Abstract
A temperature-sensitive mutant of Corynebacterium glutamicum is widely used as the fermentation strain in L-glutamate industrial production. Rapid synthesis of L-glutamate in this type of strain can be triggered by raising the culture temperature. However, the molecular mechanism of the temperature-sensitivity trait and L-glutamate excretion has not been clarified. In this study, we compared the genome sequence of an industrial L-glutamate-producing strain C. glutamicum TCCC11822 with the wild-type strain C. glutamicum ATCC13032. Seven mutant genes related to cell membrane synthesis were screened and separately integrated into the genome of C. glutamticum ATCC13032 to replace the corresponding genes. All the recombinant strains showed temperature-sensitivity and increased L-glutamate excretion. Electron microscopy showed that there was no significant change in cell morphology of recombinant strains at different temperatures. Genotypes found in this article can be applied to the construction of temperature-sensitive engineering strains and the regulation of cell permeability.
Introduction
L-glutamate is a bulk fermented product with a large market demand. Corynebacterium glutamicum is a Gram-positive bacterium that was originally isolated as an L-glutamate producer, but the wild-type strain does not usually excrete L-glutamate [Citation1, Citation2]. Induction of L-glutamate excretion of C. glutamicum usually requires biotin limitation or treatment with antibiotics or surfactants [Citation3–6]. However, due to the poor L-glutamate yields, these methods cannot achieve the requirement for industrial-scale fermentation [Citation7].
To further improve the performance of L-glutamate production, temperature-sensitive mutants of C. glutamicum were generated and a temperature-triggered process was adopted [Citation8–10]. The whole fermentation process can be divided into two phases. The first phase corresponds to the growth of bacteria at normal temperature, while the second phase is L-glutamate production period at higher temperature. Six-fold increase in L-glutamate excretion flux was measured when the L-glutamate export system was activated by a temperature shift [Citation8, Citation11]. The activity of this transport system was proposed to be altered by modifications in cell membrane or cell wall properties. It was demonstrated that the fatty acids unsaturation ratio was increased and the cytoplasmic membrane and fluidity were changed during the L-glutamate production phase of the temperature triggered process [Citation12]. However, because these temperature-sensitive mutants were commonly generated by classical whole-cell mutagenesis, the molecular mechanisms underlying the induction of L-glutamate excretion have not been yet understood.
Omics-based approaches can solve the bottleneck problems by providing comprehensive genetic information on the intracellular metabolic processes for further metabolic engineering [Citation13]. C. glutamicum TCCC 11822 is a temperature-sensitive strain which was obtained by repeated random mutation and selection from the wild-type strain C. glutamicum ATCC 13032. This strain certainly has the ability to produce high levels of L-glutamate at a temperature of 39 °C, higher than its optimal growth temperature. However, few data on the reasons for the formation of temperature-sensitive trait have been reported. In this study, the whole genome of C. glutamicum TCCC 11822 was sequenced and compared with C. glutamicum ATCC 13032. Seven mutant genes related to cell membrane synthesis were screened and their functions were studied. These mutant genes were separately integrated into the genome of C. glutamticum ATCC 13032 to replace the corresponding genes. The fermentation results showed that mutation in the genes involved in cell membrane synthesis can cause temperature-sensitivity and promote L-glutamate production but has no influence on the cell morphology of C. glutamticum ATCC 13032. These new findings can provide some evidences for understanding the mechanism of L-glutamate excretion of C. glutamicum at the genetic level and improve the fermentation efficiency and controllability of L-glutamate production.
Materials and methods
Strains, plasmids, primers and culture medium
The L-glutamate producing strain C. glutamicum TCCC 11822 is stored at the China General Microbiological Culture Collection Center with the identifiers of CGMCC 1.16145. Wild-type strain C. glutamticum ATCC13032 was used as the control strain. Plasmids and primers used for molecular biological manipulation were listed in and Supplemental Table S1, respectively.
Table 1. Strains and plasmids used in this work.
The seed medium contained 40 g/L glucose, 20 mL/L corn steep liquor, 2 g/L KH2PO4, 2 g/L MgSO4, 2.5 mg/L MnSO4, 5 mg/L FeSO4, 0.5 g/L methionine, 0.2 mg/L vitamin B1 and 0.5 mg/L biotin. The fermentation medium contained 50 g/L glucose, 30 mL/L corn steep liquor, 4.5 g/L KH2PO4, 2 g/L MgSO4, 30 mg/L MnSO4, 30 mg/L FeSO4, 0.3 mg vitamin B1 and 0.3 mg/L biotin.
Construction of plasmids and recombinant strains
Chromosomal DNA manipulations were achieved via a markerless system using the suicide vector pK18mobsacB as described previously [Citation14]. PCR and DNA digestion and ligation were performed according to the standard protocols [Citation15]. For chromosomal replacement of the native gene in C. glutamticum ATCC 13032 with the corresponding mutant gene in C. glutamicum TCCC 11822, the upstream and downstream homologous arm of mutant gene fragments containing promoter tuf were ligated into the linearised pK18mobsacB plasmid by over-lapping PCR. The recombinant pK18mobascB plasmid was transformed into C. glutamicum ATCC 13032 by electroporation, and the positive transformates were obtained via homologous recombination in the form of a double crossover. The recombinant strain of AT-90, AT-39, AT-G, AT-86, AT-66, AT-28 and AT-88 was obtained by integrating the mutant gene of cgl2090, cgl2139, fabG, cgl0286, cgl0366, cgl0628 and cgl0688 into the genome of C. glutamicum ATCC 13032, respectively. All DNA manipulations, including chromosomal editing and plasmid construction, performed in this work were verified by Sanger sequencing to ensure no extra DNA changes were introduced. The recombinant strains were tested by electrophoresis analysis of PCR amplification products (Supplemental Figures S1–S7).
Batch fermentation in shake flasks and 5 L bioreactor
Characteristics of the recombinant strains were tested by shake-flask fermentation. An appropriate amount of agar-slant cultured cells was transferred into a 500 mL flask containing 30 mL of seed medium. The culture was incubated in a rotary shaker at 32 °C and 200 rpm/min for 6–8 h. Then 5 mL seed culture liquid was inoculated into a 500 mL flask containing 30 mL fermentation culture medium. The shake-flask fermentation was performed in a rotary shaker at 200 rpm/min for 28–32 h. The fermentation temperature was controlled 32 °C for cell growth and 39 °C for L-glutamate production.
L-glutamate production was carried out in a 5 L bioreactor. The seed medium in the bioreactor was the same as that used for shake-flask fermentation. An aliquot of 250 mL seed culture liquid was inoculated into a 5 L bioreactor (New Brunswick, Germany) containing 2.5 L fermentation culture medium. The pH was automatically controlled at 7.0–7.2 by the addition of liquid ammonia. Dissolved oxygen was maintained above 30% by varying stirrer speed and the aeration rate. The fermentation temperature was increased from 32 to 39 °C when the concentration of dried cell weight (DCW) reached 5–6 g/L. When glucose in the fermentation medium was consumed, 60% (w/v) glucose solution was added at an appropriate rate to maintain a glucose concentration of 5 g/L.
Analytical procedure
Cell concentration was monitored by measuring OD600 using an ultraviolet spectrophotometer (Shimadzu UVmini-1240, Japan) and converted to DCW. Glucose concentration was measured using an SBA-40E biological sensor (Shandong Academy of Sciences, China). L-glutamate concentration was quantified by isocratic HPLC (Thermo Scientific UltiMate 3000, Golden Valley, MN) using precolumn derivatisation method [Citation16]. The samples were derivatized using 2,4-dinitrofluorobenzene and then injected into a Gemini C18 column (Phenomenex, Torrance, CA). An acetonitrile/water mixture (50:50 v/v) with 50 mM ammonium acetate was used as the mobile phase in gradient mixing mode at a flow rate of 1 mL/min. The wavelength of the UV detector and the temperature of column oven were 360 nm and 30 °C, respectively. Cellular morphology was observed by JCM-7000 scanning electron microscopy (JEOL Ltd., Tokyo, Japan).
Results and discussion
Comparison of growth and L-glutamate production between C. glutamicum TCCC 11822 and C. glutamicum ATCC 13032 during the temperature-triggered process
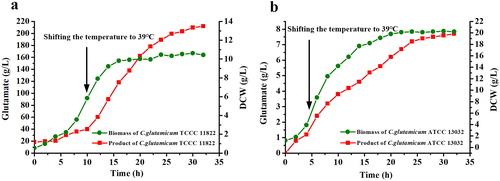
First, we compared the growth and L-glutamate production of the two strains in the temperature-triggered fermentation process in 5 L bioreactor. As shown in , the two strains showed some remarkable differences under certain L-glutamate-inducing condition. A temperature shift inhibited the growth of the mutant strain more strongly than that of the wild type strain. The growth rates of C. glutamicum TCCC 11822 and C. glutamicum ATCC 13032 were 0.58 and 1.22 gDCW/L/h at a temperature of 32 °C, but decreased to 0.21 and 0.84 gDCW/L/h at a temperature of 39 °C, respectively. Meanwhile, after a temperature shift, L-glutamate production of C. glutamicum TCCC11822 increased intensively as the growth rate diminished, reaching a synthesis rate of 7.73 g/L/h. As a comparison, the L-glutamate synthesis rate of C. glutamicum ATCC 13032 was only 0.24 g/L/h after a temperature shift.
Genome analysis of C. glutamicum TCCC 11822
C. glutamicum TCCC11822 has an obvious temperature-sensitive trait and was able to produce 210 g/L L-glutamate in 32 h fed-batch fermentation by shifting the temperature from 32 to 39 °C. To understand the molecular mechanism of the temperature-sensitivity trait and L-glutamate excretion, the whole genome of C. glutamicum TCCC 11822 was sequenced and assembled completely using PacBio single-molecule real-time sequencing technology and Illumina HiSeq 2500 technology. A total of 136,775 long reads (521.53 Mb) produced by PacBio platform were assembled to complete genome using Celera Assember version 8.0 (http://wgs-assembler.sourceforge.net/) [Citation17] with a depth of 163-fold coverage of the genome. In addition, 647 Mb paired-end reads produced by Illumina platform were used to sequence correction. Coding genes were predicted and annotated using the NCBI Prokaryotic Genome Annotation Pipeline (PGAP) version 4.1 (https://www.ncbi.nlm.nih.gov/genome/annotation_prok). The complete genome of TCCC 11822 was a circular chromosome of 3,309,401 bp with GC contents of 54.28% (). In total, 3031 protein-coding sequences (CDS), 60 tRNA, 15 rRNA, 3 tmRNA and 148 pseudo genes were identified (). All CDS were annotated with the KEGG orthology (KO) identifiers [Citation18] using KEGG Automatic Annotation Server (KAAS) version 2.1 (http://www.genome.jp/tools/kaas).
Figure 2. Genome analysis of C. glutamicum TCCC 11822. (a) Genome information of C. glutamicum TCCC 11822; (b) Comparison of genome of C. glutamicum TCCC 11822 and C. glutamicum ATCC 13032.
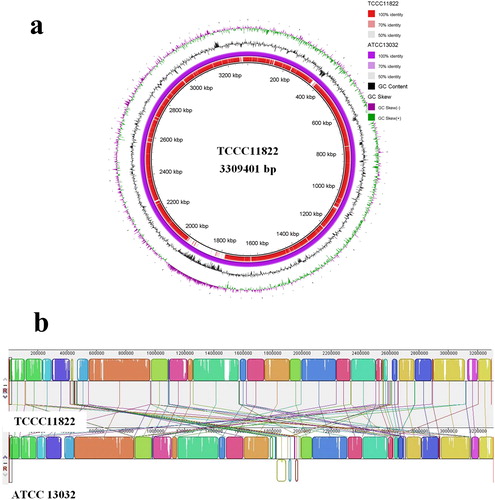
Table 2. Basic features of Corynebacterium glutamicumTCCC11822 genome.
Comparative genomics analysis of C. glutamicum TCCC11822 was performed with the wild-type strain C. glutamicum ATCC 13032 as a reference (). The unique proteins were identified using BLASTP (E-value < 10−6). In total, 337 genes were only in the genome of ATCC 13032 with no homologs in TCCC 11822, and conversely, 361 genes were only in TCCC 11822. Most of the unique proteins were annotated as hypothetical proteins. According to the KO annotation of KAAS database, we analyzed the genes missing or mutated in relevant metabolic pathways to explore the related genotypes of the temperature-sensitivity trait and L-glutamate excretion.
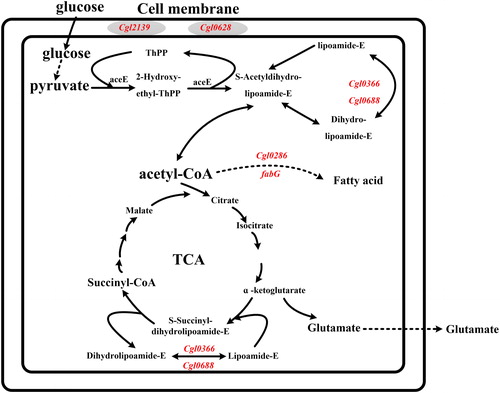
The formation of the temperature-sensitivity trait was most likely to be contributed to the mutations in the genes involved in biosynthesis of cell membrane or cell wall [Citation19]. In C. glutamicum, acetyl-CoA and malonyl-CoA are exclusively provided for the synthesis of fatty acids, which in turn are required for cell wall and cell membrane synthesis [Citation20]. The genes involving acetyl-CoA biosynthesis (cgl0366 and cgl0688) and fatty acid biosynthesis (cgl0286 and fabG) in C. glutamicum TCCC 11822 were mutated. Peptidoglycan is the main component of the gram-positive bacterial cell wall, but the UDP-N-acetylglucosamine enolpyruvyl transferase gene (cgl0352) and UDP-N-acetylmuramate dehydrogenase gene (cgl0353) involved in peptidoglycan synthesis were missing in C. glutamicum TCCC 11822 [Citation21]. Besides, peptidoglycan biosynthesis relevant genes murDEFG, mraY, mviN, pbpB, cgl0680 and cgl2699 in C. glutamicum TCCC 11822 were also mutated. Teichoic acid is a special component of the Gram-positive bacterial cell wall. The mutations of teichoic acid membrane export protein-coding gene (cgl0351) and glyceride metabolism gene (ugpABC and cgl2090) has a negative effect on the integrity of the cell wall structure. In addition, the mutation of glycosyltransferase coding gene (cgl0354 and cgl0363), lipoprotein signal peptidase coding gene (cgl2139) and lipocalin family protein-protein-coding gene (cgl0628) might also be one of the reasons for the improvementof the robustness of C. glutamicum to various stresses [Citation22–24].
Functional verification of mutant genes for cell membrane synthesis
According to the results of genomic analysis, seven mutant genes related to cell membrane synthesis were screened and their functions were studied (). We took C. glutamicum ATCC 13032 (AT) as the starting strain and separately integrated the mutant gene of cgl2090, cgl2139, fabG, cgl0286, cgl0366, cgl0628 or cgl0688 into the genome to construct the recombinant strain AT-90, AT-39, AT-G, AT-86, AT-66, AT-28 and AT-88, respectively. In order to confirm whether the mutation of these genes actually causes temperature sensitivity, the recombinant strain was cultured in shaking flask with different temperature control strategy.
Table 3. Information of mutant genes for cell membrane synthesis in C. glutamicum TCCC 11822.
As shown in , in the 32 °C fermentation process, the recombinant strains and the wild-type strain AT exhibited comparable cell growth and L-glutamate titre. The cell concentration of AT was a little higher than that of the other recombinant strains except AT-G. The L-glutamate titre of the recombinant strains except AT-G was slightly increased, but all less than 0.3 g/L. The L-glutamate titre of AT-90 was the highest, and increased by 188.9% compared to AT.
Figure 3. Temperature-triggered L-glutamate fermentation inshake-flask by recombinant strains. (a) The growth curve of 32 °C fermentation; (b) The L-glutamate production curve of 32 °C fermentation; (c) The growth curve of 32–39 °C fermentation; (d) The L-glutamate production curve of 32–39 °C fermentation.
Note: Experiments were conducted in triplicate, and values are presented as means ± SD.
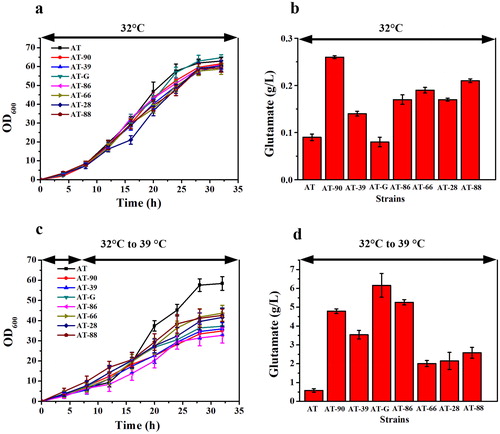
In the 32–39 °C fermentation process, the cell growth and L-glutamate titre of the recombinant strains and the wild-type strain exhibited a significant difference. The cell growth of wild-type strain AT was almost unaffected by temperature shift. The final OD600 value of AT reached 58.4, which was a decrease of only 7.1% compared to that in the constant temperature fermentation process. However, for the recombinant strains, cell growth was significantly inhibited by temperature rising. The final OD600 value of AT-90, AT-39, AT-G, AT-86, AT-66, AT-28 and AT-88 was 34.9, 35.9, 37.2, 32.7, 43.7, 41.6 and 42.6, respectively, which was a decrease of 43.7%, 41.0%, 42.5%, 46.3%, 25.5%, 31.0% and 28.3% compared to that in the constant temperature fermentation process. Interestingly, the L-glutamate titre of the recombinant strains was significantly improved with the increase of temperature. The maximum L-glutamate titre of AT-90, AT-39, AT-G, AT-86, AT-66, AT-28 and AT-88 was 4.79, 3.5, 6.2, 5.3, 2.0, 2.1 and 2.6 g/L, respectively, which was 8.2, 6.1, 10.6, 9.1, 3.5, 3.7 and 4.4-fold increase over the wild-type strain AT.
The fermentation results showed that the mutant genes in C. glutamicum TCCC 11822 () may all relate to the formation of temperature-sensitivity, and they all promoted L-glutamate excretion more or less. As shown in , the mutant genes fabG and cgl0286 of the two recombinant strains AT-G and AT-86 with highest L-glutamate titre are both related to the synthesis of fatty acids. It can be speculated that L-glutamate excretion is closely related to the variation of fatty acids content and the composition of lipids. Although the L-glutamate titre of AT-86 was lower than that of AT-G, the final OD600 value of AT-86 was the lowest, indicating that the mutant gene of cgl0286 also affects the cell growth to a large extent. The third high L-glutamate titre of recombinant strain was AT-90, and the mutant Cgl2090 catalyses the transfer of the diacylglyceryl group from phosphatidylglycerol to the sulfhydryl group of the N-terminal cysteine of a prolipoprotein, which is the first step in the formation of mature lipoproteins. Furthermore, Cgl0688 and Cgl0366 in the recombinant strains AT-88 and AT-66 are essential components of the pyruvate dehydrogenase (PDH) that related to the acetyl-CoA synthesis, and their overexpression mainly affected the cell growth rather than L-glutamate synthesis when the temperature was increased.
Electron micrograph of C. glutamicum ATCC 13032 and the recombinant strains
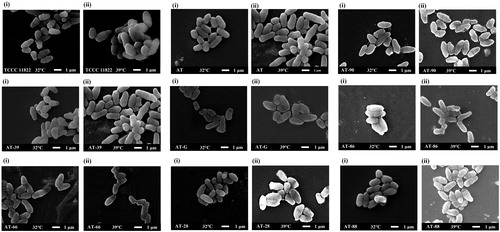
As a temperature-sensitive L-glutamate producing strain, the cell morphology of C. glutamiucm TCCC 11822 changed with temperature shift. As shown in , the cell morphology of C. glutamiucm TCCC 11822 was a typical rod shape at 32 °C, but changed to an irregular oval shape at 39 °C. However, the change of cell morphology with temperature shift was not present in the wild-type and recombinant strains. Although the surface of AT-90, AT-G, AT-28 and AT-88 cell becomes a little rough at 39 °C, the overall shape of the cell did not change, respectively. The results indicated that the mutation of genes related to cell membrane synthesis is not the main reason for the cause of the morphological changes of temperature-sensitive C. glutamicum TCCC 11822.
Relationship between cell membrane synthesis and L-glutamate production in C. glutamticum
In recent years, the production process of L-glutamate excretion caused by temperature rise has gradually replaced the fermentation method of adding biotin or other processes, due to its unique advantages such as high conversion rate, simple operation, low cost, little contamination by miscellaneous bacteria and easy industrial application [Citation25–28]. However, there are few studies on the understanding of the controlling factors involved in the temperature-sensitive L-glutamate excretion mechanism. In the currently reported L-glutamate industral producing strains, C. glutamicum TCCC 11822 is the first genome-sequenced temperature-sensitive strain [Citation29]. Seven mutant genes which related to cell membrane synthesis were screened by comparative genomics analysis and separately integrated into the genome of C. glutamticum ATCC 13032 to replace the corresponding genes. The fermentation results showed that mutation in these genes can cause temperature-sensitive trait and promote L-glutamate excretion and production in varying degrees. Nampoothiri et al. [Citation30] found that the changes in expression of the genes related to lipid or fatty acid biosynthesis caused severe alteration of phospholipid composition and temperature-sensitive growth. The strains overexpressing pgsA2 (phosphatidyl glycerophosphatesynthase) and cdsA (CDP-diacylglycerol synthase) gene exhibited an increased temperature sensitivity and showed higher amounts of L-glutamate excretion. Since the fabG, cgl0286, cgl0366 and cgl0688 genes existed in C. glutamicum TCCC 11822 are all related to fatty acids synthesis and have one or more mutation sites (Supplemental Figure S8), we inferred that their overexpression in C. glutamticum ATCC 13032 also resulted in a temperature-sensitive L-glutamate excretion via changing the phospholipid composition. However, the mechanism of the effect of other mutant genes including cg2090, cgl2139 and cgl0628 on the temperature-sensitive L-glutamate excretion was still unclear.
Regulation of cell permeability at the gene level has advantages of stability and controllability
Structure and function changes of cell membrane can directly affect the morphology and permeability of cells and further affect the metabolic process of cells. Nakamura et al. [Citation27] suggested that the structural changes of cell membranes resulted in the conformational changes of the mechanical sensitive channel protein carrier Ncgl1221, which triggered its L-glutamate transport function. Bokas et al. [Citation12] found that there were differences in the membrane fluidity of C. glutamicum 2262 at different temperatures, and that L-glutamate production was positively correlated to the membrane fluidity, suggesting that the induction of L-glutamate excretion by temperature rise was closely related to the membrane permeability. Hoischen and Krämer [Citation7] showed that excretion of L-glutamate in biotin-limited cells of C. glutamicum is mediated by carrier systems in the plasma membrane. High excretion activity could only be induced when the total lipid content of the membrane was reduced. Although traditional physical and chemical methods can regulate the permeability of cells to some extent, it is difficult to control and easy to damage the cell structure and disturb normal metabolism [Citation31]. In comparison, the regulation of cell permeability at the gene level has advantages of stability and controllability [Citation32–34]. We proved that the mutation of genes for cell membrane synthesis in C. glutamicum ATCC 13032 can promote the L-glutamate excretion without affect the cell morphology. Although the L-glutamate titre of the recombinant strains was still far less than that of the mutant strain C. glutamicum TCCC 11822, the genotype of recombinant strains is clear and easy to be obtained by modification. The effect of cooperative modification of multiple genes on the temperature-sensitive L-glutamate excretion and cell permeability will be studied in the future works.
Conclusion
In this study, we analysed the genome of temperature-sensitive C. glutamicum TCCC 11822 and determined the basic information of the genome. The existence of mutated genes involved in the cell membrane synthesis was found and the mutated genes were studied by comparing the wild-type C. glutamicum ATCC 13032 genome. It was determined that these mutant genes were some of the reasons that cause temperature-sensitivity and promote L-glutamate excretion and production. Through further electron microscope observation, it was determined that mutation of the genes related to cell membrane synthesis was not the main reason for the cause of the morphological changes of temperature-sensitive C. glutamicum TCCC 11822. Overall, comparative genome analysis and gene function verification in this study can provide reference for the research of the temperature-sensitive L-glutamate excretion mechanism.
Nucleotide sequence and strain accession number
The complete genome sequence of C. glutamicum TCCC11822 has been deposited at GenBank under the accession number CP020033. The strain is accessible in China General Microbiological Culture Collection Center with an identifier of CGMCC 1.16145.
Supplemental Material
Download PDF (1.5 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Stansen C, Uy D, Delaunay S, et al. Characterization of a Corynebacterium glutamicum lactate utilization operon induced during temperature-triggered glutamate production. Appl Environ Microbiol. 2005;71(10):5920–5928.
- Yu W, Guoqiang C, Deyu X, et al. A novel Corynebacterium glutamicum l-glutamate exporter. Appl Environ Microbiol. 2018;84(6):e02691–e02717. [cited 2019 Dec 17],
- Kataoka M, Hashimoto KI, Yoshida M, et al. Gene expression of Corynebacterium glutamicum in response to the conditions inducing glutamate overproduction. Lett Appl Microbiol. 2006;42(5):471–476.
- Eggeling L, Krumbach K, Sahm H. L-Glutamate excretion with Corynebacterium glutamicum: why is penicillin treatment or Tween addition doing the same? J Mol Microbiol Biotechnol. 2001;3:67–68.
- Ciement Y, Laneelle G. Glutamate excretion mechanism in Corynebacterium glutamicum: triggering by biotin starvation or by surfactant addition. Microbiology 1986;132:925–929.
- Radmacher E, Stansen KC, Besra GS, et al. Ethambutol, a cell wall inhibitor of Mycobacterium tuberculosis, elicits L-glutamate excretion of Corynebacterium glutamicum. Microbiology. 2005;151(5):1359–1368.
- Hoischen C, Krämer R. Membrane alteration is necessary but not sufficient for effective glutamate secretion in Corynebacterium glutamicum. J Bacteriol. 1990;172(6):3409–3416.
- Delaunay S, Gourdon P, Lapujade P, et al. An improved temperature triggered process for glutamate production with Corynebacterium glutamicum. Enzyme Microb Technol. 1999;25(8–9):762–768.
- Delaunay S, Uy D, Baucher MF, et al. Importance of phosphoenolpyruvate carboxylase of Corynebacterium glutamicum during the temperature triggered glutamic acid fermentation. Metab Eng. 1999;1(4):334–343.
- Uy D, Delaunay S, Germain P, et al. Instability of glutamate production by Corynebacterium glutamicum 2262 in continuous culture using the temperature-triggered process. J Biotechnol. 2003;104(1–3):173–184.
- Lapujade P, Goergen JL, Engasser JM. Glutamate excretion as a major kinetic bottleneck for the thermally triggered production of glutamic acid by Corynebacterium glutamicum. Metab Eng. 1999;1(3):255–261.
- Bokas D, Uy D, Grattepanche F, et al. Cell envelope fluidity modification for an effective glutamate excretion in Corynebacterium glutamicum 2262. Appl Microbiol Biotechnol. 2007;76(4):773–781.
- Qian M, Quanwei Z, Qingyang X, et al. Systems metabolic engineering strategies for the production of amino acids. Synth Syst Biotechnol. 2017; 2(2):87–96.
- Schäfer A, Tauch A, Jäger W, et al. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145(1):69–73.
- Sambrook J, MacCallum P, Russell D. Molecular cloning: a laboratory manual. New York (NY): Cold Spring Harbor Laboratory Press; 2001.
- Chenglin Z, Yanjun L, Jie M, et al. High production of 4-hydroxyisoleucine in Corynebacterium glutamicum by multistep metabolic engineering. Metab Eng. 2018;49:287–298.
- Denisov G, Walenz B, Halpern AL, et al. Consensus generation and variant detection by Celera Assembler. Bioinformatics. 2008;24(8):1035–1040.
- Moriya Y, Itoh M, Okuda S, et al. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007;35:W182–W185.
- Hirasawa T, Wachi M, Nagai K. A mutation in the Corynebacterium glutamicum ltsA gene causes susceptibility to lysozyme, temperature-sensitive growth, and L-glutamate production. J Bacteriol. 2000;182(10):2696–2701.
- Irzik K, van Ooyen J, Gätgens J, et al. Acyl-CoA sensing by FasR to adjust fatty acid synthesis in Corynebacterium glutamicum. J Biotechnol. 2014;192:96–101.
- Tuo S, Qian M, Xiaoqian L, et al. Double deletion of murA and murB induced temperature sensitivity in Corynebacterium glutamicum. Bioengineered 2019;10(1):561–573.
- Liu YB, Chen C, Chaudhry MT, et al. Enhancing Corynebacterium glutamicum robustness by over-expressing a gene, mshA, for mycothiol glycosyltransferase. Biotechnol Lett. 2014;36(7):1453–1459.
- Loi VV, Rossius M, Antelmann H. Redox regulation by reversible protein S-thiolation in bacteria. Front Microbiol. 2015;6:187.
- Berterame NM, Bertagnoli S, Codazzi V, et al. Temperature-induced lipocalin (TIL): a shield against stress-inducing environmental shocks in Saccharomyces cerevisiae. FEMS Yeast Res 2017;17(6):fox056. [cited 2019 Dec 17],
- Nakayama Y, Hashimoto K, Sawada Y, et al. Corynebacterium glutamicum mechanosensitive channels: towards unpuzzling “glutamate efflux” for amino acid production. Biophys Rev. 2018;10(5):1359–1369.
- Becker M, Börngen K, Nomura T, et al. Glutamate efflux mediated by Corynebacterium glutamicum MscCG, Escherichia coli MscS, and their derivatives. Biochim Biophys Acta. 2013;1828(4):1230–1240.
- Nakamura J, Hirano S, Ito H, et al. Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce l-glutamic acid production. Appl Environ Microbiol. 2007;73(14):4491–4498.
- Wei ZH, Wu H, Bai LQ, et al. Temperature shift-induced reactive oxygen species enhanced validamycin A production in fermentation of Streptomyces hygroscopicus 5008. Bioproc Biosyst Eng. 2012;35(8):1309–1316.
- Wendisch VF, Jorge JMP, Pérez-García F, et al. Updates on industrial production of amino acids using Corynebacterium glutamicum. World J Microbiol Biotechnol. 2016;32(6):105.
- Nampoothiri K, Hoischen C, Bathe B, et al. Expression of genes of lipid synthesis and altered lipid composition modulates L-glutamate efflux of Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2002;58(1):89–96.
- Fontanille P, Larroche C. Optimization of isonovalal production from α-pinene oxide using permeabilized cells of Pseudomonas rhodesiae CIP107491. Appl Microbiol Biotechnol. 2003;60(5):534–540.
- Patel TN, Park AHA, Banta S. Genetic manipulation of outer membrane permeability: generating porous heterogeneous catalyst analogs in Escherichia coli. ACS Synth Biol. 2014;3(12):848–854.
- Ni Y, Mao Z, Chen RR. Outer membrane mutation effects on UDP-glucose permeability and whole-cell catalysis rate. Appl Microbiol Biotechnol. 2006;73(2):384–393.
- Ni Y, Chen RR. Accelerating whole-cell biocatalysis by reducing outer membrane permeability barrier. Biotechnol Bioeng. 2004;87(6):804–811.