on apoptosis of human esophageal carcinoma Eca-109 cells and its molecular mechanism. Thorac Cancer. 2017; 8(4):296–303.
- Munagala R, Aqil F, Jeyabalan J, et al. Tanshinone IIA inhibits viral oncogene expression leading to apoptosis and inhibition of cervical cancer. Cancer Lett. 2015; 356(2):536–546.
- Li MC, Liu W, Shao WR. Progress of study on anti-human cervical papilloma virus infection with Chinese and Western medicine. Zhongguo Zhong Xi Yi Jie He Za Zhi 2007; 27(6):573–575. (Abstract in English).
- Cao YF, Wang SF, Li X, et al. The anticancer mechanism investigation of Tanshinone IIA by pharmacological clustering in protein network. BMC Syst Biol. 2018; 12(1):90.
- Zhang RW, Liu ZG, Li MC, et al. In vitro inhibition of invasion and metastasis in colon cancer cells by TanIIA. Genet Mol Res 2016; 15(3): 1–4. gmr.153039008.
- Chien SY, Kuo SJ, Chen YL, et al. Tanshinone IIA inhibits human hepatocellular carcinoma J5 cell growth by increasing Bax and caspase 3 and decreasing CD31 expression in vivo. Mol Med Rep 2012;5(1):282–286.
- Zhuang S, Cheng TH, Shih NL, et al. Tanshinone IIA induces heme oxygenase 1 expression and inhibits cyclic strain-induced interleukin 8 expression in vascular endothelial cells. Am J Chin Med. 2016; 44(02):377–388.
- Alzhrani RM, Alhadidi Q, Bachu RD, et al. Tanshinone IIA inhibits VEGF secretion and HIF-1α expression in cultured human retinal pigment epithelial cells under hypoxia. Curr Eye Res. 2017; 42(12):1667–1673.
- Eskander RN, Tewari KS. Chemotherapy in the treatment of metastatic, persistent, and recurrent cervical cancer. Curr Opin Obstet Gynecol. 2014;26(4):314–321.
- Dong W, Zhang Y, Chen X, et al. High-dose tanshinone IIA suppresses migration and proliferation while promoting apoptosis of astrocytoma cells via Notch-1 pathway. Neurochem Res. 2018;43(9):1855–1861. 208;
- Chen C, Wang HJ, Liu FR, et al. Preparation and antitumor effects of tanshinone IIA loaded albumin nanoparticles. Zhongguo Zhong Yao Za Zhi 2017; 42(4):696–701. (Abstract in English).
- Yun SM, Jeong SJ, Kim JH, et al. Activation of c-JunN-terminal kinase mediates tanshinone IIA-induced apoptosis in KBM-5 chronic myeloid leukemia cells. Bio Pharm Bull 2013; 36(2):208–214.
- Zaker A, Asili J, Abrishamchi P, et al. Cytotoxic and apoptotic effects of root extract and tanshinones isolated from Perovskiaabrotanoides Kar. Iran J Basic Med Sci. 2017; 20(12):1377–1384.
- Cui ZT, Liu JP, Wei WL. The effects of tanshinone IIA on hypoxia/reoxygenation-induced myocardial microvascular endothelial cell apoptosis in rats via the JAK2/STAT3 signaling pathway. Biomed Pharmacother. 2016; 83:1116–1126.
- Ren X, Wang C, Xie B, et al. Tanshinone IIA induced cell death via miR30b-p53-PTPN11/SHP2 signaling pathway in human hepatocellular carcinoma cells. Eur J Pharmacol.2017; 796:233–241.
- Zhu YQ, Wang BY, Wu F, et al. Influence of Tanshinone IIA on the apoptosis of human esophageal Ec-109 cells. Nat Prod Commun. 2016; 11(1):17–19.
- Hao W, Chen L, Wu LF, et al. Tanshinone IIA exerts an antinociceptive effect in rats with cancer-induced bone pain. Pain Physician. 2016; 19(7):465–476.
- Wang N, Chang Y, Chen L, et al. Tanshinone IIA protects against chronic intermittent hypoxia-induced myocardial injury via activating the endothelin 1 pathway. Biomed Pharmacother. 2017; 95:1013–1020.
- Huang J, Lin H, Hong Y. In vitro anti-tumor activity of the tanshinone IIA against SKOV3 cells. Nat Prod Res. 2016; 30(16):1844–1846.
- Park YK, Obiang-Obounou BW, Lee J, et al. Anti-adipogenic effects on 3T3-L1 Cells and zebrafish by tanshinone IIA. Int. J. Mol. Sci. 2017;18(2065): ijms18102065/1-24.
- Schneider-Gädicke A, Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986; 5(9):2285–2292.
- Feng FB, Qiu HY. Neuroprotective effect of tanshinone IIA against neuropathic pain in diabetic rats through the Nrf2/ARE and NF-κB signaling pathways. Kaohsiung J Med Sci. 2018; 34(8):428–437.
- Chao A, Lin CT, Lai CH. Updates in systemic treatment for metastatic cervical cancer. Curr treat Options in Oncol. 2014;15(1):1–13.
