?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Ischemia reperfusion (IR) injury is defined as a pathological process which initiates with tissue deoxygenation, continues with oxidative stress, and spreads with inflammation. Increased intracellular calcium is responsible for oxidative stress and inflammation during this process. Benidipine is an antihypertensive drug acting as an T/L-type calcium channel blocker. The effect of benidipine on cyclooxygenase-2 (COX-2) activity is not known. Benidipine being a calcium channel blocker suggests that it may suppress oxidant and proinflammatory COX-2 production. The aim of this study was to examine the effect of benidipine on IR induced liver injury in albino Wistar male rats. Animals were divided into three groups: liver ischemia reperfusion (LIR), 2 mg/kg benidipine + liver ischemia reperfusion (BLIR), and sham surgery (SHAM). Hepatic artery, portal vein and bile duct were clamped to obtain ischemia for one hour and reperfusion for six hours. Malondialdehyde (MDA), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and COX-2 levels were higher, whereas total glutathione (tGSH) and cyclooxygenase-1 (COX-1) levels were lower in the LIR group. Liver tissue of the LIR group showed marked dilated congested blood vessels and increased bile duct proliferation, polymorphonuclear leukocyte infiltration, edema, hemorrhage and destruction. MDA, ALT, AST and COX-2 levels were lower, and tGSH and COX-1 levels were higher in the BLIR group treated with benidipine. Liver tissue of the BLIR group was found to be normal except for mild dilated blood vessels. These experimental findings indicate that benidipine may be useful in the treatment of liver IR injury.
Keywords:
Introduction
Ischemia is defined as the deoxygenation of tissues or organs as a result of decreased or completely ceased blood flow to the tissues for various reasons [Citation1]. In the ischemia period, lipid peroxidation is initiated in the cell membranes with the increase of calcium intake to the cells. These cells undergo necrosis unless they are treated [Citation2]. Therefore, the first intervention to ischemic tissues is to ensure that blood supply of the tissues is reinitiated (reperfusion). However, reperfusion of ischemic tissue paradoxically leads to more serious damage to the tissue compared to ischemia [Citation3]. This is because xanthine oxidase that accumulates during ischemia reacts with the abundant molecular oxygen introduced to the tissue during reperfusion and converts hypoxanthine to xanthine. This reaction causes the production of excessive reactive oxygen species (ROS) [Citation4]. These ROS, known as reperfusion mediators, oxidize cell membrane lipids, thereby creating toxic products such as malondialdehyde (MDA) [Citation5]. Another mechanism that leads to tissue damage during ischemia reperfusion (IR) is the activation of phospholipase A2 by intracellular Ca2+ increase during ischemia. Phospholipase A2 increases arachidonic acid production from membrane phospholipids. During IR, the cyclooxygenage-2 (COX-2) enzyme is activated and leads to release of pro-inflammatory prostaglandins and ROS from arachidonic acid [Citation6,Citation7]. This suggests that the use of antioxidant, anti-inflammatory drugs and calcium channel blockers may be helpful in preventing liver IR injury and related complications. The aim of this study was to analyze the potential protective effect of benidipine against liver IR injury in rats. Benidipine is an antihypertensive, T/L-type calcium channel blocker [Citation8]. Benidipine has been reported to have antioxidant activity [Citation9]. In addition, it has been reported that benidipine prevents ovarian IR injury by antioxidant activity [Citation10]. However, the effect of benidipine on COX-2 activity is unknown. Benidipine being a calcium channel blocker suggests that COX-2 activity may be affected by benidipine. All this suggests that benidipine may be useful in the treatment of liver IR injury. There are no studies on the use of benidipine in the treatment of liver IR injury in the literature.
Materials and methods
Ethics statement
Animal experiments were performed according to the National Guidelines for the Use and Care of Laboratory Animals. The protocols and procedures were approved by the local Animal Experimentation Ethics Committee (Date: 31.01.2019, meeting no: 2).
Animals
A total of 18 male albino Wistar rats weighing 285–297 grams were used in this study. All rats were obtained from Atatürk University Medical Experimental Application and Research Center. The animals were kept and fed in batches at room temperature (22 °C) under appropriate conditions before the experiment.
Chemicals
Benipin, the commercial form of benidipine used in the experiments, was obtained from Deva (Turkey) and thiopental sodium was obtained from I.E ULAGAY (Turkey).
Experimental groups
Animals were divided into three groups, six rats each: liver ischemia reperfusion (LIR), 2 mg/kg benidipine + liver ischemia reperfusion (BLIR), and sham surgery (SHAM) groups.
Experimental procedures
Anesthesia application
Surgical procedures on rats were performed under sterile conditions, with 25 mg/kg intraperitoneal (i.p.) thiopental sodium anesthesia and xylazine sniffing at appropriate intervals. After thiopental sodium injection, the rats were allowed to wait for the appropriate period for surgical intervention. The period in which the animals are immobilized in the supine position is considered to be the appropriate period of anesthesia for surgical intervention [Citation11].
Surgical and pharmacological procedures
One hour prior to thiopental sodium anesthesia, 2 mg/kg benidipine was administered by oral gavage to the BLIR group (n = 6). LIR and SHAM groups of rats were treated with distilled water by the same method. During anesthesia, all rats were placed in the supine position and the anterior abdominal part was opened vertically at a length of 3.5–4 cm and laparotomy was performed. In the SHAM group, the abdominal cavity was closed with a surgical suture without any procedure. In the BLIR and LIR groups, ischemia was induced by clamping the hepatic artery, portal vein and bile duct for one hour, followed by six hours reperfusion, to create total hepatic ischemia. At the end of this period, rats were sacrificed with a high dose of anesthesia and liver tissues were excised. Biochemical and histopathological examinations were performed on the excised liver tissues. In addition, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were measured in blood samples taken from animals.
Biochemical analyses
Measurement of oxidative stress parameters
Rat’s livers were kept in −80 °C for 3 days to determine tissue total glutathione (tGSH) and MDA levels. To prepare the tissue homogenates, the liver tissues were ground with liquid nitrogen in a mortar; 0.1 g was weighed and then treated with 4.5 mL of an appropriate buffer. The tissues were treated with RIPA buffer for MDA measurement and then homogenized on ice by an Ultra-Turrax homogenizer at 9500 rpm. Homogenates were filtered and centrifuged by using a refrigerator centrifuge at 4° C. These supernatants were then used to determine MDA levels with highly sensitive enzyme-linked immunosorbent assay (ELISA) kits (Cayman Chemical, Cell BiolabsOxiSelect™ TBARS Assay STA-330 Kit, respectively).
MDA analysis
MDA was used as an indicator of oxidative stress in cells and tissues. The measurement of thiobarbituric acid (TBA) reactive substances (TBARS) was used as a well-established method for screening and monitoring lipid peroxidation. The MDA-TBA adduct formed by the reaction of MDA and TBA under high temperature (90–100 °C) and acidic conditions was measured colorimetrically at 530–540 nm [Citation12,Citation13].
tGSH analysis
The tGSH levels in the liver tissues were measured with the method created by Sedlak and Lindsay [Citation14]. For this assay, the liver tissue homogenized in 2 mL of 50 mmol/L Tris-HCl buffer containing 20 mmol/L ethylenediaminetetraacetic acid (EDTA) and 0.2 mol/L sucrose, pH 7.5. The homogenate was centrifuged at 10,000× g min at 4 °C (Hettich Zentrifugen, Universal 32 R, Germany, and then the supernatant was used to determine GSH using 5,5-dithiobis(2-nitrobenzoic acid). Absorbance was measured by spectrophotometric method at 412 nm (Epoch Microplate Spectrophotometer, Biotek, USA). Tissue protein levels were determined by the method of Bradford [Citation15]. The levels of tGSH and MDA in the tissues were expressed as µmol/(L mg) protein (nmol/mg protein) and µmol/(L mg) protein (nmol/mg protein), respectively.
Measurement of COX activity
The analysis buffer was prepared by diluting 3 mL of the analysis buffer in 27 mL of high performance liquid chromatography (HPLC)-grade water. The hem reagent was prepared by diluting 88 µL of the hem solution in 1.912 mL of the previously prepared analysis buffer. The arachidonic acid solution was prepared by adding 100 µL of KOH to 100 µL of arachidonic acid, vortexing and diluting with 1.8 mL of HPLC-grade water. The other substances used were the COX standard, colorimetric substrate, DuP697 (COX-2 inhibitor), and SC-560 (COX-1 inhibitor), which are available in commercial kits.
The procedure was performed as follows. In a 96-well microplate, the wells for the samples, which are blind for each sample, inhibitors and standards were labeled. The samples were removed from the deep freezer and allowed to defrost. To prepare the blind sample, a quantity of 50 µL from each sample was transferred into a microcentrifuge tube. The tubes were then boiled in a waterbath for 5 min and centrifuged at 8000 g for 1 min (Hettich Zentrifugen, Universal 32 R, Germany). The supernatant was used as the blind sample. Then, 150 µL of the analysis buffer and 10 µL of the hem solution were transferred into each COX standard, sample, and blind sample well. Next, 10 µL of the standard, sample, and active samples were added to the wells. Each of the inhibitor wells received 140 µL of the analysis buffer, 10 µL of the hem solution, 10 µL of the sample, and 10 µL of the SC 560 solution. The plates were rotated in a plate rotator for a few seconds and then incubated at 25 °C for 5 min. After incubation, first, 20 µL of the colorimetric substrate and then 20 µL of the arachidonic acid solution were added into each well. After rotating the plate for a few seconds and incubating it at 25 °C for 5 min, the absorbances at a wavelength of 590 nm were read. Using the formula given below, the total COX activity and the activities of COX-1 and COX-2 were calculated. After calculating the total COX activity of each sample, the COX activities of the SC 560 treated samples were calculated using the same formula to identify the COX-2 activities. The COX-2 activity was subtracted from the total COX activity to determine the COX-1 activity. The enzyme quantity that oxidized 1 nmol of TMPD (N,N,N′,N′-tetramethyl-p-phenylenediamine) at 25 °C in 1 min was accepted as one enzyme unit, and the enzyme activity in the tissues is provided as enzyme units per gram of wet tissue.
where ΔA590 is the measured change in absorbance; tassay is the assay time (tassay = 5 min); Vtotal is the total volume (Vtotal =0.21 mL); Vspecimen is the specimen volume (Vspecimen = 0.01 mL); ε is the TMPD extinction coefficient (ε = 0.00826 L/µmol). The results are divided by two because 2 mol of TMPD are required to reduce PGG2 to PGH2. The activity of COX in the tissue was expressed as nmol/(min mg) protein (U/mg protein).
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) analysis
Venous blood samples were collected into tubes without an anticoagulant. After clotting, the serum was separated by centrifugation and stored at −80 °C until assay. Using a Cobas 8000 autoanalyzer (Roche Diagnostics GmBH, Mannheim, Germany) with commercially available kits (Roche Diagnostics), serum AST and ALT activities were measured spectrophotometrically for the liver function tests.
Histopathological examination
Liver tissues removed from the rats were fixed in 10% formalin solution for 24 h. Sections of 4 μm thickness were obtained from the paraffin blocks that were obtained following the routine tissue monitoring and stained with hematoxylin & eosin. All sections were evaluated under a light microscope (Olympus BX 52, Tokyo, Japan) by a pathologist who was not aware of the treatment protocols.
Statistical analysis
The results are expressed as the mean values with standard error of the mean (± SEM). The significance level of the inter-group difference was identified using one-way analysis of variance (ANOVA). Then, Tukey’s test was performed as post-hoc. All statistical analyses were performed using SPSS version 18.0 software (IBM Corporation, Armonk, NY, USA) and differences were considered statistically significant at a level of p < 0.05.
Results and discussion
In this study, the effect of benidipine on liver IR injury was examined biochemically and histopathologically in rats. In clinical practice, IR injury is an important problem that arises in cases where tumor excision, transplantation, trauma and other liver surgeries are performed [Citation16]. The blood flow to the liver has to be partially or completely interrupted during surgery in order to prevent bleeding and loss of blood [Citation17]. Interruption of blood flow to the tissue causes ischemia of the tissue. During ischemia, calcium intake into the cells increases and lipid peroxidation occurs in biological membranes [Citation18]. After surgery, tissue re-vascularization and reperfusion is performed. However, high levels of oxygen supplied to the ischemic area with blood during reperfusion lead to the formation of ROS [Citation3]. This is the case of cellular damage that occurs after re-oxygenation of the hypoxic organ [Citation19]. ROS oxidize cell membrane lipids and create toxic products such as MDA from lipids [Citation20]. MDA causes cross-linking of cell compounds, deterioration of ion permeability and enzyme activity. It also exacerbates the existing cellular damage [Citation21].
MDA and tGSH levels
As can be seen from , IR treatment caused an increase in MDA levels in liver tissue according to sham surgery group (p < 0.001), while benidipine significantly reduced IR associated MDA increase in liver tissue (p < 0.001). The difference in MDA levels between benidipine and sham surgery group was found to be statistically insignificant (p > 0.05). Studies investigating the cause of tissue MDA increase have suggested that intracellular calcium increase is associated with the formation of xanthine oxidase involved in ROS production [Citation22,Citation23]. This information shows that calcium channel blockage is important in reducing the damage caused by lipid peroxidation. In our study, the significant increase in the amount of MDA in liver tissue treated with IR compared to benidipine and sham surgery group indicates that our experimental results are consistent with the literature. Recent studies have reported that benidipine prevents the increase of IR-induced MDA in ovarian tissue. Moreover, it has been reported that benidipine suppresses oxidative stress by preventing MDA increase in tissues [Citation9,Citation24].
Figure 1. MDA (A) and tGSH (B) levels in the liver tissues of SHAM, LIR and BLIR groups.
Note: n = 6; *p < 0.001 vs. SHAM group; **p < 0.001 vs. LIR group; α, p > 0.05 vs. SHAM group.
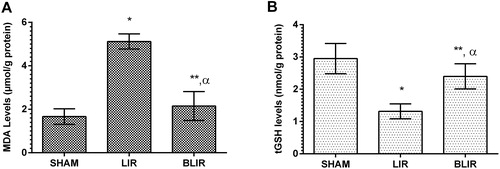
Our results showed that the amount of tGSH decreased in IR treated liver tissue with high levels of MDA. The IR procedure caused a significant decrease in the amount of tGSH in liver tissue compared to the sham surgery group (p < 0.001). Benidipine suppressed the reduction of IR-associated tGSH (p < 0.001). The difference between the amount of tGSH in the benidipine group and the sham surgery group was insignificant (p > 0.05) (. According to these results, the IR procedure disrupted the oxidant antioxidant balance in liver tissue in favor of oxidants. tGSH and MDA levels in the benidipine group were very close to those of the sham surgery group, suggesting that benidipine maintained the oxidant/antioxidant balance in liver tissue at physiological levels. In the literature, it has been reported that tGSH decreases significantly in IR treated liver tissue with high MDA levels [Citation11,Citation25]. In addition, some authors have argued that benidipine protects ovarian tissue from IR oxidative damage by preventing MDA increase and tGSH decrease [Citation9,Citation24].
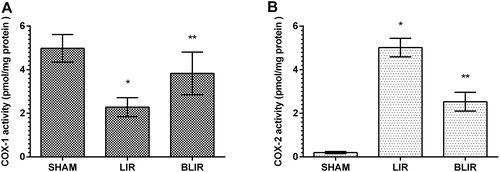
COX-1 and COX-2 activities
In IR treated liver tissue where the oxidant/antioxidant balance was disrupted in favor of oxidants, the balance between COX-1 and COX-2 activities changed in favor of COX-2. IR treatment of liver tissue was associated with decreased COX-1 activity in tissue and increased COX-2 activity compared to the sham surgery group (p < 0.001) as seen in . In contrast, COX-1 activity was high and COX-2 activity was low in the benedipine group. Benidipine significantly prevented IR-associated COX-1 reduction and COX-2 increase in the liver tissue of the experimental animals (p = 0.005, p < 0.001 respectively). Demiryilmaz et al. [Citation11] reported that there is a relationship between COX-1, COX-2, oxidant and antioxidant parameters in the pathogenesis of liver IR injury. Furthermore, it is understood that hepatoprotective activity is caused by inhibition of oxidant and COX-2 production [Citation11]. This is because COX-1 is the enzyme responsible for the synthesis of cytoprotective prostaglandins, whereas COX-2 is the enzyme responsible for the synthesis of proinflammatory prostaglandins [Citation26,Citation27]. In the studies of Kunak et al. [Citation28], COX-2 activity was high in liver tissue with IR injury. It is not known whether the inhibitory effect of benidipine on COX-2 is direct or indirect. However, it has been argued that the increase in COX-2 activity in IR injury is due to increased permeability of the cell membrane against calcium ions during ischemia [Citation21]. Benidipine being a calcium channel antagonist suggests that its inhibitory effect on COX-2 is indirect.
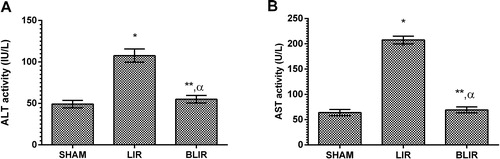
ALT and AST activities
Another parameter in this study supporting that IR caused liver damage was ALT and AST. Tuncer et al. [Citation29] reported that ALT and AST increased in liver IR injury. ROS, whose production is induced by liver IR injury, are responsible for the increased ALT and AST activities [Citation30]. As shown in , IR treatment of liver tissue significantly increased the ALT and AST activity in the blood (p < 0.001). Benedipine significantly inhibited this IR-induced increase in ALT and AST activity (p < 0.001). The difference in ALT and AST activity between the benedipine group and the sham surgery group was statistically non-significant (p > 0.05). The decrease in the ALT and AST activities associated with benedipine application may be due to its antioxidant effect.
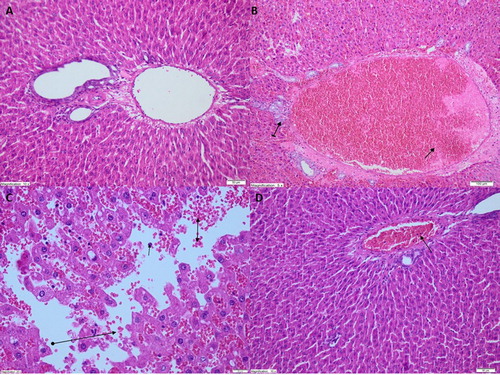
Histopatholocial observations
As shown in , no histopathological findings were found in the liver tissue of the sham surgery animal group. However, IR treated animals showed marked dilated congested blood vessels and increased bile duct proliferation in the liver tissue (. In addition, marked polymophonuclear leukocyte infiltration, edema, hemorrhage, and destruction were observed in the liver tissue of this group (. Near-normal appearance was recorded in the liver tissue of the benidipine group except for progressing mild dilated blood vessels (. These observations indicated that benidipine alleviated the development of marked dilated congested blood vessels in the liver due to IR. Benidipine also prevented bile duct proliferation, polymorphonuclear leukocyte (PNL) infiltration, edema, hemorrhage and destruction. These histopathological findings are perfectly consistent with our biochemical findings. In previous experimental studies, it has been shown that significant histopathological damage occurs in liver tissue with high MDA levels [Citation31]. In their study, Uylaş et al. [Citation32] observed pathological findings such as congestion, edema, degeneration in liver tissue with increased MDA production. Akbari et al. [Citation33] also found that the increase of ALT and AST in liver IR injury was associated with decreased antioxidant parameters and histopathological damage. Our results indicate that histopathological damage is also associated with increased COX-2. The results from our study support the demonstration that parecoxib, a selective inhibitor of COX-2, prevents liver IR injury [Citation34]. Studies in the literature have reported that COX-2 activity results in deteriorated liver function after I/R injury associated with transplantation, and selective COX-2 inhibition improves liver graft function [Citation35].
Figure 4. Light microscope images of liver tissue in SHAM (A) group, LIR group (B,C) and BLIR group (D).
Note: A. Normal view of liver tissue structure in SHAM animal group (HEX200), B. Dilated congested blood vessels (straight arrow) and increased bile duct proliferation (tailed arrow) were observed in liver tissue of LIR group (HEX100), C. Polymorphonucleated leukocytes (straight arrows), edema and hemorrhage (bilateral arrows), and destruction (round tailed arrows) were observed in liver tissue of LIR group (HEX400), D. Near-normal appearance of liver tissue in BLIR group except for progressing dilated blood vessels (HEX200).
Conclusions
The obtained results demonstrated that the IR procedure changed the oxidant/antioxidant balance in the liver tissue of rats in favor of oxidants. Furthermore, the balance between COX-1 and COX-2 activity in IR treated liver tissue changed in favor of COX-2. Benidipine prevented this change in the oxidant/antioxidant balance and COX-1/COX-2 balance in damaged liver tissue subjected to IR. These experimental findings indicate that benidipine may be useful in the treatment of liver IR injury.
Declaration of interest
The authors declare no conflict of interest.
References
- Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol Gastrointest Liver Physiol. 1986;250(6):G749–G753.
- Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181(2):160–166.
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am. 1992;72(1):65–83.
- Lindsay TF, Liauw S, Romaschin AD, et al. The effect of ischemia/reperfusion on adenine nucleotide metabolism and xanthine oxidase production in skeletal muscle. J Vasc Surg. 1990;12(1):8–15.
- Del RM. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl. 1980;492:153–168.
- Yapca OE, Borekci B, Suleyman H. Ischemia-reperfusion damage. Eurasian J Med. 2013;45(2):126–127.
- El-Marasy SA, Abdel-Rahman RF, Abd-Elsalam RM. Neuroprotective effect of vildagliptin against cerebral ischemia in rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391(10):1133–1145.
- Saito I, Suzuki H, Kageyama S, et al. Effect of antihypertensive treatment on cardiovascular events in elderly hypertensive patients: Japan’s benidipine research on antihypertensive effects in the elderly (J-BRAVE). Clin Exp Hypertens. 2011;33(2):133–140.
- Hassan MQ, Akhtar MS, Akhtar M, et al. Edaravone, a potent free radical scavenger and a calcium channel blocker attenuate isoproterenol induced myocardial infarction by suppressing oxidative stress, apoptotic signaling and ultrastructural damage. Ther Adv Cardiovasc Dis. 2016;10(4):214–223.
- Unlubilgin E, Suleyman B, Balci G, et al. Prevention of infertility induced by ovarian ischemia reperfusion injury by benidipine in rats: biochemical, gene expression, histopathological and immunohistochemical evaluation. J Gynecol Obstet Hum Reprod. 2017;46(3):267–273.
- Demiryilmaz I, Turan MI, Kisaoglu A, et al. Protective effect of nimesulide against hepatic ischemia/reperfusion injury in rats: effects on oxidant/antioxidants, DNA mutation and COX-1/COX-2 levels. Pharmacol Rep. 2014;66(4):647–652.
- Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol. 1998;108:101–106.
- Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol. 1994;366:43–58.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254.
- Olthoff KM. Can reperfusion injury of the liver be prevented? Trying to improve on a good thing. Pediatr Transplant. 2001;5(6):390–393.
- Hasselgren PO. Prevention and treatment of ischemia of the liver. Surg Gynecol Obstet. 1987;164(2):187–196.
- Serracino-Inglott F, Virlos IT, Habib NA, et al. Differential nitric oxide synthase expression during hepatic ischemia-reperfusion. Am J Surg. 2003;185(6):589–595.
- Toledo-Pereyra LH, Simmons RL, Najarian JS. Protection of the ischemic liver by donor pretreatment before transplantation. Am J Surg. 1975;129(5):513–517.
- Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21(3):361–370.
- Caraceni P, Rosenblum ER, Van Thiel DH, et al. Reoxygenation injury in isolated rat hepatocytes: relation to oxygen free radicals and lipid peroxidation. Am J Physiol. 1994;266(5 Pt 1):G799–G806.
- De Martino GN. Calcium-dependent proteolytic activity in rat liver: identification of two proteases with different calcium requirements. Arch Biochem Biophys. 1981;211(1):253–257.
- Schaffer SW, Roy RS, McMcord JM. Possible role for calmodulin in calcium paradox-induced heart failure. Eur Heart J. 1983;4(Suppl H):81–87.
- Ohtani K, Usui S, Kaneko S, et al. Benidipine reduces ischemia reperfusion-induced systemic oxidative stress through suppression of aldosterone production in mice. Hypertens Res. 2012;35(3):287–294.
- Abdelsameea AA, Abbas NA, Abdel Raouf SM. Liraglutide attenuates partial warm ischemia-reperfusion injury in rat livers. Naunyn Schmiedebergs Arch Pharmacol. 2017;390(3):311–319.
- Castillo Alcalá F, Lizárraga Madrigal I, Sumano López H. Inhibidores selectivos de la ciclooxigenasa-2: usos potenciales en perros. Vet Mexico 2002;33(3):285–307.
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and− 2. J Biol Chem. 1996;271(52):33157–33160.
- Kunak CS, Kukula O, Mutlu E, et al. The effect of etoricoxib on hepatic ischemia-reperfusion injury in rats. Oxid Med Cell Longev. 2015;2015:598162.
- Tuncer MC, Ozturk H, Buyukbayram H, et al. Interaction of L-arginine-methyl ester and Sonic hedgehog in liver ischemia-reperfusion injury in the rats. World J Gastroenterol. 2007;13(28):3841–3846.
- Yabe Y, Kobayashi N, Nishihashi T, et al. Prevention of neutrophil-mediated hepatic ischemia/reperfusion injury by superoxide dismutase and catalase derivatives. J Pharmacol Exp Ther. 2001;298(3):894–899.
- Chen J, Liu H, Zhang X. [Protective effects of rosiglitazone on hepatic ischemia reperfusion injury in rats]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43(7):732–737.
- Uylaş MU, Şahin A, Şahintürk V, et al. Quercetin dose affects the fate of hepatic ischemia and reperfusion injury in rats: an experimental research. Int J Surg. 2018;53:117–121.
- Akbari G, Mard SA, Dianat M, et al. The hepatoprotective and microRNAs downregulatory effects of crocin following hepatic ischemia-reperfusion injury in rats. Oxidative medicine and cellular longevity. 2017;2017:1702967.
- Zhang T, Ma Y, Xu K-Q, et al. Pretreatment of parecoxib attenuates hepatic ischemia/reperfusion injury in rats. BMC Anesthesiol. 2015;15(1):165.
- Oshima K, Yabata Y, Yoshinari D, et al. The effects of cyclooxygenase (COX)-2 inhibition on ischemia-reperfusion injury in liver transplantation. J Invest Surg. 2009;22(4):239–245.