Abstract
The present study investigated the role and mechanism of SUMO2/3 in the protection of vascular endothelial cells by propofol. An ischemia-reperfusion (I/R) injury model of human umbilical vein endothelial cells (HUVECs) was constructed. After treatment with propofol, we determined the expression of SUMO2/3 proteins by Western blotting, cell proliferation by CCK-8 assay, cell migration by Transwell assay and apoptosis by flow cytometry. After interference of SUMO2/3, the biological functions of HUVECs were observed. Using Western blotting and laser scanning confocal microscopy, the autophagy of HUVECs was examined. Using immunoprecipitation, the SUMO modification site of Beclin1 was examined. The results showed that I/R injury decreased the proliferation and migration and promoted the apoptosis of HUVECs, and propofol restored the normal biological functions to HUVECs. I/R injury inhibited the expression of SUMO2/3 in HUVECs, and propofol increased the expression of SUMO2/3 in HUVECs. Interference of SUMO2/3 expression suppressed the proliferation and migration, and promoted the apoptosis of HUVECs. Propofol exerted its protective effect on HUVECs via autophagy mediated by SUMO2/3 proteins. Beclin1 was modified by SUMO2/3 at the K26 site. SUMO modification of the K26 site in Beclin1 was involved in the regulation of autophagy of HUVECs, and K26R Beclin1 had reduced effect in activating autophagy of HUVECs compared with wild-type Beclin1. These results demonstrate that SUMO2/3 participates in regulating the protective effect of propofol on HUVECs. SUMO2/3 activates the autophagy activity of cells by modifying the K26 site of Beclin1, thus inhibiting I/R injury to vascular endothelial cells.
Introduction
Ischemia-reperfusion (I/R) injury is the most common tissue and organ injury in surgery [Citation1]. If not properly treated, it can easily damage blood vessels and multiple organs, affect prognosis and even endanger life [Citation2]. Blood vessels are the major component of the circulatory system and the main place for nutrient exchange between tissue cells and blood [Citation3]. In fact, the first pathological changes occur in blood vessels during I/R injury. The vascular wall is covered by a layer of endothelial cells, which is an important barrier between blood vessels and tissues [Citation4]. When vascular injury occurs, the function of vascular endothelial cells is disordered or even damaged, which leads to the direct entry of inflammatory cells and molecules into tissues, aggravating the injury of tissue cells [Citation5, Citation6]. Therefore, it is of great clinical value to study the molecular mechanism of I/R injury of vascular endothelial cells.
Anesthesia is an important step in surgery, which can reduce the body’s stress response to trauma and pain [Citation7]. It is discovered that anesthetic drugs can also play a protective role in tissues and organs, and the protective effect of propofol has attracted the attention of researchers [Citation8]. Intraoperative use of propofol is closely related to the protection of various organs, such as brain, lung, spinal cord, blood vessel and kidney [Citation9, Citation10]. Although the molecular mechanism of propofol’s involvement in organ protection has been subject of studies, the exact mechanism of propofol’s involvement in organ protection remains unclear, and new genes and related mechanisms involved in this process remain to be discovered. This is of great significance for the development and clinical application of protective drugs against I/R injury.
Small ubiquitin-like modifier (SUMO) modification is a reversible ATP-dependent post-translational modification process similar to ubiquitination [Citation11, Citation12]. SUMO protein is a typical representative of ubiquitin-like modified protein subfamily, and SUMO modification is one of the conservative post-translational modifications in eukaryotes [Citation13]. By now, four SUMO isomers have been found in mammalian cells, namely SUMO1, SUMO2, SUMO3 and SUMO4 [Citation14, Citation15]. Different SUMO proteins differ in amino acid sequence, but their three-dimensional structures are very similar [Citation16]. Enzymes that participate in SUMO modification include activating enzyme SAE1/SAE2 heterodimer (E1) and unique ligase Ubc9 (E2), as well as protein ligase (E3) [Citation17, Citation18]. SUMO modification widely exists in various physiological and pathological processes of the body. For example, the expression of SUMO2/3 protein is increased significantly when cells were exposed to stress [Citation19]. In the present study, we investigated whether SUMO2/3 protein participates in the process of propofol inhibiting I/R injury.
Materials and methods
Cells
Human umbilical vein endothelial cells (HUVECs; Cell Bank, Chinese Academy of Sciences, Shanghai, China) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin and 100 IU/mL streptomycin at 37 °C, 5% CO2 and 70% humidity. HUVECs were passaged every three days, and those in logarithmic growth were collected for experiments.
HUVECs (1 × 106) were seeded into 6-well plates, and divided into normal control group, I/R group and propofol group. The cells in the normal control group were cultured in DMEM supplemented with 10% FBS, 100 IU/mL penicillin and 100 IU/mL streptomycin at 37 °C, 5% CO2 and 70% humidity. The cells in the I/R group were cultured in low-glucose and serum-free DMEM placed in an airtight container. After ventilation with mixed gases of 5% CO2 and 95% N2 for 30 min, the container was sealed and placed in an incubator with 5% CO2 at 37 °C. Fourteen hours later, the medium was discarded, and 100 μL DMEM supplemented with 10% FBS, 100 IU/mL penicillin and 100 IU/mL streptomycin were added into each well for culture at 37 °C and 5% CO2 for 4 h. The cells in the propofol group were treated according to the same protocol as used for the I/R group, and the difference was that 12.3 μmol/L propofol was present during the culture of 14 h.
To transfect HUVECs, the cells (2 × 105) in logarithmic growth were seeded onto 24-well plates one day before transfection, and cultured in antibiotics-free DMEM supplemented with 10% FBS until reached 70% confluency. In the first vial, 1.5 µL SUMO2/SUMO3 small interference RNA (siRNA; 20 pmol/µL; Hanbio, Shanghai, China) was mixed with 50 µL Opti Mem medium (Thermo Fisher Scientific, Waltham, MA, USA). In the second vial, 1 µL Lipofectamine 2000 (Thermo Fisher Scientific) was mixed with 50 µL Opti Mem medium. After standing still for 5 min, the two vials were combined for additional incubation at room temperature for 20 min. Then, the mixtures were added onto cells in the respective groups. Six hours later, the medium was replaced with DMEM containing 10% FBS. After cultivation for 48 h, the cells were collected for further assays.
Transfection of HUVECs using p-CMV-FLAG-SUMO2, p-CMV-FLAG-SUMO3, p-CMV-HA-Beclin1 and p-CMV-HA-K26R plasmids (Hanbio, Shanghai, China) was performed according to the same protocol as described for SUMO2/3 interference sequences in 6-well plates (2 μg plasmids/well). After cultivation for 48 h, the cells were collected for further assays.
Quantitative real-time polymerase chain reaction
HUVECs (1 × 106) were lysed using 1 mL TRIzol reagent following the manufacturer’s manual (Thermo Fisher Scientific, Waltham, MA, USA). Total RNA was extracted using the phenol/chloroform method. The concentration and quality of RNA was measured using ultraviolet spectrophotometry (Nanodrop ND2000, Thermo Scientific, Waltham, MA, USA). Then, cDNA was obtained by reverse transcription from 1 μg RNA and stored at –20 °C. Reverse transcription of mRNA was performed using Ipsogen RT Kit (Qiagen, Hilden, Germany).
QuantiNova SYBR Green PCR Kit (Qiagen, Hilden, Germany) was used to detect mRNA expression of SUMO2 and SUMO3, using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as internal reference. The primer sequences for SUMO2 were 5′-CTGACAGACTGATACTGATGCCA-3′ (forward) and 5′-TCCTTGCCACATGTGCTACC-3′ (reverse). The primer sequences for SUMO3 were 5′-GAGAGGCAGGGCTTGTCAAT-3′ (forward) and 5′-AACCTTGCCCCCAATACCTG-3′ (reverse). The primer sequences for GAPDH were 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ (forward) and 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ (reverse). The reaction system (20 μL) was composed of 10 μL quantitative real-time polymerase chain reaction (qRT-PCR)-Mix, 0.5 μL upstream primer, 0.5 μL downstream primer, 2 μL cDNA and 7 μL ddH2O. The PCR program was: initial denaturation at 95 °C for 10 min; 95 °C for 1 min and 60 °C for 30 s (40 cycles) (iQ5; Bio-Rad, Hercules, CA, USA). The 2–ΔΔCq method was used to calculate the relative expression of SUMO2 and SUMO3 mRNA against GAPDH. Each sample was tested in triplicate.
Laser scanning confocal microscopy
At 24 h after transfection, HUVECs in each group were seeded onto culture plates at a density of 1 × 105/well. When reached 70% confluency, the cells were infected with Lv-RFP-LC3B lentivirus (titer, 1 × 108; Hanbio, Shanghai, China) at a ratio of MOI = 20. Forty-eight hours later, the cells were observed under a laser confocal microscope (SP8; Leica, Wetzlar, Germany), and five fields were selected randomly. Green and red vesicles represented autophagosomes. After autophagosomes fused with lysosomes, green fluorescence disappeared, and only red fluorescence remained. The number of autophagosomes in HUVECs was counted to evaluate autophagy activity.
CCK-8 assay
HUVECs were seeded at a density of 2000/well in 96-well plates. At 0, 24, 48 and 72 h, 20 μL CCK-8 reagent (5 g/L; Beyotime, Shanghai, China) was added onto the cells. On the last day, 150 μL CCK-8 reaction solution was added and the cells were incubated at 37 °C for 2 h. Then, the absorbance of each well was measured at 490 nm for plotting cell proliferation curves. Each group was tested in three replicate wells and the values were averaged.
Transwell assay
Transwell chambers (Corning Inc., Corning, NY, USA) were used to evaluate the migration ability of HUVECs. The cells (1 × 105) from each group were seeded into the upper chamber containing 200 μL serum-free DMEM medium. In addition, 500 μL DMEM medium supplemented with 10% FBS was added into the lower chamber. After 24 h, the chamber was removed and the cells in the upper chamber were wiped off. After being fixed with 4% formaldehyde for 10 min, the membrane was stained using Giemsa method for microscopic observation of five random fields (200×). The number of transwell cells was calculated for the evaluation of cell migration ability. All procedures were carried out on ice with pipetting tips being cooled at 4 °C.
Flow cytometry
HUVECs (1 × 106) in each group were washed with precooled phosphate-buffered saline twice and subjected to flow cytometry using ANXN V FITC APOPTOSIS DTEC KIT I (BD Biosciences, Franklin Lakes, NJ, USA) following the manufacturer’s manual to detect cell apoptosis. Cells with ANNEXIN V-positive values were early apoptotic cells, those with PI-positive values were necrotic cells, and those with double positive values were late apoptotic cells.
Western blotting
HUVECs (1 × 106) were trypsinized and collected, and then lysed with precooled Radio-Immunoprecipitation Assay (RIPA) lysis buffer (600 μL; 50 mmol/L Tris-base, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate, 1% TritonX-100, 1% sodium deoxycholate; Beyotime Institute of Biotechnology, Shanghai, China) for 30 min on ice. The mixture was centrifuged at 13,000 rpm and 4 °C for 10 min. The supernatant was used to determine protein concentration by bicinchoninic acid (BCA) protein concentration determination kit (RTP7102, Real-Times Biotechnology Co., Ltd., Beijing, China). The samples were then mixed with 5× sodium dodecyl sulfate loading buffer before denaturation in boiling water bath for 10 min. Afterwards, the samples (20 µg) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 100 V. The resolved proteins were transferred to polyvinylidene difluoride membranes on ice (250 mA, 1 h) and blocked with 5% skimmed milk at room temperature for 1 h. Then, the membranes were incubated with rabbit anti-human SUMO2/3 (1:1000; ab3742; Abcam, Cambridge, UK), LC3B (1:1000; Abcam, Cambridge, UK), p62 (1:1000; Abcam, Cambridge, UK) or GAPDH (1:4000; Abcam, Cambridge, UK) monoclonal primary antibodies at 4 °C overnight. After five times of extensive washing with phosphate-buffered saline with Tween 20 for 5 min, the membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:4000; Abcam, Cambridge, UK) for 1 h at room temperature before washing five times with phosphate-buffered saline with Tween 20 for 5 min. Then, the membrane was developed with an enhanced chemiluminescence detection kit (Beyotime, Shanghai, China) for imaging. Image lab v3.0 software (Bio-Rad, Hercules, CA, USA) was used to acquire and analyze the imaging signals. The relative contents of target proteins were expressed against GAPDH.
Protein immunoprecipitation
293T cells were co-transfected p-CMV-FLAG-SUMO2/3 and p-CMV-HA-Beclin1, or p-CMV-FLAG-SUMO2/3 and p-CMV-HA-K26R, and cultured at 37 °C and 5% CO2for 48 h. Cell sediments were collected, and 106 cells were mixed with 1 mL lysis buffer containing 1% phenylmethylsulfonyl fluoride before being kept on ice for 30 min with shaking. Then, the mixture was centrifuged at 4 °C and 12,000 × g for 15 min before collecting the supernatant. The supernatant with proteins was mixed with 1 μL antibodies (anti-Flag), and gently shaken at 4 °C overnight. During shaking, 10 μL protein A/G agarose beads were washed with lysis buffer, and centrifuged at 3000 × g for 3 min before removing supernatant. The beads were washed three times. Then, the beads were transferred into cell lysates incubated with antibodies before gentle shaking at 4 °C for 2 h. After the beads were coupled with antibodies, the mixture was centrifuged at 4 °C and 2000 × g for 1 min. After removing the supernatant, the beads were mixed with 1 mL lysis buffer before centrifugation at 4 °C and 2000 × g for 1 min. This washing step was repeated 3–4 times before completely removing the supernatant. Then, 5× sodium dodecyl sulfate loading buffer was added onto the beads and boiled at 100 °C for 5 min. The proteins captured on the beads were examined by Western blotting.
Statistical analysis
The results were analyzed using SPSS 20.0 statistical software (IBM, Armonk, NY, USA). The data were expressed as mean values with standard deviations (±SD). Data were tested for normality. Multi-group measurement data were analyzed using one-way analysis of variance (ANOVA). In case of homogeneity of variance, least significant difference and Student–Newman–Keuls methods were used; in case of heterogeneity of variance, Tamhane’s T2 or Dunnett’s T3 method were used. Comparison between two groups was carried out using Student’s t-test. p < 0.05 indicated statistically significant differences.
Results and discussion
I/R injury decreases the proliferation and migration and promotes the apoptosis of HUVECs, and propofol restores normal biological functions to HUVECs
Vascular injury is the most common pathological change in clinical surgery, which is often caused by I/R, operation and so on [Citation20]. As an important barrier to the inner wall of blood vessels, vascular endothelial cells maintain and participate in the regulation of the cardiovascular system through powerful repair and endocrine functions [Citation21]. Propofol is one of the commonly used intravenous anesthetics in clinic. It has the advantages of quick onset and rapid metabolism, and is often used in the induction stage of anesthesia for surgery [Citation22]. Propofol has also been reported to protect tissues and organs [Citation23]. However, how propofol protects tissues and cells remains to be further studied.
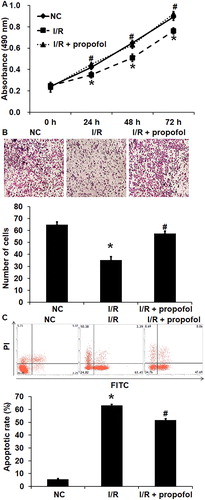
I/R injury is one of the main causes of tissue and cell injury in clinical operation [Citation24]. It has been discovered that propofol can affect the biological functions of tissues and cells through different mechanisms, especially inhibiting I/R-induced cell injury. For example, propofol can inhibit calcium overload induced by calcineurin and activate the YAP signaling pathway [Citation25]. In addition, propofol inhibits I/R-induced apoptosis of human brain vascular smooth muscle cells by regulating the expression of Bcl-2, BAX and Caspase 3 [Citation26]. To examine the effect of propofol on an I/R injury model of HUVECs, we performed CCK-8 assay, Transwell assay and flow cytometry. CCK-8 assay showed that the absorbance of HUVECs in the I/R group was significantly lower than that of the normal control group at 24, 48 and 72 h (p < 0.05 for all; . By contrast, propofol treatment significantly increased the absorbance of HUVECs with I/R injury to levels similar to that of the normal control group (p < 0.05 for 24, 48 and 72 h; . Transwell assay showed that the migration ability of HUVECs in the I/R group was significantly decreased than that of the normal control group (p < 0.05), and propofol treatment significantly increased the migration ability of HUVECs with I/R injury to a level similar to that of the normal control group (p < 0.05) (. Flow cytometry showed that the apoptotic rate of HUVECs in the I/R group was significantly higher than that of the normal control group (p < 0.05), and propofol treatment significantly reduced the apoptotic rate of HUVECs with I/R injury (p < 0.05) (. These results suggest that I/R injury decreases the proliferation and migration and promotes the apoptosis of HUVECs, and propofol restores normal biological functions to HUVECs.
Figure 1. Effect of propofol on the biological functions of HUVECs with I/R. (A) Proliferation of HUVECs without I/R (NC group), HUVECs with I/R (I/R group) and HUVECs with I/R that were treated with propofol. CCK-8 assay was used to determine cell proliferation. *p < 0.05 compared with NC group; #p < 0.05 compared with I/R group. (B) Migration ability of HUVECs in NC group, I/R group and I/R + propofol group. Transwell assay was used to determine cell migration. *p < 0.05 compared with NC group; #p < 0.05 compared with I/R group. (C) Apoptosis of HUVECs NC group, I/R group and I/R + propofol group. Flow cytometry was used to determine cell apoptosis. *p < 0.05 compared with NC group; #p < 0.05 compared with I/R group.
I/R injury inhibits the expression of SUMO2/3 in HUVECs, and propofol increases the expression of SUMO2/3 in HUVECs
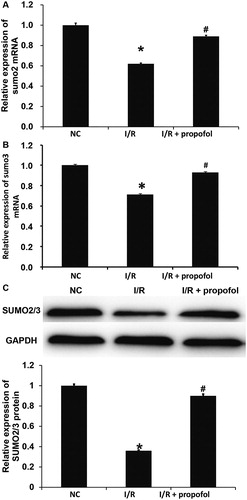
SUMO is an important member of the ubiquitin-like protein family, and it can bind to many proteins to exert its biological functions [Citation27]. The dysregulation of SUMO modification may be related to many diseases. For example, SUMO modification of lbd30 by six1 regulates secondary cell wall formation in Arabidopsis thaliana [Citation28]. Trim28 prolongs HIV-1 latency by promoting SUMO modification of CDK9 and inhibiting p-tefb [Citation29]. To determine the expression of SUMO2 and SUMO3, qRT-PCR and Western blotting were performed. The data showed that the expression of SUMO2/3 mRNA and protein in HUVECs of the I/R group was significantly lower than that in the negative control group (p < 0.05), and treatment with propofol significantly elevated the expression of SUMO2/3 mRNA and protein in HUVECs with I/R injury to levels similar to the negative control group (p < 0.05) (). The results indicate that I/R injury inhibits the expression of SUMO2/3 in HUVECs, and propofol increases the expression of SUMO2/3 in HUVECs. Vascular injury includes endothelial cell injury, whereas normal endothelial cells migrate to the injured site, proliferate in situ and repair the injured site in time [Citation30, Citation31]. Therefore, we believe that SUMO2/3 can affect the ability of vascular endothelial cells to repair vascular injury.
Figure 2. Effect of propofol on the expression of SUMO2/3 in HUVECs with I/R. (A,B) Expression of SUMO2 (A) and SUMO3 (B) mRNA in HUVECs without I/R (NC group), HUVECs with I/R (I/R group) and HUVECs with I/R that were treated with propofol. qRT-PCR was used to determine mRNA expression. *p < 0.05 compared with NC group; #p < 0.05 compared with I/R group. (B) Expression of SUMO2/3 proteins in HUVECs of NC group, I/R group and I/R + propofol group. Western blotting was used to determine protein expression. *p < 0.05 compared with NC group; #p < 0.05 compared with I/R group.
Interference of SUMO2/3 expression suppresses the proliferation and migration, and promotes the apoptosis of HUVECs
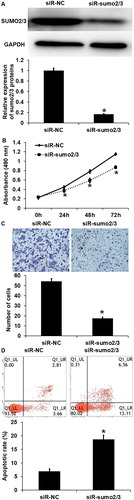
To further examine the protective effect of SUMO2/3 proteins on HUVECs, we interfered the expression of SUMO2/3 proteins using their siRNA. Western blotting showed that the expression of SUMO2/3 proteins in the siR-SUMO2/3 group was significantly lower than that in the siR-NC group (p < 0.05) (. CCK-8 assay showed that the absorbance of HUVECs in the siR-SUMO2/3 group was significantly lower than that in the siR-NC group at 24, 48 and 72 h (p < 0.05 for all) (. Transwell assay showed that the number of cross-membrane HUVECs in the siR-SUMO2/3 group was significantly smaller than that in the siR-NC group (p < 0.05) (. Flow cytometry showed that the apoptotic rate of HUVECs in the siR-SUMO2/3 group was significantly higher than that in the siR-NC group (p < 0.05) (. These results suggest that interference of SUMO2/3 expression suppresses the proliferation and migration, and promotes the apoptosis of HUVECs.
Figure 3. The effect of SUMO2/3 inhibition on the biological functions of HUVECs. (A) Expression of SUMO2/3 proteins in HUVECs transfected with siR-NC or siR-SUMO2/3. Western blotting was used to determine protein expression. *p < 0.05 compared with siR-NC group. (B) Proliferation of HUVECs transfected with siR-NC or siR-SUMO2/3. CCK-8 assay was used to examine cell proliferation. *p < 0.05 compared with siR-NC group. (C) Migration ability of HUVECs transfected with siR-NC or siR-SUMO2/3. Transwell assay was used to examine cell migration. *p < 0.05 compared with siR-NC group. (C) Apoptosis of HUVECs transfected with siR-NC or siR-SUMO2/3. Flow cytometry was used to examine cell apoptosis. *p < 0.05 compared with siR-NC group.
Propofol exerts its protective effect on HUVECs via autophagy mediated by SUMO2/3 proteins
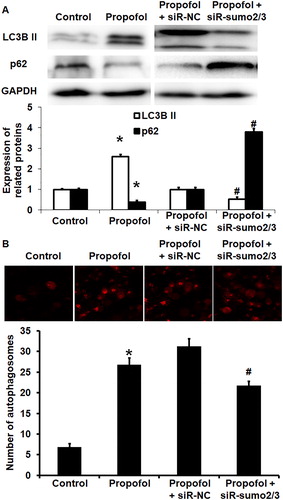
The mechanism by which propofol affects the biological functions of HUVECs via the SUMO2/3 pathway remains unclear. Previous reports have shown that propofol protects cells by activating autophagy. However, more recent studies have shown that SUMO modification is also involved in autophagy regulation. For example, SUMO modification of VPS34 can activate autophagy and promote phenotypic transformation of vascular smooth muscle cells, thus participating in the formation of pulmonary artery hypertension [Citation32]. To investigate how propofol exerts its protective effect on HUVECs, we examined the autophagy of HUVECs using Western blotting and laser scanning confocal microscopy. Western blotting showed that LC3B II expression was enhanced, whereas p62 expression was decreased in untransfected HUVECs after treatment with propofol (p < 0.05 for both). In addition, transfection with siR-SUMO2/3 decreased the expression of LC3B II and increased the expression of p62 compared with siR-NC in the presence of propofol (p < 0.05 for both) (. Laser scanning confocal microscopy showed that treatment with propofol increased the number of autophagosomes in untransfected HUVECs (p < 0.05), and transfection with siR-SUMO2/3 decreased the number of autophagosomes in HUVECs compared with siR-NC in the presence of propofol (p < 0.05) (. These results indicate that propofol exerts its protective effect on HUVECs via autophagy mediated by SUMO2/3 proteins.
Figure 4. Effect of propofol on the autophagy of untransfected HUVECs and those without or with interference of SUMO2/3 expression. (A) Expression of LC3B II and p62 proteins in HUVECs of control group, propofol group, propofol + siR-NC group and propofol + siR-SUMO2/3 group. Western blotting was used to determine protein expression. *p < 0.05 compared with control group; #p < 0.05 compared with propofol + siR-NC group. (B) Number of autophagosomes in HUVECs of control group, propofol group, propofol + siR-NC group and propofol + siR-SUMO2/3 group. Laser scanning confocal microscopy was used to determine the number of autophagosomes. *p < 0.05 compared with control group; #p < 0.05 compared with propofol + siR-NC group.
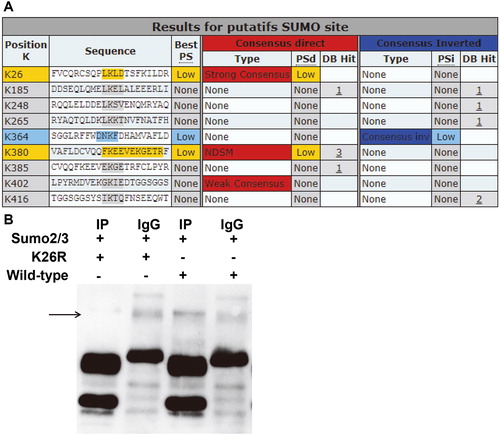
Beclin1 can be modified by SUMO2/3 at K26 site
To test whether SUMO2/3 can modify Beclin1, a key gene in autophagy, bioinformatics prediction was first employed. The data showed that the K26 site of Beclin1 was very likely to be modified by SUMO (. Therefore, 293T cells were co-transfected with p-CMV-FLAG-SUMO2/3 and p-CMV-HA-Beclin1, or p-CMV-FLAG-SUMO2/3 and p-CMV-HA-K26R before protein immunoprecipitation (IP) was carried out. The data showed that, compared with the IgG group, the IP group had a new protein band, and the band corresponding to Beclin1 had increased molecular weight. By contrast, the new protein band disappeared after mutation of K26R in Beclin1 (. These results suggest that Beclin1 can be modified by SUMO2/3 at the K26 site.
SUMO modification of K26 site in Beclin1 is involved in the regulation of autophagy of HUVECs, and K26R Beclin1 has reduced effect in activating autophagy of HUVECs compared with wild-type Beclin1
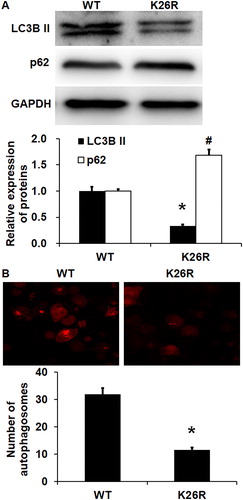
To test the effect of K26R of Beclin1 on autophagy of HUVECs, the expression of LC3B II and p62 proteins was measured by Western blotting, and autophagosomes were determined by laser scanning confocal microscopy. Western blotting showed that the LC3B II protein expression in HUVECs with K26R mutation of Beclin1 was significantly lower than that in HUVECs with wild-type Beclin1 (p < 0.05), whereas the p62 protein expression in HUVECs with K26R mutation of Beclin1 was significantly higher than that in HUVECs with wild-type Beclin1 (p < 0.05) (. Laser scanning confocal microscopy showed that the number of autophagosomes in HUVECs with K26R mutation of Beclin1 was significantly smaller than that in HUVECs with wild-type Beclin1 (p < 0.05) (. The results indicate that SUMO modification of K26 site in Beclin1 is involved in the regulation of autophagy of HUVECs, and K26R Beclin1 has a reduced effect in activating autophagy of HUVECs compared with wild-type Beclin1.
Figure 6. Effect of wild-type (WT) or K26R Beclin1 on the autophagy of HUVECs. (A) Expression of LC3B II protein and p62 protein in HUVECs with WT or K26R Beclin1. Western blotting was used to determine protein expression. *p < 0.05 compared with WT group for LC3B II protein; #p < 0.05 compared with WT group of p62 protein. (B) Number of autophagosomes in HUVECs with WT or K26R Beclin1. Laser scanning confocal microscopy was used to determine the number of autophagosomes. *p < 0.05 compared with WT group for LC3B II protein; #p < 0.05 compared with WT group of p62 protein.
Conclusions
The present study demonstrated that SUMO2/3 participates in regulating the protective effect of propofol on HUVECs. Propofol promotes the expression of SUMO2/3, which results in the modification of K26 site of Beclin1 and activation of autophagy activity of HUVECs, thus producing protective effect on cells.
Author contributions
The final version of the manuscript has been read and approved by all authors, and each author believes that the manuscript represents honest work. PL, LL and HC collaborated to design the study. PL, LL and TW were responsible for performing experiments. PL, LL and HC analyzed the data. All authors collaborated to interpret results and develop the manuscript.
Ethical approval and consent to participate
All procedures performed in this study were approved by the Ethics Committee of Weifang Yidu Central Hospital.
Acknowledgement
The authors wish to thank Drs. Siyan Zhang, Hao Zhang and Dongdong Li in the Department for their help and support.
Disclosure statement
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed in this study are available from the corresponding author upon reasonable request.
References
- Hu S, Cao S, Tong Z, et al. FGF21 protects myocardial ischemia-reperfusion injury through reduction of miR-145-mediated autophagy. Am J Transl Res. 2018;10(11):3677–3688.
- Jiang Y, Xie H, Tu W, et al. Exosomes secreted by HUVECs attenuate hypoxia/reoxygenation-induced apoptosis in neural cells by suppressing miR-21-3p. Am J Transl Res. 2018;10(11):3529–3541.
- Diebel LN, Liberati DM, Martin JV. Acute hyperglycemia increases sepsis related glycocalyx degradation and endothelial cellular injury: a microfluidic study. Am J Surg. 2019;217(6):1076–1082.
- Shukla K, Sonowal H, Saxena A, et al. Didymin by suppressing NF-kappaB activation prevents VEGF-induced angiogenesis in vitro and in vivo. Vasc Pharmacol. 2019;115:18–25.
- Li S, Zhan JK, Wang YJ, et al. Exosomes from hyperglycemia-stimulated vascular endothelial cells contain versican that regulate calcification/senescence in vascular smooth muscle cells. Cell Biosci. 2019;9:1.
- Wang Y, La M, Pham T, et al. High levels of HtrA4 detected in preeclamptic circulation may disrupt endothelial cell function by cleaving the main VEGFA receptor KDR. FASEB J. 2019;33(4):5058–5066.
- Elsharkawy H, Ince I, Pawa A. Rhomboid intercostal and sub-serratus (RISS) plane block for analgesia after lung transplant. J Clin Anesth. 2019;56:85–87.
- Chen F, Li M, Zhu X. Propofol suppresses proliferation and migration of papillary thyroid cancer cells by down-regulation of lncRNA ANRIL. Exp Mol Pathol. 2019;107:68–76.
- Coppens M, Van Limmen JGM, Schnider T, et al. Corrigendum to ‘Study of the time course of the clinical effect of propofol compared with the time course of the predicted effect-site concentration: performance of three pharmacokinetic-dynamic models’ [Br J Anaesth 2010;104:452–458]. Br J Anaesth. 2019;122(2):287.
- Coppens MJ, Versichelen LFM, Mortier EP, et al. Corrigendum to ‘Do we need inhaled anaesthetics to blunt arousal, haemodynamic responses to intubation after i.v. induction with propofol, remifentanil, rocuronium?’ [Br J Anaesth 2006;97:835–841]. Br J Anaesth. 2019;122(2):286.
- Gong X, Nie Q, Xiao Y, et al. Localization patterns of SUMOylation enzymes E1, E2 and E3 in ocular cell lines predict their functional importance. Curr Mol Med. 2019;18(8):516–522.
- Nie Q, Gong X, Gong L, et al. Sodium iodate-induced mouse model of age-related macular degeneration displayed altered expression patterns of SUMOylation enzymes E1, E2 and E3. Curr Mol Med. 2019;18(8):550–555.
- Nie Q, Wang L, Gong X, et al. Altered expression patterns of the SUMOylation enzymes E1, E2 and E3 are associated with glucose oxidase- and UVA-induced cataractogenesis. Curr Mol Med. 2019;18(8):542–549.
- Niu D, Lin X-L, Kong X, et al. SIZ1-mediated SUMOylation of TPR1 suppresses plant immunity in arabidopsis. Mol Plant. 2019;12(2):215–228.
- Orosa B, Yates G, Verma V, et al. SUMO conjugation to the pattern recognition receptor FLS2 triggers intracellular signalling in plant innate immunity. Nat Commun. 2018;9(1):5185.
- Parker AR, Forster LA, Baro DJ. Modulator-gated, SUMOylation-mediated, activity-dependent regulation of ionic current densities contributes to short-term activity homeostasis. J Neurosci. 2019;39(4):596–611.
- Gujrati M, Mittal R, Ekal L, et al. SUMOylation of periplakin is critical for efficient reorganization of keratin filament network. Mol Biol Cell. 2019;30(3):357–369.
- Mohiuddin M, Evans TJ, Rahman MM, et al. SUMOylation of PCNA by PIAS1 and PIAS4 promotes template switch in the chicken and human B cell lines. Proc Natl Acad Sci USA. 2018;115(50):12793–12798.
- Nie Q, Yang L, Qing W, et al. Differential expression of SUMOylation enzymes SAE1, U BA2, UBC9, PIAS1 and RanBP2 in major ocular tissues of mouse eye. Curr Mol Med. 2019;18(6):376–382.
- Sanders TL, Johnson NR, Levy NM, et al. Effect of vascular injury on functional outcome in knees with multi-ligament injury: a matched-cohort analysis. J Bone Joint Surg Am Vol. 2017;99(18):1565–1571.
- Li L, Zhao Y, Guo L, et al. Ultrasound guidance enhances the efficiency of brachial plexus block and ameliorates the vascular injury compared with nerve stimulator guidance in hand surgery patients. J Invest Surg. 2019;28:1–6.
- Shin DJ, Germann AL, Johnson AD, et al. Propofol is an allosteric agonist with multiple binding sites on concatemeric ternary GABAA receptors. Mol Pharmacol. 2018;93(2):178–189.
- Dogan MF, Arslan SO, Yildiz O, et al. Propofol-induced vasodilation in human internal mammary artery: role of potassium channels. J Cardiothorac Vasc Anesth. 2019;33(8):2183–2191.
- Huang Z, Zheng D, Pu J, et al. MicroRNA-125b protects liver from ischemia/reperfusion injury via inhibiting TRAF6 and NF-kappaB pathway. Biosci Biotechnol Biochem. 2019;83(5):829–835.
- Li X, Yao L, Liang Q, et al. Propofol protects hippocampal neurons from hypoxia-reoxygenation injury by decreasing calcineurin-induced calcium overload and activating YAP signaling. Oxid Med Cell Longev. 2018;2018:1725191.
- Zhang J, Xia Y, Xu Z, et al. Propofol suppressed hypoxia/reoxygenation-induced apoptosis in HBVSMC by regulation of the expression of Bcl-2, Bax, caspase3, Kir6.1, and p-JNK. Oxid Med Cell Longev. 2016;2016:1518738.
- Verma V, Croley F, Sadanandom A. Fifty shades of SUMO: its role in immunity and at the fulcrum of the growth-defence balance. Mol Plant Pathol. 2018;19(6):1537–1544.
- Liu C, Yu H, Li L. SUMO modification of LBD30 by SIZ1 regulates secondary cell wall formation in Arabidopsis thaliana. PLoS Genet. 2019;15(1):e1007928.
- Ma X, Yang T, Luo Y, et al. TRIM28 promotes HIV-1 latency by SUMOylating CDK9 and inhibiting P-TEFb. eLife. 2019;8:pii:e42426.
- Barhoumi T, Fraulob-Aquino JC, Mian MOR, et al. Matrix metalloproteinase-2 knockout prevents angiotensin II-induced vascular injury. Cardiovasc Res. 2017;113(14):1753–1762.
- Marcia L, Kim DY. Predictors of peripheral vascular injury in patients with blunt lower extremity fractures. Ann Vasc Surg. 2019;57:35–40.
- Yao Y, Li H, Da X, et al. SUMOylation of Vps34 by SUMO1 promotes phenotypic switching of vascular smooth muscle cells by activating autophagy in pulmonary arterial hypertension. Pulm Pharmacol Ther. 2019;55:38–49.