Abstract
Picea wilsonii is widely used for a forestation and landscaping in China. However, the molecular mechanisms underlying abiotic stress tolerance in this species remain elusive. In this study, we examined the transcriptomic changes induced by drought and salt stress in 8-week-old seedlings of P. wilsonii via RNA-Seq analysis; seedlings grown under normal conditions (without abiotic stress) served as a control. Approximately 36.26 Gb of transcriptome data were obtained from the three treatments. A total of 4171 (2579 up-regulated and 1732 down-regulated) and 3438 (1592 up-regulated and 1706 down-regulated) differentially expressed genes were identified under drought and salt treatments, respectively. Both RNA-Seq and qRT-PCR analyses showed that the expression of PwNAC genes, including PwNAC11, 24, 36, 38, 45, 47, 51 and 52, was greatly induced not only by salt stress but also by drought treatment, suggesting that these genes are involved in both salt and drought stress resistance in P. wilsonii. This is the first report of the transcriptome analysis of P. wilsonii. Our data will serve as a valuable genomic resource for the further characterization of the molecular mechanisms underlying abiotic stress tolerance in P. wilsonii.
Introduction
Picea wilsonii (Pinaceae) is a dominant coniferous forest tree species in northern China characterised by cold and shade tolerance. Given its broad environmental adaptability, it could serve as a good raw material for the timber industry. However, compared to other congeneric species, the natural distribution range of P. wilsonii is declining because of climate change and anthropogenic interference.
Transcription factors play an important role in plant growth and development and response to external stimuli [Citation1]. A large number of transcription factor families are widely implicated in plant development and stress response, including NAC (short for NAM, ATAF and CUC), Arabidopsis transcription activation factor (ATAF), cup-shaped cotyledon (CUC), v-myb avian myeloblastosis viral oncogene homolog (MYB), basicregion-leucine zipper (bZIP) and dehydration responsive element binding factor. NAC is a large transcription factor family that plays a pivotal role in plant growth, development, stress resistance and hormone signaling [Citation2–9]. To date, 117 NAC genes have been identified in Arabidopsis thaliana, 151 in rice (Oryza sativa), 79 in grapes (Vitis vinifera), 32 in white spruce (Picea glauca), 163 in poplar (Populus spp.), 152 in soybean (Glycine max) and 152 in tobacco (Nicotiana tabacum) [Citation8,Citation10–13]. In Arabidopsis, overexpression of NAC genes increases resistance to abiotic stresses [Citation14,Citation15]. For example, the ATAF-1 overexpressing plants showed greater resistance to drought stress than ataf-1 and ataf-2 mutant plants [Citation3]. The expression of ANAC019, ANAC055 and ANAC072 (RD26) genes is induced by salt, drought and abscisic acid (ABA) treatments, and overexpression of these genes enhances drought resistance in plants [Citation11]. In poplar, overexpression of the NAC13 gene significantly enhances salt tolerance, whereas suppression of NAC13 expression by silencing significantly reduces salt tolerance [Citation16]. In addition to their role in abiotic stress resistance, NAC transcription factors also promote plant growth and development. In wheat (Triticum aestivum), transgenic plants overexpressing the TaNAC6 gene showed not only stronger resistance to drought stress but also higher yield than the wild type [Citation17]. In white birch (Betula papyrifera), overexpression of BpNAC012 enhanced lignin accumulation in transgenic lines and improved resistance to salt and osmotic stresses [Citation18]. However, Arabidopsis thaliana ANAC069 was reported to negatively regulate salt and osmotic stress tolerance [Citation19], suggesting functional divergence of NACs. Despite the numerous studies on NAC transcription factors in model plant species and a variety of crops, the information available on NAC function in woody plants, and especially spruce, is limited.
In this study, we aimed to determine the molecular basis of abiotic stress response in P. wilsonii. Deep transcriptome sequencing of P. wilsonii seedlings was performed using the Illumina technology to investigate the effect of salt and drought stresses on gene expression. Based on the analysis of differentially expressed genes (DEGs), we further examined the expression of 13 candidate NAC genes in P. wilsonii plants treated with low temperature, hydrogen peroxide (H2O2), phytohormones and wounding. The results of this study will provide the basis for the improvement of agronomic traits in P. wilsonii.
Materials and methods
Plant materials and treatments
Eight-week-old seedlings of P. wilsonii were used for transcriptome sequencing. Seeds were germinated in the greenhouse, and seedlings were grown at 19 °C and 16 h light/8 h dark photoperiod [Citation20]. To carry out the salt treatment, seedlings were grown on Murashige and Skoog (MS) liquid medium supplemented with 300 mM NaCl for 6 h [Citation20,Citation21]. To conduct the drought treatment, seedlings were placed on dry filter paper with 35% relative humidity at 20 °C for 6 h [Citation22,Citation23]. Seedlings grown on MS liquid medium for 6 h were used as a control.
Additionally, qRT-PCR experiments were performed using 8-week-old P. wilsonii seedlings treated with various abiotic stresses. Briefly, seedlings grown on solid matrices were supplied with water (control) or water containing 300 mM NaCl, 100 μM ABA, 20 mM H2O2, 100 μM gibberellin (GA3), 100 μM indole-3-acetic acid (IAA), 100 μM methyljasmonate (Me-JA), 200 μM salicylic acid (SA) and ethylene (ET) released from 5 mM ethephon (ETH) solution for 0, 3, 6 and 12 h [Citation20,Citation24–26]. To perform the drought treatment, watering was withheld for 0, 7, 14 and 21 days. To determine the effect of low temperature, seedlings were subjected to 4 °C for 0, 3, 6 and 12 h [Citation27]. To conduct the wounding treatment, seedlings were wounded with a sharp needle and harvested at 0, 0.5, 1, 3 and 6 h. All treatments were performed in three biological replications, with 100 seedlings per replicate.
RNA-Seq library construction and transcriptome sequencing
Total RNA was extracted from P. wilsonii seedlings (Aidlab, Beijing) and analyzed using the Nanodrop Spectrophotometer. Then, mRNA was purified from total RNA samples using magnetic oligo (dT) beads. First-strand cDNA was synthesized from RNA fragments using random hexamers, and the second strand of cDNA was synthesized using buffer, dNTPs, DNA polymerase I and RNase H. The double-stranded cDNA was purified using AMPure XP beads, end-repaired and ligated to sequencing adaptors. Subsequently, the cDNA fragments (200 ± 25 bp) were separated on 2% TAE-agarose gels. RNA-Seq libraries were constructed using the Illumina’s mRNA-seq Sample Preparation Kit (Illumina Inc., San Diego, CA, USA) and sequenced using the Illumina HiSeq™ 2500 platform.
Sequence assembly and annotation
Clean sequence reads were assembled using the Trinity software program [Citation28]. These fragments were then extended to generate longer fragments (contigs), which were clustered and further assembled according to the paired-end information. The longest transcripts in the cluster were defined as unigenes. The online BLAST search tool [Citation29] was used to compare unigene sequences with non-redundant (Nr) [Citation30], Swiss-Prot [Citation31], Gene Ontology (GO) [Citation32], Clusters of Orthologous Groups (COG) [Citation33], euKaryotic Orthologous Groups (KOG) [Citation34] and Kyoto Encyclopedia of Genes and Genomes (KEGG) [Citation35]databases. Aminoacid sequences encoded by the unigenes were annotated with the HMMER [Citation36] software using the Pfam database [Citation37].
qRT-PCR
To validate RNA-Seq data, the expression of selected unigenes was examined by qRT-PCR using gene-specific primers designed with the Primer Premier version 3.0 software; the PwEF-1α gene was used as an internal reference (Supplementary material, Table S1) [Citation38]. The qRT-PCR was performed on a Step One Plus Real-time PCR system (ABI, USA) with the Power Up SYBR Green Kit (ABI) using the following conditions: 5 °C for 2 min; 95 °C for 3 min; 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Melting curve analysis was performed from 60 °C to 95 °C by increasing the temperature at a rate of 1.6 °C s−1. All qRT-PCR experiments were performed in three biological replicates, each comprising three technical replications. Relative transcript levels were calculated using the 2−ΔΔCT method [Citation39,Citation40].
Table 1. Length distribution of the contigs, transcripts, unigenes from the Trinity de novo assembly.
Results and discussion
RNA-Seq and transcriptome assembly
After removing adaptor sequences and low-quality reads, 36.26 Gb of clean reads were obtained from three treatments, including salt, drought and control, each comprising 42,328,519, 38,526,704 and 40,026,038 reads, respectively (Supplementary material, ).
Table 2. Functional annotation of Picea wilsonii transcriptome.
Shortread sequences from the three treatments were assembled into 19,509,185 contigs greater than 200 bp in length (). These contigs were further connected into 104,537 transcripts (>500 bp), with N50 value of 1046 bp and a mean length of 692.40 bp. Cluster analysis and assembly of these transcripts revealed 39,897 unigenes (>500 bp), which accounted for 17.81% of the total unigene number (223,925). The N50 value and mean length of unigenes were 572 bp and 467.86 bp, respectively.
Functional annotation and classification of unigenes
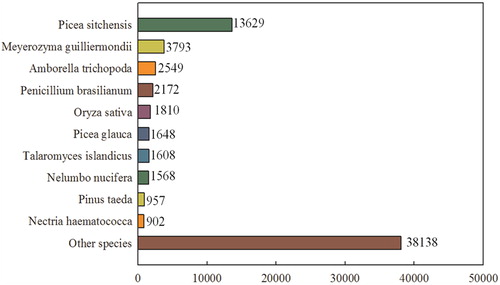
In the NCBI Nr database, homologs of P. wilsonii unigenes were identified in a wide range of plant species. Approximately 30.01% of the annotated unigenes, with the best score, showed matches in Picea sitchensis, Meyeozyma guilliermondii and Amborella trichopoda ().
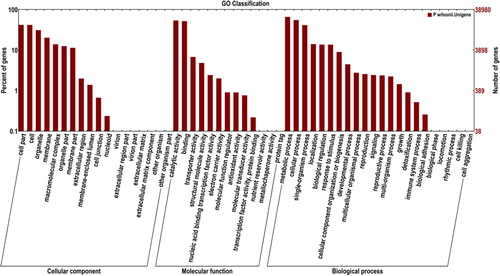
Diverse protein databases (COG, GO, KEGG, KOG, Pfam, Swiss-Prot and Nr) were used to annotate the unigenes by BLAST, with an E-value of less than 10−5 (). According to GO annotation results, 89,968 (43.27%), 48,655 (23.40%) and 69,278 (33.32%) unigenes were assigned to the biological process, molecular function and cellular component categories, respectively (, Supplementary material, Table S3).
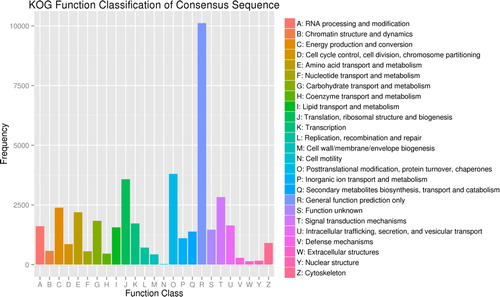
According to COG annotations, 223,925 unigenes were assigned to functional prediction and classification (, Supplemental Table S4). The largest group was the general function prediction (5187 unigenes, 16.83%), followed by translation, ribosomal structure and biogenesis (3363 unigenes, 10.91%), replication, recombination and repair (2446 unigenes, 7.29%), transcription (2194 unigenes, 7.12%), posttranslational modification, protein turnover and chaperones (2049 unigenes, 6.79%), signal transduction mechanisms (1544 unigenes, 5.01%) and carbohydrate transport and metabolism (1805, unigenes 5.86%). Notably, no unigene was assigned to extracellular structures.
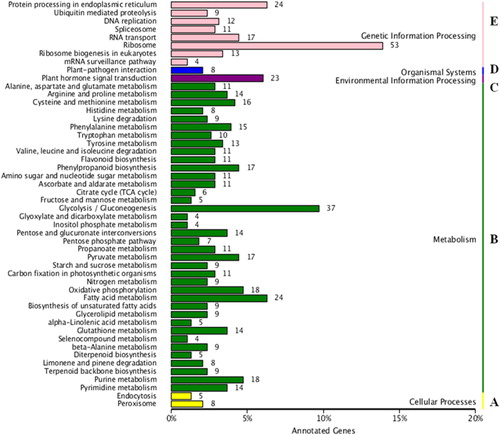
The KEGG pathway database was used to predict the pathway annotations of unigenes. A total of 18,983 unigenes were assigned to 120 biological pathways. The enriched pathways included cellular processes (13 unigenes), gene information processing (143 unigenes), metabolism (437 unigenes), organismal systems (8 unigenes) and environmental information processing (23 unigenes) (). Since the characteristics of many genes have not yet been elucidated, the present gene annotation information was incomplete, and only 33.02% of all the assembled unigenes could be annotated. Nevertheless, the data set generated in this study undoubtedly serves as a valuable resource for the investigation of specific processes, functions and pathways in P. wilsonii.
Expression and phylogenetic analyses of 13 candidate NAC genes
A total of 4171 (2579 up-regulated and 1732 down-regulated) and 3438 (1592 up-regulated and 1706 down-regulated) DEGs were identified under drought and salt treatments, respectively. Commonly regulated by drought and salt stress of these were 1364 genes.
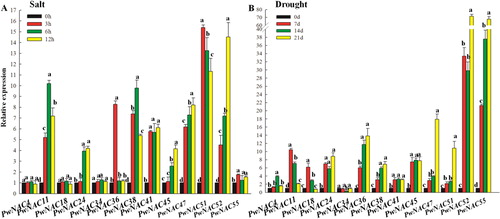
NAC transcription factors play an important role in plant growth, development, hormone signal transduction and abiotic stress response [Citation41,Citation42]. Transcriptome analysis of Arabidopsis and rice showed that 20–25% of the NAC genes are induced by at least one kind of abiotic stress such as salt, drought, cold or phytohormones [Citation9,Citation42]. In this study, to validate RNA-Seq data and perform sequence alignments, we selected 13 candidate NAC genes probably involved in salt or drought stress tolerance. In plants subjected to salt stress, expression levels of PwNAC11, 24, 36, 38, 41, 45, 47, 51 and 52 were highly up-regulated throughout the treatment. The peak expression time points were at 3 h for PwNAC36 and 51 (>7-fold higher than the control), 6 h for PwNAC11 and 38 (∼8-fold higher than the control) and 12 h for PwNAC24, 45, 47, 52 (>3-fold higher than the control). Unlike other NAC genes, which showed a gradual increase in expression, PwNAC41 expression peaked at 3 h during the salt treatment and remained unchanged thereafter (). Overall, these results showed that PwNACs potentially function at different stages to regulate salt stress resistance in the plant.
In plants treated with drought stress, PwNAC11 and 18 showed the highest expression at day 7, which was >5-fold greater than that in the control. The expression of PwNAC52 and 55 increased rapidly; compared to the control, both these genes showed 20-fold higher expression at 7 days and >66-fold higher expression (peak) at 21 days. On the contrary, the expression of PwNAC34 declined during the drought treatment, reaching the lowest level at 14 days (), suggesting that PwNAC34 plays a negative role in plant response to drought stress.
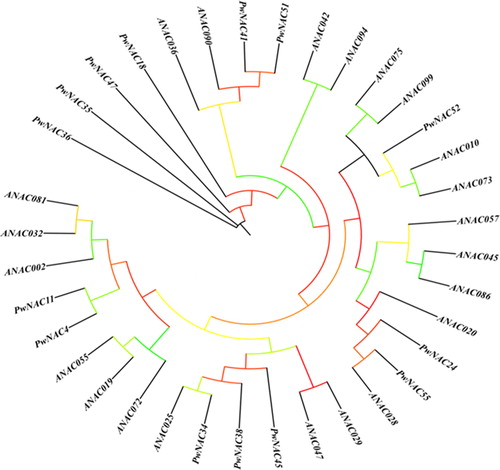
A phylogenetic tree of 13 P. wilsonii NACs and 21 Arabidopsis homologs was constructed using the neighbor-joining method with the MEGA software (). PwNAC4 and PwNAC11 were close to ANAC019, ANAC055, ANAC072 (RD26), ANAC002, ANAC032 and ANAC081 (ATAF2). PwNAC52 was similar to ANAC010 and ANAC073. PwNAC41 and PwNAC51 showed high homology to ANAC090 and ANAC036. PwNAC34, PwNAC38 and PwNAC45 were similar to ANAC025, ANAC029 and ANAC047.
Expression profiles of 13 candidate PwNAC genes under low temperature and H2O2 treatments
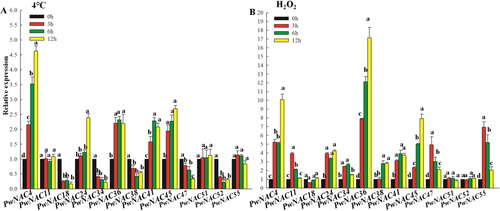
In plants subjected to low temperature (4 °C), expression levels of PwNAC4, 24, 36, 41 and 45 were up-regulated, whereas those of PwNAC18, 34, 38, 47 and 52 were repressed; three genes including PwNAC11, 51 and 55 showed almost unchanged expression in response to low temperature (). Notably, the expression level of PwNAC4 in cold-treated plants was 3.63-fold greater than that in the control at 12 h (peak expression). In plants treated with H2O2, the expression of PwNAC4,11, 24, 34, 36, 41, 45, 47 and 55 was induced rapidly; the expression level of these genes in H2O2-treated plants was >1.3-fold higher at 3 h and that of PwNAC36 was 16.12-fold higher than that in the control (). In contrast to the substantial changes in gene expression levels observed under salt and drought treatments, the low temperature treatment resulted in minimal changes in gene expression, suggesting that the NAC genes investigated in this study play more important roles in drought and salt stresses than in cold stress.
Expression profiles of 13 candidate PwNAC genes in response to phytohormones and wounding
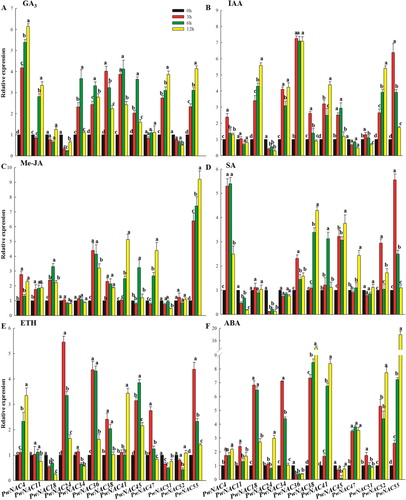
We examined the effect of five phytohormones including GA3, IAA, SA, ETH, JA and ABA on the expression of PwNAC genes. GA3 treatment induced the expression of PwNAC4, 11, 34, 36, 38, 41, 45, 51 and 55, while IAA treatment up-regulated the expression of PwNAC4, 18, 34, 36, 38, 41, 45, 52 and 55. Previously, SA, JA and ETH have been reported as three key signaling molecules involved in plant defense responses [Citation43]. Here, we found that PwNAC4, 36, 38, 41, 45, 47 and 55 were induced by Me-JA, SA and ETH (), suggesting these genes are potentially involved in plant defense against pathogens. NAC transcription factors are reported to participate in drought stress via the ABA-dependent or ABA-independent pathway [Citation42]. Our results showed that ABA enhanced the expression of PwNAC4, 11, 18, 24, 38, 41, 47, 51, 52 and 55, and the expression patterns of these genes under ABA treatment were similar to those in the drought treatment, suggesting the presence of ABA-dependent pathway in P. wilsonii, which is involved in the response to drought stress ().
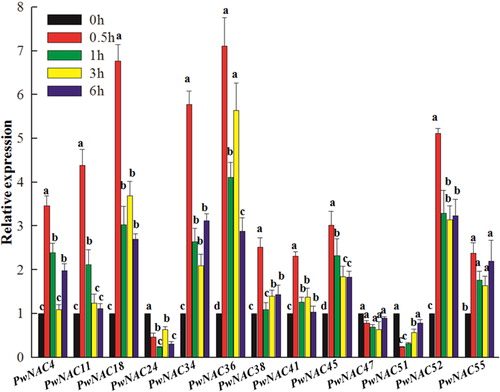
Additionally, we detected the expression pattern of NAC genes in plants subjected to wounding stress (). The expression of PwNAC4, 11, 18, 34, 36, 38, 41, 45, 52 and 55 was rapidly induced by wounding, suggesting the possibility that these genes act as early regulators of the response to mechanical damage.
Conclusions
In this study, we investigated the transcriptome of P. wilsonii plants under salt and drought treatments using the Illumina sequencing technology. Approximately 36.26 Gb of data were generated from three treatments (control, salt and drought). A total of 4171 and 3438 DEGs were identified in plants exposed to drought and salt treatments, respectively. The RNA-Seq data of PwNAC genes were validated using the qRT-PCR assay. The expression of PwNAC11, 18, 24, 36, 38, 45, 47, 51, 52 and 55 was greatly induced by drought stress, whereas that of PwNAC11, 24, 36, 38, 41, 45, 47, 51 and 52 was induced by salt stress, suggesting the involvement of these genes in abiotic stress resistance in P. wilsonii. Overall, this study provides a database for further functional genomic research on woody plants. Data generated in this study represent a rich genetic resource for breeding.
Supplemental Material
Download PDF (927.4 KB)Acknowledgment
We thank Biobreeding Biotechnology Corporation (Shaanxi, China) for conducting RNA-Seq and data analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adam E, Kozma-Bognar L, Kolar C, et al. The tissue-specific expression of a tobacco phytochrome B gene. Plant Physiol. 1996;110(4):1081–1088.
- Ooka H, Satoh K, Doi K, et al. Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 2003;10(6):239–247.
- Lu PL, Chen NZ, An R, et al. A novel drought-inducible gene, ATAF1, encodes a NAC family protein that negatively regulates the expression of stress-responsive genes in Arabidopsis. Plant Mol Biol. 2007;63(2):289–305.
- Kim SG, Kim SY, Park CM. A membrane-associated NAC transcription factor regulates salt-responsive flowering via FLOWERING LOCUS T in Arabidopsis. Planta 2007;226(3):647–654.
- Jeong JS, Kim YS, Baek KH, et al. Root-specific expression of OSNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 2010;153(1):185–197.
- Hao YJ, Wei W, Song QX, et al. Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J. 2011;68(2):302–313.
- Shao H, Wang H, Tang X. Nac transcription factors in plant multiple abiotic stress responses: progress and prospects. Front Plant Sci. 2015;6:902.
- Nakashima K, Takasaki H, Mizoi J, et al. Nac transcription factors in plant abiotic stress responses. Biochim Biophys Acta. 2012;1819(2):97–103.
- Puranik S, Sahu PP, Srivastava PS, et al. Nac proteins: regulation and role in stress tolerance. Trends Plant Sci. 2012;17(6):369–381.
- Le DT, Nishiyama R, Watanabe Y, et al. Genome-wide survey and expression analysis of the plant-specific NAC transcription factor family in soybean during development and dehydration stress. DNA Res. 2011;18(4):263–276.
- Hu H, Dai M, Yao J, et al. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci USA. 2006;103(35):12987–12992.
- Rushton PJ, Bokowiec MT, Han S, et al. Tobacco transcription factors: novel insights into transcriptional regulation in the Solanaceae. Plant Physiol. 2008;147(1):280–295.
- Pascual MB, Canovas FM, Avila C. The NAC transcription factor family in maritime pine (Pinus pinaster): molecular regulation of two genes involved in stress responses. BMC Plant Biol. 2015;15(1):254.
- Yoon HK, Kim SG, Kim SY, et al. Regulation of leaf senescence by NTL9-mediated osmotic stress signaling in Arabidopsis. Mol Cells 2008;25(3):438–445.
- Liu QL, Xu KD, Zhao LJ, et al. Overexpression of a novel chrysanthemum NAC transcription factor gene enhances salt tolerance in tobacco. Biotechnol Lett. 2011;33(10):2073–2082.
- Zhang XM, Cheng ZH, Zhao K, et al. Functional characterization of poplar NAC13 gene in salt tolerance. Plant Sci. 2019;281:1–8.
- Tang Y, Liu M, Gao S, et al. Molecular characterization of novel TaNAC genes in wheat and overexpression of TaNAC2a confers drought tolerance in tobacco. Physiol Plant. 2012;144(3):210–224.
- Hu P, Zhang KM, Yang CP. BpNAC012 positively regulates abiotic stress responses and secondary wall biosynthesis. Plant Physiol. 2019;179(2):700–717.
- He L, Shi X, Wang Y, et al. Arabidopsis ANAC069 binds to C[A/G]CG[T/G] sequences to negatively regulate salt and osmotic stress tolerance. Plant Mol Biol. 2017;93(4–5):369–387.
- Zhang H, Cui X, Guo Y, et al. Picea wilsonii transcription factor NAC2 enhanced plant tolerance to abiotic stress and participated in RFCP1-regulated flowering time. Plant Mol Biol. 2018;98(6):471–493.
- Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant cell. 1994;6:251–264.
- Li L, Yu Y, Wei J, et al. Homologous HAP5 subunit from Picea wilsonii improved tolerance to salt and decreased sensitivity to ABA in transformed Arabidopsis. Planta 2013;238(2):345–356.
- Lu M, Sun QP, Zhang DF, et al. Identification of 7 stress-related NAC transcription factor members in maize (Zea mays L.) and characterization of the expression pattern of these genes. Biochem Biophys Res Commun. 2015;462(2):144–150.
- Zhang T, Zhang D, Liu Y, et al. Overexpression of a NF-YB3 transcription factor from Picea wilsonii confers tolerance to salinity and drought stress in transformed Arabidopsis thaliana. Plant Physiol Biochem. 2015;94:153–164.
- Ma NN, Zuo YQ, Liang XQ, et al. The multiple stress-responsive transcription factor SLNAC1 improves the chilling tolerance of tomato. Physiol Plantarum. 2013;149(4):474–486.
- Song SY, Chen Y, Chen J, et al. Physiological mechanisms underlying OSNAC5-dependent tolerance of rice plants to abiotic stress. Planta 2011;234(2):331–345.
- Zhang T, Li YF, Zhou YN, et al. Cloning and expression analysis of a homologous expansin gene EXP2 in Picea wilsonii. J For Res. 2016;27(2):247–255.
- Grabherr MG, Haas BJ, Yassour M, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–652.
- Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402.
- Deng Y, Li J, Wu S. Integrated nr database in protein annotation system and its localization. Comput Engin. 2006;32:71–74.
- Apweiler R, Bairoch A, Wu CH, et al. Uniprot: the universal protein knowledgebase. Nucleic Acids Res. 2004;32(Database issue):D115–D119.
- Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet. 2000;25(1):25–29.
- Tatusov RL, Galperin MY, Natale DA, et al. The cog database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28(1):33–36.
- Koonin EV, Fedorova ND, Jackson JD, et al. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 2004;5(2):R7.
- Kanehisa M, Goto S, Kawashima S, et al. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32(Database issue):D277–D280.
- Eddy SR. Profile hidden markov models. Bioinformatics 1998;14(9):755–763.
- Finn RD, Bateman A, Clements J, et al. Pfam: the protein families database. Nucl Acids Res. 2014;42(D1):D222–D230.
- Yu Y, Li Y, Huang G, et al. PwHAP5, a CCAAT-binding transcription factor, interacts with PwFKBP12 and plays a role in pollen tube growth orientation in Picea wilsonii. J Exp Bot. 2011;62(14):4805–4817.
- Czechowski T, Stitt M, Altmann T, et al. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005;139(1):5–17.
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108.
- Tran LS, Nakashima K, Sakuma Y, et al. Isolation and functional analysis of arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell. 2004;16(9):2481–2498.
- Fujita M, Fujita Y, Maruyama K, et al. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 2004;39(6):863–876.
- Doornbos RF, Geraats BP, Kuramae EE, et al. Effects of jasmonic acid, ethylene, and salicylic acid signaling on the rhizosphere bacterial community of Arabidopsis thaliana. MPMI. 2011;24(4):395–407.