?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The testes are a sensitive organ to electromagnetic pollution and people are concerned about the harmful effects of the radiofrequency radiation (RFR) emitted from cellular phones. Therefore, the purpose of this study was to investigate the effects of long-term exposure to different RFR frequencies on single-strand DNA breaks and oxidative changes in rat testicular tissue. Twenty-eight male Sprague–Dawley rats were divided randomly into four groups. Three groups were exposed to radiation emitted from 900, 1800 and 2100 MHz RF generators, 2 h/day for 6 months. The sham-control group was kept under the same experimental conditions but the RFR generator was turned off. Immediately after the last exposure, testes were removed and DNA damage, 8-hydroxydeoxyguanosine (8-OHdG), malondialdehyde (MDA), total antioxidant status (TAS), total oxidant status (TOS) and oxidative stress index (OSI) were analyzed. The results of this study indicated that RFR increased TOS, OSI, MDA and 8-OHdG (p < 0.05). TAS levels in the exposed group were lower than in the sham group (p < 0.05). In terms of DNA damage, the tail intensities in the comet assay were higher in the exposure groups (p < 0.05). This study demonstrated that long-term exposure to RFR emitted by cellular phones may cause oxidative stress and oxidative DNA damage in rat testicular tissue and may generate DNA single-strand breaks at high frequencies (1800 and 2100 MHz). Our results showed that some RFR emitted from cellular phones has potential to lead to cell damage in the testes.
Introduction
Cellular phones have become an indispensable part of modern telecommunication. It was estimated in 2014 that there were 6.9 billion cellular phone memberships globally which continues to increase with the development of new generation mobile communication systems such as 4 G-5G. Given the large number of cellular phone users, it is important to examine, understand and monitor the possible impacts of radiofrequency radiation (RFR) on public health [Citation1]. Cellular phones with a communication basis emit radiofrequency and electromagnetic waves (EMW) in the 800 and 2200 MHz frequency range [Citation2]. It is known that biological systems put forth different biological responses to electromagnetic wave exposure at different frequency and intensities [Citation3]. In recent years, wireless or corded headsets have been preferred when talking on the phone instead of cellular phones directly up to the ear. However, when people use cellular phones in this way, they usually carry the phone in the front pocket of their pants, in their hand or on their belt. Thus, while trying to protect the brain from the effect of the phone in talk mode, the testes are left in the domain. The antenna of the cellular phone gets too close to the testes when it is carried on the belt or in trouser pockets. Due to the sensitivity of the testes to electromagnetic radiation, concerns about the negative impact of RFR on them have risen.
Wave frequency and exposure duration are among the primary factors that play a role in the harmful impacts of cellular phone radiation on the reproductive system of males [Citation4, Citation5]. There are reports that being subjected to cellular phone radiation may lead to infertility and undesired impacts in the reproductive system [Citation6, Citation7] such as single and double strand DNA breaks [Citation8], decrease in sperm concentration and serum testosterone levels [Citation9, Citation10], reduction in sperm vitality and motility [Citation11], increased ROS (reactive oxygen species) [Citation12], decrease in seminiferous tubule diameter and epithelium thickness [Citation13]. It is known that excessive production of ROS and lack of antioxidants in the cells lead to imbalances resulting in molecular oxidative damage, cellular membrane peroxidation, DNA damage and amino acid modifications [Citation14–16].
Contrary to the afore mentioned findings, some researchers have asserted that being subject to the non-ionizing radiation emitted by cellular phones has no undesired impacts on the testes and other biological tissues [Citation17–19]. The contradictory reports on the effects on testes tissue exposed to non-ionizing radiation emitted from cellular phones determined in previous studies, along with the rapid increase of cellular phone usage, encouraged us to carry out this study. The purpose of the present study was to evaluate the possible impacts of exposure to 900, 1800 and 2100 MHz chronic radiation emitted by cellular phones on the DNA integrity and oxidative status in the testes tissue of rats and to compare the impacts of radiation at these frequencies on testes.
Materials and methods
Ethics statement
All tentative protocols applied on the rats were carried out with respect to the Principles of Laboratory Animal Care and the rules of Scientific and Ethics Committee of Dicle University Health Research Center. This research was confirmed by the ethics committee of the Dicle University.
Animals
Twenty-eight male Sprague–Dawley adult rats (mean weight of 282.42 ± 16.18 g) were obtained from the Health Sciences Research and Application Center of Dicle University. Following a week of adaptation period, they were set apart into four random groups (seven rats per group): Group 1 (sham-control), Group 2 (900 MHz), Group 3 (1800 MHz) and Group 4 (2100 MHz). Rats were kept in laboratory conditions under a 12-h light/dark period at 22 ± 1 °C temperature and 45% relative humidity during the study. The rats were fed with normal tap water and tablet nutrient ad libitum (TAVAS Inc. Adana, Turkey).
Exposure and field measurements
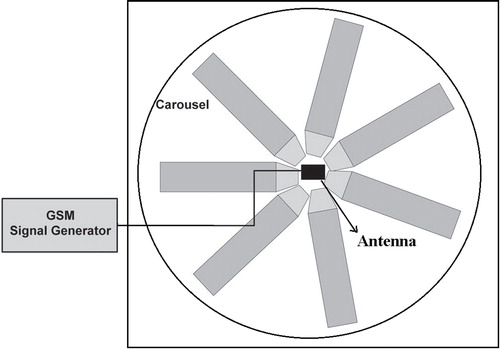
The 3 GSM signal generating set (PM10 type Everest, Adapazari, Turkey), emitting cellular phone signals at the 900 MHz, 1800 MHz and 2100 MHz frequency band, was utilized to model an exposure system. During the RFR application, the rats were put within plexiglass cages and the antenna of the generating set was set in the plexiglass carousel center to ensure ideal exposure ().
The RF exposure groups of rats were exposed to 900 MHz (Group 2), 1800 MHz (Group 3) and 2100 MHz (Group 4) RFR emitted by the RF signal generator 2 h/d for 6 months. Identical experimental conditions were applied to the sham-control group, except that the RFR generator was turned off. Electric field (V/m) and electromagnetic power density (mW/cm2) within the plexiglas was measured by using EMR 300 (NARDA, Pfullingen, Germany) active field probe. At the end of the 6th month, all the rats studied were euthanized by Ketalar anesthesia. Immediately, the testicular tissues of the rats were taken and kept at -80 °C for analysis. Afterwards, the testicle tissue specimens of the rats were homogenized in PBS (Phosphate Buffer Saline, pH: 7.4) solution and the damage to DNA was determined by the Comet assay. In addition, total oxidant status (TOS), total antioxidant status (TAS), 8-hydroxydeoxyguanosine (8-OHdG) and malondialdehyde (MDA) analyses were conducted with the testicular tissue as endpoints for oxidative stress index (OSI), oxidative DNA damage and lipid peroxidation. The total protein content was determined using the Bradford method in all tissue specimens by means of a spectrophotometer (V-630 UV-VIS, Jasco) [Citation20].
Specific absorption rate (SAR) measurement
The parameters of the electromagnetic field close to and around the antenna in the experimental setup were measured by an EMR 300 field probe. These measurements provided accurate operation of the signal-generating set, and formed a basis for the simulations of electromagnetic exposure of the rats using SAR analysis. The measured values were used as reference for simulating the mean input power of the antenna. CST Microwave Studio was used for electromagnetic field simulations. This is based on finite integration technique (FIT) which resembles the generally used finite-difference time domain (FDTD) technique, apart from relocating spatial derivatives with integration in the simulation area. It is claimed by CST that charge and energy conversation is preserved in FIT using integral form of Maxwell’s equations contrary to the differential forms used in FDTD. This particular formulation leads to stable numerical results in time-domain.
The rat model plays a vital role in SAR simulations. Volumetric pixel (voxel) rat model which was formed used computerized tomography scans of a rat was commercially available and acquired for this study. In the voxel model, every tissue has its electrical and thermal properties measured at cellular phone frequencies. Thus, this voxel model is extremely accurate and similar voxel sets for several human models also exist for industry standard SAR calculations. Electric field and SAR distribution were simulated using CST with this rat voxel model. The simulated electric field values around the rats were consistent with the measured data using electric field probe. A sample rat with 282 g body weight was used for SAR calculations. The weight of the sample rat was nearly equal to the mean weight of the rats that were used in the experiments. SAR values are summarized in ; for 1 g, 10 g average and point SAR. The maximum point SAR values for the whole body at 900, 1800 and 2100 MHz frequency were 0.638, 0.166 and 0.174 W/kg, respectively.
Table 1. Simulated SAR values for rat testicles at three different frequencies.
Measurement of total antioxidant status (TAS)
TAS measurement in the testicular tissue was carried out by a fully automatic colorimetric measurement method in accordance with Erel [Citation21]. This method is based on color change resulting from reactions. There is an inverse relationship between the color change and the amount of antioxidants in the sample. Oxidation reactions and color formation are suppressed by antioxidants in the sample. The results of the reaction are measured spectrophotometrically and given in micromolar Trolox Equivalent per liter [Citation21]. The measurement of TAS was conducted by a TAS kit (Rel Assay, Turkey, Catalog No. RL0017) and the values were obtained on a plate reader (Thermo Scientific Multiskan FC, 2011-06, USA) at 660 nm wave length.
Measurement of total oxidant status (TOS)
The measurement of TOS was carried out by a fully automatic colorimetric measurement method in accordance with Erel [Citation22]. The spectrophotometrically measured color intensity is directly proportional to the total oxidant molecule amount in the sample. Hydrogen peroxide was used for calibrating the assay. The findings are defined in equivalents of micromolar hydrogen peroxide per liter (μmol H2O2 Equiv./L). TOS measurement was carried out by a TOS kit (Rel Assay, Turkey, Catalog No. RL0024) and the values were obtained on a plate reader (Thermo Scientific Multiskan FC, 2011 -06, USA) at 530 nm wave length.
Calculation of oxidative stress index (OSI)
OSI is an index of oxidative stress level and is equal to the percentage of the TOS to the TAS. It was found according to the formula below [Citation22]:
where TOS is presented as μmol H2O2 Eq./L and TAS is presented as μmol troloks Eq./L.
Measurement of malondialdehyde (MDA) content
The measurement of the MDA content was carried out using the commercial MDA ELISA (enzyme-linked immunosorbent assay) kit (Eastbiopharm, catalog no. CK-E10376) and the values were obtained on a plate reader (Thermo Scientific Multiskan FC, 2011-06, USA) using ELISA.
Measurement of 8-hydroxydeoxyguanosine (8-OHdG)
The measurement of 8-OHdG was conducted by means of a commercial 8-OHdG ELISA kit (Eastbiopharm, Cat. No. CK-E11652) in a plate reader (Thermo Scientific Multiskan FC, 2011-06, USA) using ELISA technique. In this method, color development is stopped and the intensity of the color is measured; the color develops inversely proportional to the amount of 8-OHdG in the sample. The data are defined in nanograms per milliliter (ng/mL).
Comet assay
DNA damage was measured using alkaline comet assay (single-cell gel electrophoresis) [Citation23, Citation24]. The alkaline version of the method is used to measure single-strand damage of DNA [Citation23, Citation24]. However, double strand DNA damage can also be observed in case the comet assay is used under neutral conditions [Citation25]. For the analysis, the supernatant of testicular tissue homogenate was used [Citation26]. All microscope slides were covered with a coat of agarose (0.5%, normal melting point) and dehydrated at room temperature. Then, 10 mL-cell emulsion was mixed with 100 mL of 0.8% low melting point agarose in PBS at 37 °C and dropped onto the bottom layer. Slides were kept for 5 min at 4 °C in a humid box to solidify. The cover glass was taken and the slides were put into freshly prepared cold lysis buffer (25 g sodium dodecyl sulfate (SDS) in tris-borate-EDTA (TBE) buffer) at 4 °C for 50 min. In order to unwind the DNA, the slides were taken from the lysis tampon, then dehydrated and put in a horizontal electrophoresis unit being laden with new alkaline electrophoresis solution (TBE, pH 13) which contained 54 g Tris, 27.5 g boric acid, 20 mL ethylenediaminetetraacetic acid (EDTA) for 20 min. Electrophoresis was performed at 300 mA and 20 V for 30 min at room temperature. Next, the slides were bathed with pure water for 5 min for extracting the alkali ions and detergents. Following neutralization, the slides were stained with 50 mL of ethidium bromide (1 mg/mL) and coated with the cover glass. All phases were conducted under dimmed light to avoid additional DNA damage [Citation27]. The examination was made by a fluorescent microscope (Olympus, Japan) at a magnification of 400x. For each sample, 100 nuclei images chosen randomly were analyzed using comet assay software (CASP, publicly available, http://www.casp.of.pl, Wroclaw, Poland) [Citation28]. The variables of tail moment (tail diameter/head diameter or DNA% in tail × tail length) and tail intensity (DNA% in the tail of the comet) were examined in this study. Undamaged DNA does not lose its integrity and does not form a comet tail, whereas fragments of damaged DNA move at different speed in the electrical field and create a ‘comet’ pattern because they have different molecular weights and different electrical charges due to damage [Citation24].
Statistical analysis of data
SAS (Statistical Analysis System) 9.4.1 was utilized for statistical evaluation of research data. The Kolmogorov–Smirnov test was applied to define whether the data had normal distribution and it was determined that the data had normal distrubution. To assess whether there were statistically significant differences between the groups, one-way analysis of variance (ANOVA) was used. In accordance with the variance analysis, the Duncan multiple comparison tests were applied to specify which groups presented difference with regards to variables. Results were presented as mean values with standard deviation (±SD) and differences were considered statistically significant at p < 0.05 level.
Results and discussion
DNA damage evaluated by comet assay
Tail moment and tail intensity (%) as the comet parameters which demonstrate DNA damage level formed by distinct RFR in the testicles of the experimental groups. Comparisons were made with the sham-control group and among the RFR groups.
As shown in , the tail intensity and tail moment, which are the DNA damage indicators, increased with frequency. Whereas the increase in tail intensity was statistically significant (p < 0.05), the incease in tail moment was found non-significant (p > 0.05). Duncan multiple comparison test showed that the increase in the tail intensity was statistically significant among groups when compared with each other and with the sham control only in Group 3 and Group 4 (p < 0.05). These results indicate that the exposure of male rats to 1800 and 2100 MHz RFR for six months was associated with an increase in DNA damage in the testes as detected by alkaline comet assay.
Table 2. DNA damage in rat testes evaluated based on tail moment and tail intensity (%) in comet assay.
Analysis of TAS, TOS, OSI, 8-OHdG and MDA
The TOS value in the testicles in the exposure groups was significantly higher than that in the sham-control group (). There were also statistically significant differences between the exposure groups (p < 0.05). The TAS value in the testicles significantly decreased in the exposure groups as compared to the sham-control group (p < 0.05). When the experimental groups were compared among themselves, the decrease in Group 3 was statistically non-significant (p > 0.05). A statistically significant increasing in the OSI parameter was observed in all exposure groups as compared to the sham control group, but the difference between Group 3 and Group 4 was statistically non-significant (p > 0.05). The RFR exposure groups showed a statistically significant increase in 8-OHdG and MDA parameters in comparison to the sham control group (p < 0.05). The RFR exposure groups showed statistically significant differences between each other. These results showed that 900, 1800 and 2100 MHz RFRs could cause a change in the biochemical parameters of testicular cells.
Table 3. TOS, TAS, OSI, 8-OHdG and MDA parameters in rat testes.
Testicles are the male reproductive organs where sperm production takes place. When cellular phones are carried inside the trouser pockets or on the belt, they are very close to the testicles. This results in the testicles being exposed to the EMF waves emitted from the cellular phones for hours during the day. Electromagnetic fields may increase the reactive oxygen species (ROS) production by changing the levels of energy and spin orientations of electrons [Citation29]. Since testicles contain large amounts of peroxidation-sensitive polyunsaturated fatty acids (PUFAs), they are hypersensitive to ROS induced oxidative damage [Citation30]. The electromagnetic energy emitted from cellular phones penetrates live tissue and may result in degenerative effects on different biological systems [Citation31]. DNA is important for cellular functions, reproduction, viability and inheritance of genetic material from generation to the generation. Therefore, many studies have focused on the potential damages that may be induced in the DNA molecule as a result of cellular phone radiation.
Even though the number of studies on the influence of cellular phones on reproductive functions has increased in recent years, no consensus has been reached. It has been put forth in many studies that exposure to RFR emitted by cellular phones may lead to various negative effects [Citation31–33]. Akdag et al. [Citation28] examined whether 2.4 GHz (Wi-Fi) RFR causes DNA damage in rats brain, skin, liver, kidney and testicle tissue through the Comet assay method. They found that long exposure (12 months) to 2.4 GHz RFR was associated with induced DNA damage in the testicle tissue, but did not cause significant DNA damage in brain, skin, liver and kidney tissues. Thus, it has been suggested that testicles are more sensitive to RFR. Kesari et al. [Citation34] reported that RF-EMF might cause oxidative stress with an increased level of reactive oxygen species, which may provoke infertility. Another study reported that electromagnetic radiation emitted from Wi-Fi (2450 MHz) and cellular phones (900 and 1800 MHz) causes an increase in lipid peroxidation and a decrease in the levels of antioxidant trace elements (copper and zinc) along with TAS and glutathione (GSH) in the kidneys and testicles of rats in their development periods, and thus leads to oxidative stress [Citation29]. Saygın et al. [Citation35] also reported that exposure to electromagnetic radiation emitted by wireless devices (2.45 GHz; 3 h/day, 130 days) led to an increase in the MDA content and TOS value of Sprague–Dawley rats in addition to a decrease in the TAS value. Rago et al. [Citation36] reported a statistically significant relationship between the SAR and oxidative DNA damage biomarker 8-OHdG and DNA fragmentation, after exposure to RF-EMR. Even though there are many similar studies in the literature which report the harmful impacts of cellular phones on reproductive functions, there are also studies which report no impacts on testicles functions [Citation17, Citation37, Citation38]. It is known that degenerative effects such as DNA damage and oxidative stress which may be due to RFR depend on the type of cell and experiment setting (exposure time, frequency, SAR, continuous wave, pulsed wave, being exposed as a cellular phone owner etc.) [Citation39, Citation40].
In the present study, the TAS levels of all RFR exposure groups decreased statistically significantly in comparison to the sham-control group; however the TOS, OSI, MDA and 8-OHdG values were increased significantly. The changes in these parameter levels support some previous observations of the impact of cellular phone radiation on the testicles. Our findings are in accordance with the findings of many different studies [Citation28, Citation35]. The significant increases in the lipid peroxidation indicator MDA and oxidative DNA damage bio-marker 8-OHdG in the testicles tissue in this study may be due to ROS generated from RFR emitted from cellular phones. It is known that even though testicles contain high amounts of PUFAs, which are sensitive to peroxidation, they also contain low amounts of antioxidants and are thus sensitive to oxidative damage caused by ROS [Citation28]. Lipid peroxidation may lead to cellular damage in the spermatozoa because of the increase in the fluidity of sperm cell membrane. The damage of sperm membrane resulting from lipid peroxidation may be due to the increase in ROS levels, like superoxide anion, hydrogen peroxide and hydroxyl radicals, caused by cellular phone radiation [Citation8]. It is also believed that RFR may induce DNA base modification by increasing the activation of free radicals and suppressing antioxidants. Dasdag et al. [Citation41] observed that there were increased levels of MDA and TOS due to exposure to 900 MHz RFR emitted by cellular phones leading to an increase in lipid peroxidation and oxidative stress thus causing oxidative damage. Tas et al. [Citation42] reported that long-term exposure (3 h per day, 7 d a week for one year) to 900 MHz RFR could alter some reproductive parameters in adult Wistar Albino rats. Increased ROS production resulting from cellular phone radiation may lead to changes in macromolecules like membrane lipids, proteins and DNA in human spermatozoa in vitro [Citation43].
A statistically significant increase was observed in DNA strand breakage in the testicle tissues of rats subject to long term RFR similar to the RFR emitted from cellular phones. The comet assay findings of this study indicated that exposure to RFR at high frequencies (1800 and 2100 MHz) caused DNA single strand breakage in testicles. Since electromagnetic energy is directly proportional to the frequency of the wave, higher frequencies mean higher energies [Citation39]. The statistically significant increase in the damage to testicular tissues subjected to RFR was in accordance with the findings of various other studies [Citation44, Citation45]. The significant increase in DNA fragmentation seen in testicular tissue indicates that men should be more careful when using or keeping cellular phones for long periods of times near their testicles because DNA strand breaks have been associated with necrosis, aging and cancer [Citation46]. The ROS derived from oxidative damage owing to RFR emitted by signal generators may be the primary reason for the significant increase in the DNA strand breakage in the testicular tissue of rats observed in this study. This is because the free radical assaults on the DNA are a probable cause of DNA damage in the cells [Citation28]. Owing to the existence of numerous negatively loaded phosphate groups in DNA, Fe and Cu transition metal ions are constantly bound to DNA, and Fe and Cu ions released from proteins in the cell owing to the effect of oxidative stress could also bind to DNA. The binding of Fe and Cu metals to the deoxyribose sugars of DNA transforms the DNA into a target for hydrogen peroxide (H2O2), which in turn leads to Haber-Weiss and Fenton reactions generating hydroxyl radicals (OH•) with high reactivity, resulting in significant damage in the cell [Citation14, Citation46]. Also, it has been reported in various studies that electromagnetic fields (EMF) induce the Fenton reaction and free radical activity in the cell [Citation47]. According to our findings, cellular phone radiation especially emited at high frequencies may cause various types of damage in testes. When a wireless bluetooth or corded headset is used, the effects may be reduced if the cellular phone is distant enough from the body. Hence, the use of cellular phones as far away from the body as possible is required. Especially, cellular phones should be kept away from testicles.
Conclusions
The results from the present study indicated that there was a higher level of oxidative stress and DNA damage in the testes of rats exposed to the different cellular phone frequencies than in the sham-control group. In addition, the study findings demonstrated that long-time exposure to RFR emitted by cellular phones might provoke oxidative stress and oxidative DNA damage in rat testicular tissue and may generate DNA single strand breaks at high frequencies (1800 and 2100 MHz). Based on the results from this study, we speculate that RFR exposure might cause infertility; however, more elaborate long-term studies at the molecular level are required.
Disclosure statement
The authors report no conflicts of interest.
References
- Pandey N, Giri S, Das S, et al. Radiofrequency radiation (900 MHz)-induced DNA damage and cell cycle arrest in testicular germ cells in swiss albino mice. Toxicol Ind Health. 2017;33(4):373–384.
- Al-Bayyari N. The effect of cell phone usage on semen quality and fertility among Jordanian males. Middle East Fert Soci J. 2017;22:178–182.
- Topal Z, Hanci H, Mercantepe T. The effects of prenatal long-duration exposure to 900-MHz electromagnetic field on the 21-day-old newborn male rat liver. Turk J Med Sci. 2015; 45:291–297.
- Yahyazadeh A, Deniz OG, Kaplan AA, et al. The genomic effects of cell phone exposure on the reproductive system. Environ Res. 2018; 167:684–693.
- Kesari KK, Kumar S, Behari J. Effects of radiofrequency electromagnetic waves exposure from cellular phone on reproductive pattern in male Wistar rats. Appl Biochem Biotechnol. 2011;164(4):546–559.
- Dasdag S, Taş M, Akdag MZ, et al. Effect of long-term exposure of 2.4GHz radiofrequency radiation emitted from Wi-Fi equipment on testes functions. Electromagn Biol Med. 2015;34(1):37–42.,
- Vignera SL, Condorelli RA, Vicari E, et al. Effects of the exposure to mobile phones on male reproduction: a review of the literature. J Androl. 2012; 33:350–356.
- Kumar S, Nirala JP, Behari J, et al. Effect of electromagnetic irradiation produced by 3G mobile phone on male rat reproductive system in a simulated scenario. Indian J Exp Biol 2014;52(9):890–897.
- Pandey N, Giri S. Melatonin attenuates radiofrequency radiation (900 MHz)-induced oxidative stress, DNA damage and cell cycle arrest in germ cells of male Swiss albino mice. Toxicol Ind Health. 2018;34(5):315–327.
- Mugunthan N, Anbalagan J, Meenachi S. Effects of long term exposure to a 2G cell phone radiation (900-1900 MHz) on mouse testis. Int J Sci Res 2014; 3:523–529.
- Zalata A, El-Samanoudy AZ, Shaalan D, et al. In vitro effect of cell phone radiation on motility, DNA fragmentation and clusterin gene expression in human sperm. Int J Fertil Steril 2015;9(1):129–136.
- Saygin M, Ozmen O, Erol O, et al. The impact of electromagnetic radiation (2.45 GHz, Wi-Fi) on the female reproductive system: the role of vitamin C. Toxicol Ind Health. 2018;34(9):620–630.
- Hanci H, Odaci E, Kaya H, et al. The effect of prenatal exposure to 900 megahertz electromagnetic field on the 21 old day rat testicle. Reprod Toxicol. 2013; 42:203–209.
- Alkis ME, Bilgin HM, Akpolat V, et al. Effect of 900-, 1800-, and 2100-MHz radiofrequency radiation on DNA and oxidative stress in brain. Electromagn Biol Med. 2019;38(1):32–47.
- Aitken RJ, De Iuliis GN, Finnie JM, et al. Analysis of the relationships between oxidative stresses, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod. 2010;25(10):2415–2426.
- Agarwal A, Sekhon LH. Oxidative stress and antioxidants for idiopathic oligoasthenoteratospermia: is it justified?. Indian J Urol. 2011;27(1):74–85.
- Odacı E, Ozyılmaz C. Exposure to a 900 MHz electromagnetic field for 1 hour a day over 30 days does change the histopathology and biochemistry of the rat testis. Int J Radiat Biol. 2015;91(7):547–554.
- Imai N, Kawabe M, Hikage T, et al. Effects on rat testis of 1.95 GHZ W-CDMA for IMT-2000 cellular phones. Syst Biol Reprod Med. 2011;57(4):204–209.
- Su L, Yimaer A, Xu Z, et al. Effects of 1800 MHz RF-EMF exposure on DNA damage and cellular functions in primary cultured neurogenic cells. Int J Radiat Biol. 2018;94(3):295–305.
- Bradford MM. A rapid sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254.
- Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37(2):112–119.
- Erel O. A new automatedri colometric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–1111.
- Cam ST, Seyhan N. Single-strand DNA breaks in human hair root cells exposed to mobile phone radiation. Int J Radiat Biol. 2012; 88:420–424.
- Singh NP, McCoy MT, Tice RR, et al. A simple technique for quantization of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–191.
- Erel Y, Yazici N, Ozvatan S, et al. Detection of irradiated quail meat by using DNA comet assay and evaluation of comets by image analysis. Radiat. Phys. Chem 2009;78(9):776–781.
- Cerda H. Detection of irradiated fresh chicken: pork and fish using the DNA comet assay. LWT - Food Sci Technol. 1998;31(1):89–92.
- Haines G, Marples B, Daniel P, et al. DNA damage in human and mouse spermatozoa after in vitro-irradiation assessed by the comet assay. Adv Exp Med Biol 1998; 444:79–91.
- Akdag MZ, Dasdag S, Canturk F, et al. Does prolonged radiofrequency radiation emitted from wi-fi devices induce dna damage in various tissues of rats?. J Chem Neuroanat. 2016;75:116–122.
- Ozorak A, Naziroglu M, Celik O, et al. Wi-Fi (2.45 GHz)- and mobile phone (900 and 1800 mhz)- ınduced risks on oxidative stress and elements in kidney and testis of rats during pregnancy and the development of offspring. Biol Trace Elem Res 2013;156:221–229.
- Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 2007;77(2):190–201.
- Cetkin M, Kizilkan N, Demirel C, et al. Quantitative changes in testicular structure and function in rat exposed to mobile phone radiation. Andrologia 2017;49:e12761. [cited 2019 Oct 03]
- Ye W, Wang F, Zhang W, et al. Effect of mobile phone radiation on cardiovascular development of chick embryo. Anat Histol Embryol. 2016;45(3):197–208.
- Mugunthan N, Anbalagan J, Samy AS, et al. Effects of chronic exposure to 2G and 3G cell phone radiation on mice testis-A randomized controlled trial. Int J Cur Res Rev 2015;7:36–47.
- Kesari KK, Agarwal A, Henkel R. Radiations and male fertility. Reprod Biol Endocrinol. 2018;16(1):118. [cited 2019 Oct 03]
- Saygin M, Caliskan S, Ozguner MF, et al. Impact of L-carnitine and selenium treatment on testicular apoptosis in rats exposed to 2.45 GHz microwave energy. West Indian Med J. 2015; 64:55–61.
- Rago R, Salacone P, Caponecchia L, et al. The semen quality of the mobile phone users. J Endocrinol Invest. 2013;36(11):970–974.
- Dasdag S, Akdag MZ. The link between radiofrequencies emitted from wireless technologies and oxidative stress. J Chem Neuroanat. 2016;75:85–93.
- Dasdag S, Akdag MZ, Ulukaya E, et al. Mobile phone exposure does not induce apoptosis on spermatogenesis in rats. Arch Med Res. 2008;39(1):40–44.
- Desai NR, Kesari KK, Agarwal A. Pathophysiology of cell phone radiation: oxidative stress and carcinogenesis with focus on male reproductive system. Reprod Biol Endocrinol. 2009;7(1):114. [cited 2019 Oct 03]
- Vijayalaxmi Prihoda TJ. Genetic damage in human cells exposed to non-ionizing radiofrequency fields: a meta-analysis of the data from 88 publications (1990-2011). Mutat Res 2012;749:1–16.
- Dasdag S, Bilgin HM, Akdag MZ, et al. Effect of long term mobile phone exposure on oxidative-antioxidative processes and nitric oxide in rats. J Biotechnol Biotechnol Equip. 2008;22(4):992–997.[Mismatch005D
- Tas M, Dasdag S, Akdag MZ, et al. Long-term effects of 900 MHz radiofrequency radiation emitted from mobile phone on testicular tissue and epididymal semen quality. Electromagn Biol Med. 2014; 33(3):216–222.
- De Iuliis GN, Newey RJ, King BV, et al. Mobile phone exposure radiation induces reactive oxygen species production and DNA damage in mouse spermatozoa in vitro. PLoS One. 2009;4(7):e6446. [cited 2019 Oct 03];
- Kumar S, Kesari KK, Behari J. Evaluation of genotoxic effects in male Wistar rats following microwave exposure. Indian J Exp Biol 2010;48(6):586–592.
- Gandhi G, Kaur G, Nisar U. A cross-sectional case control study on genetic damage in individuals residing in the vicinity of a mobile phone base station. Electromagn Biol Med. 2015;34(4):344–354.
- Phillips JL, Singh NP, Lai H. Electromagnetic fields and DNA damage. Pathophysiology 2009;16(2-3):79–88.
- Lai H, Singh NP. Magnetic-field-induced DNA strand breaks in brain cells of the rat. Environ Health Perspect. 2004;112(6):687–694.