Abstract
Acromegaly is a rare disease with significant social and economic burden considering both main treatment patterns and concomitant diseases. The aim of this study was to present a systematic overview of the economic and pharmaco-economic studies of acromegaly treatment. A systematic search in PubMed and Google Scholar was performed following the validated five-step approach for preparing a systematic review of economic evaluations for informing evidence-based healthcare decision on the basis of predefined inclusion and exclusion criteria. To perform critical analysis of the selected articles, the costs on macrolevel and microlevel were extracted and inflated to 2018 for the purposes of their comparison. Of 100 studies, 33 ones were included in the present analysis, most of them being cost-of-illness studies (12), followed by cost-effectiveness analysis applying Markov modeling or decision tree models (9). The total costs for acromegaly patients in different countries as a percent of the gross domestic product (GDP) vary in the range between 0.00001% and 0.003%. The annual direct and indirect costs per patient vary in the range between 3,788 € and 93,970 € as a result of differences in the type of calculations. Acromegaly is a high-consuming rare disease, so it is urgent to provide economically based evidence in every country for the purposes of making the most suitable policy decisions in the healthcare sector.
Keywords:
Introduction
Acromegaly is a rare debilitating disease caused by increased levels of growth hormones (GH) and insulin-like growth factor 1 (IGF-1) mainly as a result of a GH-secreting pituitary adenoma [Citation1,Citation2]. It affects between 2.8 and 13.7 cases per 100,000 people and the incidence is between 0.2 and 1.1 cases per 100,000 [Citation3].
Acromegaly patients are characterized not only with the specific acral and soft tissue overgrowth but also with development of metabolic dysfunction (insulin resistance, elevated glycated hemoglobin, etc.) [Citation1]. Mortality and morbidity of various cardiovascular diseases are significantly increased as hypertension is highly prevalent (in > 40% of acromegaly patients) [Citation4], which undoubtedly poses a significant economic burden to any health insurance fund. Comorbidities should be closely monitored and treated appropriately in order to reduce the morbidity levels [Citation5]. Moreover, some medications (somatostatin receptor ligand) for acromegaly have negative impact on glucose levels, which could worsen patients’ condition. Therefore, blood glucose and glycated hemoglobin levels should be strictly monitored and managed according to individual response [Citation5].
Appropriate and effective management of acromegaly and associated complications leads to decreased morbidity and mortality as well as improved quality of life [Citation4]. Therefore, it is crucial to achieve long-term biochemical control, which is defined as a main therapeutic goal [Citation1]. Taking into consideration not only the efficacy but also the cost-effectiveness of the available therapeutic strategies (surgery, radiotherapy, medicines with various mechanism of action) could lead to significantly better therapeutic outcomes. However, there is not enough economic evidence on the cost-effectiveness of different options, which could be highlighted as a challenge for the best choice of medicines for acromegaly patients [Citation5]. Economic evidence should be taken into consideration as a crucial instrument for the purposes of decision making in the healthcare sector. Thus, the financial impact of particular diseases and innovative treatments could be identified and the scarce resources could be reallocated better. Rare diseases are an area with a lot of challenges due to the inadequate funding especially in low- and middle-income countries. Moreover, for the rare diseases, as it is the case with acromegaly, much more evidence is needed so as to make the best resource allocation decisions.
Published systematic or literature reviews have focused more on the cost-effectiveness of particular medicines (pegvisomant or somatostatin analogues) for acromegaly [Citation6,Citation7] but few combine all studies regarding the financial burden of acromegaly. Therefore, we undertook the present study so as to critically appraise, consolidate and present an overview of the scientific economic and pharmaco-economic literature focused on acromegaly treatment performing a detailed systematic literature review in the scientific databases. The findings could be informative for the society about the impact of acromegaly and its comorbidities on the total costs for rare diseases.
Materials and methods
Study design
A comprehensive systematic literature review of the published economic studies on the financial burden of acromegaly was performed. It was based on the developed and validated five-step approach for preparing a systematic review of economic evaluations for informing evidence-based healthcare decisions: (1) initiating a systematic review; (2) identifying (full) economic evaluations; (3) data extraction, risk of bias and transferability assessment; (4) reporting results; (5) discussion and interpretation of finding [Citation8].
Inclusion and exclusion criteria
Internet-based search of studies concerning direct and indirect costs associated with acromegaly and its concomitant diseases was carried out for the aims of the present study. Systematic review was conducted in the Internet-based scientific databases PubMed and Google Scholar. The keywords used were: cost of illness, acromegaly, financial burden, direct costs, indirect costs and others described below in the text. Moreover, abstracts from some distinguished scientific meetings in the area of endocrinology and economics were also reviewed. A particular consistency was followed:
Defining the study question: What are the economic and pharmaco-economic aspects of acromegaly treatment?
Input the keywords – ‘cost of illness’ OR ‘economic burden’ OR ‘cost effectiveness analysis’ OR ‘cost utility analysis’ OR ‘cost benefit analysis’ OR ‘cost-minimization analysis’ OR ‘cost outcome descriptions ‘OR ‘cost descriptions’ AND ‘acromegaly’.
The search for the available studies was initiated in the following databases: PubMed and Google Scholar with no limitation of the year of publication.
The studies were analyzed and duplicated studies were withdrawn from the analysis. Studies were selected only if they meet the following inclusion criteria: (1) The studies are observational, retrospective or prospective using bottom-up or top-down approach, incidence-based or prevalence-based; already published systematic reviews were also included; (2) Full economic evaluations (Cost-Effectiveness Analysis (CEA), Cost-Benefit Analysis (CBA), Cost-Utility Analysis (CUA) and Cost-Minimization Analysis (CMA)) or partial economic evaluations (Cost comparison/cost analysis, Cost outcome descriptions, Cost descriptions and Cost-of-illness (CoI) studies); (3) The subjects in the studies were acromegaly patients; (4) No limitation in the sample size was set; (5) Studies were in English; (6) Regardless of publication status (published, unpublished, in press, or in progress). The exclusion criteria were: (1) duplicated studies; (2) language other than English; (3) studies covering patients who do not suffer from acromegaly but from gigantism or other endocrine disorders; (4) articles which identified resource utilization but did not calculate the relevant cost; (5) articles which present only clinical outcomes, utility scores or health-related quality of life; (6) no full text available or lack of enough information in the abstract valuable for analysis.
The technological scheme PRISMA Flow Diagram was applied. This diagram presents the flow of information during different phases of the systematic review. It shows the number of the identified, included and excluded studies and the reasons for exclusion as well.
Selection of studies
Two authors (MK and YR) independently assessed the studies resulting from the searches. First, duplicated studies were removed from the list of identified articles. The copies of all relevant studies which met the inclusion criteria were reviewed. The full-text papers were read independently and, after discussions regarding their eligibility, we excluded those that did not meet the inclusion criteria. The selection was based on the titles and abstracts as well as on the basis of the predefined eligibility criteria.
Data extraction and summarization
The data from the relevant studies were extracted and summarized in tables including the following information: publication year and name of the first author, country of study, participants (sample size), types of calculated costs, time horizon, type of economic analysis performed, interventions compared and types of outcomes used where it was applicable for the purposes of economic evaluation.
Data analysis
Following the methodology of a similar study focused on the economic burden of cardiovascular diseases in type 2 diabetes [Citation9] and the five-step approach developed and validated by van Mastrigt et al. (2016) [Citation10], the costs were inflated to 2018 values taking into consideration local inflation rates and were converted in euro so as to be comparable. A free web-based tool for adjusting estimates of cost expressed in one currency and price year to a specific target currency and price year ‘CCEMG – EPPI-Centre Cost Converter’ (v.1.6 last update: 29 April 2019) was used. These costs were analyzed and the average costs per patient and the total annual financial burden of acromegaly was revealed and presented.
Assessment of the risk of bias in the included studies
The methodology quality of selected studies was assessed on the basis of the Consensus on Health Economic Criteria (CHEC) checklist [Citation11]. It is a single checklist consisting of 19 ‘yes’ or ‘no’ questions used to evaluate mainly full but also partial economic evaluations. In case of not enough information available, a ‘no’ point should be given for this category [Citation12]. The maximum score which could be attained is 19 for all categories. It is not applicable for studies based on modeling or scenario-analysis. Therefore, we applied the checklist only for cost of illness, cost effectiveness, cost minimization, cost utility, cost consequences and budget impact analysis.
Results and discussion
As a result of the literature search in both databases (PubMed and Google Scholar) and other paper-based sources, 100 studies were found. Forty-five articles were removed due to duplications. Titles and abstracts were screened for consistence with the pre-defined criteria. Twenty-two papers met the exclusion criteria and were excluded. The remaining 33 studies were finally included for data extraction and summarization: 15 out of 33 were conference abstracts and 18, full-text articles. The flow of the search process and selection of studies is presented in .
Figure 1. PRISMA flow diagram [Citation13].
![Figure 1. PRISMA flow diagram [Citation13].](/cms/asset/fa72e076-1d09-44bd-8bd5-eaa1b5f0f7b8/tbeq_a_1680317_f0001_c.jpg)
The studies that were included in this systematic review are listed in along with all required information described in the methology section. Studies carried out in Belgium (1 study), Brazil (3 studies), Bulgaria (2 studies), Canada (2 studies), China (1 study), Colombia (1 study), Finland (1 study), France (1 study), Greece (1 study), Italy (2 studies), Mexico (1 study), Netherlands (1 study), Poland (2 studies), Spain (4 studies), Sweden (2 studies), Turkey (1 study), the United Kingdom (3 studies) and the Unites States (4 studies) were selected and presented in the present review. Most of the studies are cost-of-illness studies (12 studies), followed by cost-effectiveness analysis applying Markov modeling or decision tree models (9 studies), cost effectiveness (5), cost consequences studies (4), cost-utility studies with modeling approaches (2), budget impact analysis (2), cost minimization (1), cost analysis (1), cost utility analysis (1). Some of the studies include more than one type of analysis: cost effectiveness and cost utility [Citation6,Citation22,Citation31] or cost minimization and budget impact analysis [Citation27] or cost effectiveness and budget impact analysis [Citation30]. Pegvisomant, octreotide and lanreotide as well as combination or sequential therapies (with lanreotide and octreotide LAR or vice versa [Citation20]) are the preferred interventions analyzed and compared to each other or to surgical treatment. Abrams et al. [Citation17] compares two regimes of lanreotide depot once every 4 weeks versus lanreotide depot every 6 weeks so as to analyze whether and when the next dose could be administered on a larger period of time. The cost-effectiveness of innovative treatments such as pegvisomant is tested and demonstrated as it could be a dominant alternative in comparison with octreotide LAR [Citation30]. Pasireotide cost effectiveness is also proved through five state Markov model with 30-year time horizon as it is a dominant alternative in comparison with the existing standard care [Citation31]. Cost-of-illness studies demonstrate the significant economic burden of acromegaly for different countries and form different perspectives and, logically, the complications are common and increase utilization and cost in acromegaly patients.
Table 1. Characteristics of the studies included in the systematic review.
presents results on micro-level regarding costs paid per one acromegaly patient. As it can be seen, direct costs (lab tests, medicines, visits, hospitalizations, diagnostic procedures, etc.) are included in all studies, whereas the indirect costs presented as loss of productivity due to acromegaly, unemployment, assistance to perform household chores and family member loss of income are calculated only in a few studies [Citation26,Citation37,Citation42]. This could be highlighted as a strong limitation especially in cost-of-illness studies where the societal perspective is broader and it is usually taken into account. In most studies, the costs are represented as total costs without strict differentiation by subtypes which interferes with the precise discussion and analysis. The annual costs per patient vary in the range between 3,788 € and 93,970 € as a result of differences in the type of calculated costs whether direct or indirect. Moreover, pricing medicines and other healthcare resources lists and treatment patterns and associated therapeutic schemes differ from country to country, which also led to a great variation in the costs. The perspective of the studies, which determines the type of identified resources and calculated costs, is mainly of the national insurance fund or of the society.
Table 2. Economic burdens presented as annual per patient costs of acromegaly treatment.
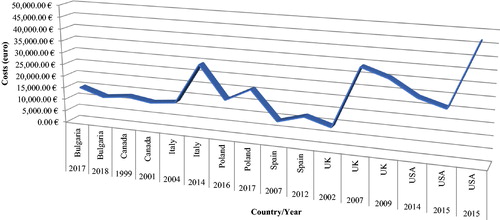
The annual per-patient costs are on the increase for the observed period of time in each country (): in Italy there is an increase from around 12,400 € in 2004 [Citation16] to around 29,260 € in 2014 [Citation28]; in Poland: from 15,573 (in 2016) [Citation35] to 20,911 € (in 2017) [Citation39]; in the United States the costs changed from around 20,000 € (2014–2015) [Citation29,Citation33] to around 93,900 € (in 2015) [Citation34]; in Spain, from 8,972 [Citation18] to 12,033 € [Citation25] for the period 2007–2012; in the UK, from 8,875 [Citation6] to around 30,000 € [Citation22] for the period 2002–2009. It should be noted that the last study carried out in the United States and included in the present review resulted in lower costs in comparison with 2017 because it takes into account only indirect costs [Citation34]. In Bulgaria, the costs differ for both years 2017 and 2018, being lower in 2018, which could be attributed to different methodology: the 2017 study applied macrocosting [Citation40], whereas the 2018 study used a microcosting approach [Citation43].
Figure 2. Annual per patient costs in different countries inflated and based on 2018 exchange rates.
Six studies reported the population-level burden of acromegaly, as the total annual costs were calculated. For the purposes of performing a comparison between the countries, we calculated the costs as a percent of the gross domestic product (GDP) value for 2018 (). The costs as a percent of GDP vary in the range between 0.003% and 0.00001%. However, the results should be analyzed with caution because some of the presented studies consider only direct medical costs associated with supplying and administration of medicines, whereas others calculate additional costs (lab tests, surgical procedures, indirect costs, etc.). Moreover, the healthcare systems in these countries differ significantly, as well as the level of outcomes and their economic development.
Table 3. Economic burden presented as annual acromegaly costs in absolute value and as % of GDP.
The methodological quality of the reviewed economic studies (excluding models) was assessed using CHEC checklist and the scores for each study are included in . Most of the studies were only abstracts with insufficiently included information regarding the way of cost valuation or measuring the outcomes. Ethical and distributional issues as well as ethic statements were also missing in 86% of the studies. The main limitation of a great number of studies was the lack of strict identification of the type of economic evaluation whether it is cost analysis, cost consequences or cost of illness. The relevant perspective was also not very clearly stated (43%). However, we can conclude that all reviewed and summarized studies are of relatively good quality, as the adjusted average scores for all studies is 10, which is more than half of the maximum 19 points. Different costs were also reported in different studies, e.g. only 3 out of 33 presented the indirect costs taking into consideration the societal perspective [Citation26,Citation37,Citation42].
The present review identified a wide range of studies regarding the costs and outcomes in a specific group of patients, those diagnosed with the rare disease acromegaly, over a period of almost 20 years (from 1999 till 2019). Not surprisingly, the number of economic evaluations for acromegaly patients has been increasing in recent years as new treatments become available and enter the market. The studies found in the literature focus mainly on the cost-effectiveness of these innovative therapies. There are some high-quality systematic reviews about cost-effectiveness analysis of these therapies [Citation7,Citation45] in recent years. The number of cost-of-illness studies, especially in some regions such as Central and Eastern European countries (CEEC) are insufficient so as to describe the full economic burden of acromegaly for the society. Cost-of-illness studies or cost analyses describing the economic burden of acromegaly in the CEEC regions have been published only for Poland and Bulgaria. However, the Bulgarian studies are only published as abstracts and do not present the types of costs, resources consumed and overall financial burden of the disease for the Bulgarian settings in details [Citation40,Citation43]. Therefore, a further step could involve more detailed economic evaluations as well as initiation of common research among the countries from the CEEC region. Economic evaluation studies of acromegaly with high methodological quality have been performed in the United States, the United Kingdom, Italy and other countries from Western Europe, as they precisely inform the society about the significant financial burden of acromegaly and its comorbidities [Citation6,Citation16,Citation18,Citation21,Citation22,Citation25,Citation26,Citation28,Citation29,Citation32–34,Citation42,Citation44]. Sliwczynski et al. [Citation35] revealed that comorbidity is associated with approximately 5 times higher costs, which highlights the importance of providing strict control over the main disease and the concomitant diseases. Broder et al. [Citation29] reported that the complications in acromegaly patients are common and this could significantly increase utilization and cost in acromegaly patients. Logically, ensuring adequate control of the acromegaly patients leads to savings for Italian Healthcare Service in comparison to poor control of patients [Citation16]. Giving scientifically-based evidence about the real economic burden of acromegaly and its complications and comorbidities, better control and management of the condition could be provided through optimization of financial resources. Therefore, the clinical and economic burden of acromegaly could be reduced.
A strength of the present study is that it combines results from various economic evaluation research on acromegaly and compares the direct or indirect costs as a percent of GDP, showing that the costs vary in a wide range between 0.00001% and 0.003%. As it was mentioned previously, these results should be analyzed with caution because of the heterogeneity of the studies included. Moreover, the healthcare systems, the level of outcomes and the economic development differ significantly around the world. Another strength is that for the first time, the annual costs per patient with acromegaly were compared on the basis of the already published data from different countries after inflating their values to 2018 values and converted in a common currency so as to be comparable. The obtained wide variation range of the costs, i.e. between 3,788 € and 93,970 € (), could be explained with some differences in the type of calculated costs (direct and/or indirect), pricing medicines and other healthcare resources lists and treatment patterns and associated therapeutic schemes that differ from country to country. Therefore, further common studies could be carried out so as to apply a common methodology and to obtain more sophisticated and significant results for the costs values.
The main limitations of the present review are related to several aspects. First, some older analyses performed before 1999 might have been missed because we narrowed the search mainly to on-line based sources. Second, risk of bias assessment was not performed for some studies applying modeling approaches for economic evaluation due to lack of appropriate assessment methods. Another limitation of the present systematic review is that it is not based on a previously developed and published protocol, as it is required according to step 1 of the 5-step approach [Citation8]. Moreover, the methodologies of the included studies differ significantly; for some of them the methods for costs valuation are not clearly stated, which might seriously interfere with the final conclusion regarding the comparison of the annual cost per patient and the annual total costs. What was shown, however, is that acromegaly treatment consumes significant resources and requires specific management and health policy decisions by the national health insurance funds.
Conclusions
Acromegaly being a rare conditions, there are limited financial resources for its treatment especially in the low- and middle-income countries. To meet the expectations of society for adequate financial coverage of expensive therapies by the public funds, there should be more economic evaluations about acromegaly patients based on a high-quality. Particularly, there is a need of studies including a wider range of consumed resources, considering the burden of concomitant diseases and testing the efficiency of available therapeutic options. Such studies could inform the healthcare decision makers and the society and could bring ideas for efficient reallocation of the limited resources. This systematic review attempts to summarize the available knowledge regarding the economic and pharmaco-economic aspects of acromegaly treatment and to present what the acromegaly management costs variation among different countries is. The results should be very carefully analyzed taking into account the heterogeneity of the summarized and reviewed studies. However, it is more than clear that acromegaly is a high-consuming rare disease and it is urgent to provide economically based evidence in every country for the purposes of making the most suitable policy decisions in the healthcare sector.
Authors’ contributions
All the authors provided valuable contributions to the manuscript. MK and YR performed the research and wrote the paper. SV, AE, SZ, ZM, MD, GP reviewed the paper. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Melmed S, Bronstein M, Chanson P, et al. A consensus statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018;14(9):552–561.
- Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119(11):3189–3202.
- Lavrentaki A, Paluzzi A, Wass JA, et al. Epidemiology of acromegaly: review of population studies. Pituitary. 2017;20(1):4–9.
- Melmed S, Casanueva FF, Klibanski A, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary. 2013;16(3):294–302.
- Giustina A, Chanson P, Kleinberg D, et al. A consensus on the medical treatment of acromegaly. Nat Rev Endocrinol. 2014;10(4):243–248.
- Moore D, Meads C, Roberts L, et al. The effectiveness and cost-effectiveness of somatostatin analogues in the treatment of acromegaly. Birmingham: West Midlands Health Technology Assessment Collaboration (WMHTAC). DPHE Report No. 37; 2002.
- Connock M, Adi Y, Bayliss S, et al. The clinical effectiveness and cost-effectiveness of pegvisomant for the treatment of acromegaly: a systematic review. In: Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet]. York (UK): Centre for Reviews and Dissemination (UK); 2007. Available from: https://www.ncbi.nlm.nih.gov/books/NBK73672/
- van Mastrigt GA, Hiligsmann M, Arts JJ, et al. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: a five-step approach (part 1/3). Expert Rev Pharmacoecon Outcomes Res. 2016;16(6):689–704. doi:10.1080/14737167.2016.1246960
- Einarson T, Acs A, Ludwig C, et al. Economic burden of cardiovascular disease in Type 2 Diabetes: a systematic review. Value Health. 2018;21(7):881–890.
- van Mastrigt G, Hiligsmann M, Arts J, et al. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: data extraction, a five-step approach (part 1/3). Expert Rev Pharmacoecon Outcomes Res. 2016;16(6):689–704.
- Evers S, Goossens M, de Vet H, et al. Criteria list for assessment of methodological quality of economic evaluations: consensus on Health Economic Criteria. Int J Technol Assess Health Care. 2005;21(2):240–245.
- Walker DG, Wilson RF, Sharma R, et al. Best practices for conducting economic evaluations in health care: a systematic review of quality assessment tools. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012. Available from: https://www.ncbi.nlm.nih.gov/books/NBK114550/
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta- analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Wilson LS, Shin JL, Ezzat S. Cost-effectiveness of acromegaly treatments. Value Health. 1999;2(5):369.
- Wilson LS, Shin JL, Ezzat S. Longitudinal assessment of economic burden and clinical outcomes in acromegaly. Endocr Pract. 2001;7(3):170–180.
- Didoni G, Grottoli S, Gasco V, et al. Cost-of-illness study in acromegalic patients in Italy. J Endocrinol Invest. 2004;27(11):1034–1039. doi:10.1007/BF03345306
- Abrams P, Alexopoulou O, Abs R, et al. Optimalization and cost management of lanreotide-Autogel therapy in acromegaly. Eur J Endocrinol. 2007;157(5):571–577.
- Luque-Ramírez M, Paramo C, Varela da Costa C, et al. Cost of management of invasive growth hormone-secreting macroadenoma. J Endocrinol Invest. 2007;30(7):541–545.
- Valentim J, Passos V, Mataveli F, et al. Cost-effectiveness analysis of somatostatin analogues in the treatment of acromegaly in Brazil. Arq Bras Endocrinol Metab. 2008;52(9):1452–1460.
- Salinas Escudero G, Idrovo J, Rivas R, et al. Economic evaluation of long-term somatostatin analogs in the treatment of acromegaly in Mexico: monotherapy vs sequential therapy. Value Health. 2009; Rio Abstracts, A506.
- Biermasz NR, Roelfsema F, Pereira A, et al. Cost–effectiveness of lanreotide Autogel® in treatment algorithms of acromegaly. Expert Rev Pharmacoeconomics Outcomes Res. 2009;9(3):223–234.
- Moore D, Adi Y, Connock MJ, et al. Clinical effectiveness and cost-effectiveness of pegvisomant for the treatment of acromegaly: a systematic review and economic evaluation. BMC Endocr Disord. 2009;9(1):20.
- Fujii RK, Mould JF, Fernandes RA, et al. Economic evaluation of pegvisomant for active acromegaly patients who failed available therapies in Brazil – Public health care system perspective. Value Health. 2012;15(4):A105.
- Alfonso-Cristancho R, Herran Diazgranados S, Maestre Martinez K, et al. Cost-effectiveness of somatostatin analogues for the treatment of acromegaly in Colombia. OJEMD. 2012;2(4):102–106.
- Roset M, Merino-Montero S, Luque-Ramírez M, et al. Cost of clinical management of acromegaly in Spain. Clin Drug Investig. 2012;32(4):235–245.
- Tasdemir E, Magestro M, Griner BP, et al. Prevalence-based measurement of the economic burden of rare diseases: case review to determine the annual cost of acromegaly in France. Value Health. 2014;17(7):A527.
- Kousoulakou K, Panayiotou-Pazaitou E, Pantazi C, et al. Economic evaluation of lanreotide autogel in the management of acromegaly in Greece. Value Health. 2014;17(7):A348–A349.
- Tasdemir E, Magestro M, Griner BP, et al. Prevalence-based measurement of the economic burden of rare diseases: case review to determine the annual cost of acromegaly in Italy. Value Health. 2014;17:A323–A686.
- Broder MS, Neary MP, Chang E, et al. Treatments, complications, and healthcare utilization associated with acromegaly: a study in two large United States databases. Pituitary. 2014;17(4):333–341.
- Ferreira CN, Rufino C, Santana CF. Acromegaly patients with inadequate response to maximum dose octreotide-lar who progress to treatment with pegvisomant: economic evaluation and incremental budget impact analysis from the public perspective to São Paulo State. Value Health. 2015;18(7):A842–A881.
- Hahl J, Kurki S, Miettinen T, et al. Cost-effectiveness of pasireotide long-acting in a treatment of acromegaly in Finland. Economic evaluation based on Finnish Auria Biobank Data on health care resource utilization. Value Health. 2015;18:A335–A766.
- Margusino-Framiñán L, Pertega-Diaz S, Pena-Bello L, et al. Cost-effectiveness analysis of preoperative treatment of acromegaly with somatostatin analogue on surgical outcome. Eur J Intern Med. 2015;26(9):736–741.
- Placzek H, Xu Y, Mu Y, et al. Clinical and economic burden of commercially insured patients with acromegaly in the United States: a retrospective analysis. JMCP. 2015;21(12):1106–1114.
- Chuang CC, Bhurke S, Chen SY, et al. Treatment patterns and economic burden in patients treated for acromegaly in the USA. Drugs Real World Outcomes. 2015;2(3):299–309.
- Sliwczynski A, Brzozowska M, Labenda A, et al. Real-world comorbidities, treatment pattern and cost of patients with acromegaly in Poland based on retrospective analysis of administrative claims data. Value Health. 2016;19(7):A347–A766.
- Carlqvist P, Wilen-Koort A. A Cost-effectiveness analysis of pasireotide long-acting compared to continued use of a first-line somatostatin antagonist for the treatment of acromegaly in Sweden. Value Health. 2016;19(7):A591.
- Lesén E, Granfeldt D, Houchard A, et al. Cost-of-illness of acromegaly in Sweden – A register-linkage population-based study. Value Health. 2016;19(7):A669.
- Xuan JW, Zhang ZY, Wang YF, et al. Cost-effectiveness analysis of octreotide long acting release and lanreotide slow release for the treatment of postoperative patients with active acromegaly in China. Zhonghua Yi Xue Za Zhi. 2017;97(10):765–769.
- Elbaum M, Bolanowski M. One-center analysis of cost associated with acromegaly management in Poland. Value Health. 2017;20(9):A477.
- Kamusheva M, Zaharieva S, Elenkova A, et al. Health-related quality of life and pharmacotherapy costs study for patients with rare endocrine diseases in Bulgaria – A pilot study. Value Health. 2017;20(9):A560.
- Peral C, Cordido F, Gimeno V, et al. Cost-effectiveness analysis of second-line pharmacological treatments of acromegaly in Spain. Value Health. 2017;20(9):A557.
- Liu S, Adelman DT, Xu Y, et al. Patient-centered assessment on disease burden, quality of life, and treatment satisfaction associated with acromegaly. J Investig Med. 2018;66(3):653–660.
- Rusenova Y, Alimanova B, Marinova D, et al. Pharmacotherapy costs for patients with acromegaly in bulgaria. Value Health. 2018;21(3):S451.
- Korkmaz O, Gurcan M, Nuhoglu Kantarci FE, et al. The effects of pre-operative somatostatin analogue therapy on treatment cost and remission in acromegaly. Pituitary. 2019;22(4):387–396.
- Leonart LP, Borba HHL, Ferreira VL, et al. Cost-effectiveness of acromegaly treatments: a systematic review. Pituitary. 2018;21(6):642. doi:10.1007/s11102-018-0908-0
