Abstract
Neonatal hypoxic-ischaemic encephalopathy (HIE) is a neurological disease that can cause neonatal death. MicroRNA-124-3p (miR-124-3p) plays an important role in the development of various diseases. In this research, our aim was to investigate the potential function of miR-124-3p in regulating HIE and to find the underlying mechanism. Here, we discovered that miR-124-3p expression was reduced and cell apoptosis was increased in HIE rat model group. Interestingly, we identified that B cell lymphoma-2-associated X protein (Bax) was a direct target gene of miR-124-3p and Bax expression was negatively modulated by miR-124-3p in HIE. Functional experiments showed that miR-124-3p expression was reduced and Bax expression was increased in oxygen and glucose deprivation (OGD)-treated neurons. In addition, the cell viability of neurons was decreased and apoptosis was increased after OGD treatment. And miR-124-3p over-expression promoted cell viability and restrained apoptosis of the OGD-treated neurons. All these effects were reversed by Bax over-expression. Our results indicated that over-expression of miR-124-3p attenuated OGD-induced neuronal injury through targeting Bax, suggesting a potential role of miR-124-3p/Bax in the pathophysiology of HIE.
Introduction
Neonatal hypoxic-ischaemic encephalopathy (HIE) is a neurological disease of newborns. It is characterized by ischaemia and hypoxia. It is the main cause of neonatal death, poor neuro-behavioural function and long-term disability [Citation1]. HIE patients not only suffer themselves, but also bring huge burdens and pressures to parents and society [Citation2]. Therefore, there is an urgent need to determine an effective treatment of neonatal HIE. In addition, it is required to find the underlying mechanism of HIE [Citation3]. Studies have reported that some of the factors that contribute to HIE include intracellular calcium overload, energy failure, oxidative stress, glutamate-mediated excitotoxicity and inflammation [Citation4–6].
Growing evidence has shown that miRNAs are involved in neonatal hypoxic-ischaemic encephalopathy and other neurological disorders [Citation7–13]. miR-124 is specifically and prominently expressed in human and rodent brains. There are two mature forms of miR-124, miR-124-3p (known as miR-124) and miR-124-5p (known as miR-124*). The expression of miR-124 in the cerebral cortex of mammals is usually high but in cancer patients with poor prognosis, it is significantly down-regulated [Citation14]. The biological function of miR-124-3p is similar to other members of the miR-124 family. According to reports, miR-124-3p is involved in the occurrence and development of various diseases [Citation15–22]. For example, miR-124-3p was reported to regulate the neural development and was an enriched miRNA in brain [Citation15,Citation16]. Over-expression of miR-124-3p can lead to cell cycle arrest in tumour cells, and inhibit their migration and invasion. It also can be used as a tumour suppressor for different cancers [Citation17–19]. The expression of miR-124-3p is significantly reduced in Alzheimer’s disease (AD) and other neurodegenerative diseases [Citation20]. In addition, miR-124-3p was reported to be involved in other diseases [Citation21,Citation22]. However, it is not clear whether miR-124-3p can regulate the pathogenesis of HIE. Therefore, the purpose of this study was to explore the role of miR-124-3p in HIE and its molecular mechanism.
Materials and methods
Animals
P7 (seven-day-old) Sprague-Dawley rats (male, 18–20 g) were purchased from the Experimental Animal Center of Kunming Medical University (China). The rats were placed in cages at a constant temperature (22–25 °C) with 40–50% humidity and a 12 h (h) light/dark cycle and food and water were available ad libitum.
Ethics statement
This experiment was approved by the Institutional Animal Care and Use Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine. Experiments were conducted in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
HIE brain injury model establishment
We divided the rats into two groups (Control and HIE model). The model of HIE brain injury in P7 rats was established by the Rice-Vannucci method [Citation23] and some modifications were made. Briefly, 3% isoflurane-oxygen mixture was used to induce anaesthesia in P7 rats and 2% isoflurane-oxygen mixture was used for maintenance. HIE model rats were permanently cut off the left common carotid artery with a bipolar electrocoagulation device (gutta cutter, Jiangsu Kanghua Medical Equipment Co., Ltd., Jiangsu, China). Then we transferred the rats into a 37 °C incubator for 10 min until they regained consciousness, and then returned them to their cages for 90 min. Afterwards, we placed the rats in a hypoxia chamber containing 8% O2 in mixture with 92% N2 for 120 min. Rats in the control group only underwent isolation and stringing of vessels without occlusion and subsequent ischaemia. On the third day after surgery, the rats were anaesthetized with pentobarbital intraperitoneal injection (40 mg/kg) and subsequently sacrificed via cervical dislocation and then brain tissues were collected.
Induction of oxygen-glucose deprivation (OGD) injury in vitro
Primary rat neurons were placed into Dulbecco’s modified Eagle’s medium (DMEM) without glucose and in an anaerobic chamber at 37 °C with 95% N2 and 5% CO2. After OGD treatment for 2 h, we updated the medium using normal medium containing glucose and cultured cells at 37 °C for 12 h under normoxic conditions (95% air and 5% CO2).
Western blotting
We lysed the treated and untreated neurons and tissue using RIPA buffer (R0010, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) containing protease and phosphatase inhibitors, and extracted the total protein. After using a BCA quantification kit (PICPI23223, Thermo Fisher Scientific, Inc., USA) to quantify the proteins, we used 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) to separate 25 µg protein from each sample, and then semi-dry transferred them onto polyvinylidene fluoride (PVDF) membranes (HATF00010, EMD Millipore, Billerica, MA, USA). We sealed the membranes at room temperature for 1 h with 5% skimmed milk (BD Biosciences, Franklin Lakes, NJ, USA), then incubated them overnight at 4 °C in primary antibody against S100B (cat no. 9550; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), NSE (cat no. Ab53025; 1:1,000; Abcam, USA), Bax (cat no. 14796; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), Bcl-2 (cat no. Ab196495; 1:1,000; Abcam, USA) and GAPDH (cat no. 5174; 1:1000; Cell Signaling Technology, Inc.). On the second day, after 6 times of Tris-buffered saline and Tween 20 (TBST) washing at room temperature, the membranes were secondarily incubated with horseradish peroxidase (HRP) conjugated goat anti-rabbit antibodies for 2 h (cat no. 7074; 1:2,000; Cell Signaling Technology, Inc.). After washing with TBST for 6 times, the chemiluminescent reagent (WBKLS0100, EMD Millipore) was applied to develop the blots for 5 min and to expose into the ECL imaging system (Tanon-5200, Tanon, Shanghai, China). Image J version 1.47 (Bethesda, MD, USA) was used to analyse and calculate the protein expression relative to the internal reference protein GAPDH (glyceraldehyde-3-phosphate dehydrogenase).
Reverse transcription-quantitative polymerase chain reaction (qRT-qPCR)
According to the manufacturer’s protocol, Trizol (Invitrogen; Thermo Fisher Scientific Inc.) was used to extract total RNA from the rat brain tissue and neurons. A PrimeScript reverse transcription reagent kit (Takara Biotechnology Co., Ltd., Dalian, China) was used for reverse transcription experiments in accordance with the manufacturers’ protocols. We used the following primers to conduct PCR: miR-124-3p, forward, 5′-GCTAAGGCACGCGGTG-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′; GAPDH, forward 5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse 5′-GTAGAGGCAGGGATGATGTTCT-3′; U6, forward 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse 5′-CGCTTCACGAATTTGCGTGTCAT-3′; Bax, forward, 5′-TTCATCCAGGATCGAGCAGG-3′ and reverse, 5′-CATCAGCAAACATGTCAGC-3′. The thermocycling conditions were as follows: 5 min at 95 °C and 40 cycles of 30 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C. We used U6 or GAPDH to normalize the gene expression with the 2−ΔΔCq method. Every experiment was repeated three times.
Bioinformatics analysis
The potential targets of miR-124-3p were predicted using TargetScan 7.1 (http://www.targetscan.org/vert_71/).
Dual-luciferase reporter assay
The potential targets of miR-124-3p were predicted using TargetScan 7.1 and Bax was proved as a promising target of miR-124-3p. We used Bax-3′-UTR-wild-type (WT) and Bax-3′-UTR-mutant (MUT) (GeneCopoeia, Inc., Rockville, MD, USA) vectors with wild-type and mutated Bax-3′-UTRs to investigate whether the 3′-UTR of Bax is targeted by miR-124-3p directly. We inoculated neuronal cells (5 × 104 cells per well) into 24-well plates and then co-transfected with Bax-3′-UTR-WT (2 µg) or Bax-3′-UTR-MUT (2 µg) plasmids and miR-124-3p mimic (50 nmol/L) or mimic control (50 nmol/L) using Lipofectamine® 2000 (Thermo Fisher Scientifc, Inc.). Twenty-four hours later, we used dual-luciferase reporter gene assay kit (Promega Corporation, Madison, WI, USA) to detect the relative firefly and Renilla luciferase activities according to the manufacturer’s plan. Firefly luciferase activity was used as a control. Every experiment was repeated three times.
Cell transfection
We transfected respectively miR-124-3p mimic, mimic control, Bax-plasmid, control-plasmid, miR-124-3p mimic + control-plasmid, or miR-124-3p mimic + Bax-plasmid into neurons with 5 µL Lipofectamine 2000 transfection reagent (Invitrogen, USA) per well following the manufacturer’s instructions. After incubation for 48 h, qRT-PCR was used to calculate the transfection efficiency. Every experiment was repeated three times.
Flow cytometry (FCM)
In this study, a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) was used to analyse cell apoptosis in rat brain tissue and neurons. Firstly, rat brain tissues and neurons were adjusted to 1 × 106 cells/mL concentration. The cell apoptosis rate was detected after adding 5 µL fluorescein isothiocyanate-labeled Annexin V and 5 µL propidium iodide (PI) (cat. no. 6592; Cell Signaling Technology, Inc., Danvers, MA, USA) for 1 h. FlowJo software 7.6.1 (Tree Star Inc., Ashland, OR, USA) was used to analyse cell apoptosis rate. Every experiment was repeated three times.
MTT assay
Treated and untreated neurons were inoculated into 96-well plates at a density of 1 × 104 cells. The final volume was 100 µL and kept in DMEM at 37 °C, then we added 20 µL of 5 mg/mL MTT solution into each well and incubated the wells in an incubator at 37 °C with 5% CO2 for 4 h. Then 150 µL dimethyl sulphoxide (DMSO) was added to each well to dissolve the crystal completely. We used automatic microplate reader (BioTek, USA) to measure the absorbance at 490 nm. We repeated every experiment three times.
Data analysis
We expressed the values as means with standard deviation (±SD), and used GraphPad Prism 6.0 (GraphPad Software, San Diego, CA) to do statistical analysis. Two-tailed Student’s t-test or one-way analysis of variance (ANOVA) were used to analyse the differences between or among groups. The differences were considered statistically significant at p < 0.05.
Results and discussion
So far, it is not completely simple for us to understand the pathogenesis of HIE, so it is important to study its potential molecular mechanism for the development of new prevention and treatment of neuronal death in neurodegenerative diseases. miRNAs have been shown to be involved in the regulation of OPC function during neonatal hypoxia-ischaemia cerebral injury [Citation24]. And studies have suggested that miRNAs may be potential therapeutic targets for neonatal hypoxic-ischaemic encephalopathy.
miR-124-3p has been confirmed to play crucial roles in many diseases. According to reports, miR-124-3p expression in ectopic endometrium was decreased, and the cell proliferation and invasion of ectopic endometrium was inhibited by up-regulation of miR-124-3p, suggesting that miR-124-3p may play an important role in endometriosis [Citation21]. And it is reported that miR-124-3p can inhibit cell invasion in endometrial cancer and thus act as a tumour inhibitor [Citation25,Citation26]. In addition, Huang et al. [Citation27] proposed that miR-124-3p might inhibit neuronal inflammation induced by traumatic brain injury and promote outward growth of neurites. Furthermore, miR-124-3p plays a neuroprotective role in the cell model of 6-hydroxydopamine-induced Parkinson’s disease by regulating ANAX5 [Citation28]. miR-124 was also shown to protect neurons from apoptosis in newborn rats with hypothyroidism [Citation29].
Based on the shown results, in this study, we hypothesized and tried to identify that miR-124-3p was a key factor for HIE, and then we explored the role of miR-124-3p in HIE and its molecular mechanism in vitro.
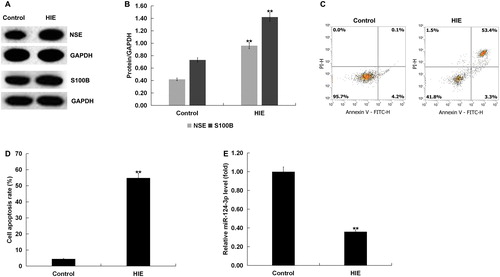
miR-124-3p expression in the brain tissue of rats with HIE
Western blot assay was used to detect the expression of S100 calcium-binding protein B (S100B) and neuron-specific enolase (NSE) in the brain tissues of rats with or without HIE to confirm the successful establishment of the HIE rat model. The results showed that the expression of NSE and S100B in HIE group was increased significantly compared with the control group (). Cell apoptosis in the brain tissues of rats with or without HIE was also analysed by FCM. The results showed that cell apoptosis was significantly increased in the HIE model group compared with the control group (). In addition, miR-124-3p expression was significantly decreased in the brain tissues of rats in the HIE model group compared with the control group ().
Figure 1. Expression of miR-124-3p in the brain tissue of rats with HIE. (A and B) Protein levels of the molecular markers related to the development of HIE, S100 calcium-binding protein B (S100B) and neuron-specific enolase (NSE), in the brain tissue of rats with or without HIE. (C and D) Cell apoptosis in the brain tissue of rats with or without HIE. (E) miR-124-3p expression in the brain tissue of rats with or without HIE.
Note: Data are mean values ± SD. **p < 0.01 vs. control group.
Bax was a target gene of miR-124b-3p
To explore the molecular mechanism of miR-124-3p in the mediation of HIE, we identified genes directly targeted and regulated by miR-124-3p. Interestingly, bioinformatics analysis indicated that Bax was a presumed target gene of miR-124-3p (). Bax was the first identified pro-apoptotic member in the protein family of the B cell lymphoma-2 (Bcl-2) [Citation30], and Bcl-2 is also a key anti-apoptotic protein [Citation31]. In order to confirm whether miR-124-3p binds directly to Bax 3′-UTR, we performed dual-luciferase reporter assay. Firstly, we found that miR-124-3p mimic significantly enhanced miR-124-3p expression in neurons (). The results of dual-luciferase reporter assay indicated that over-expression of miR-124-3p significantly reduced the luciferase activity of neurons transfected with wild-type Bax 3′-UTR reporter and miR-124-3p mimic (). However, no significant changes in luciferase activity were observed in neurons transfected with mutant Bax 3′-UTR reporter and miR-124-3p mimic (). Overall, these results suggested that Bax was a direct target gene of miR-124-3p, and miR-124-3p might affect HIE through regulating Bcl-2 expression.
Figure 2. Bax was a target gene of miR-124-3p. (A) Binding sites of miR-124-3p to Bax. (B) miR-124-3p expression in neurons transfected with miR-124-3p mimic or mimic control. (C) Dual-luciferase reporter system was used to confirm the binding sites between miR-124-3p and Bax. (D and E) Protein and mRNA expression of Bax in the brain tissue of rats with or without HIE.
Note: Data are mean values ± SD. **p < 0.01 vs. control group; ##p < 0.01 vs. mimic control group.
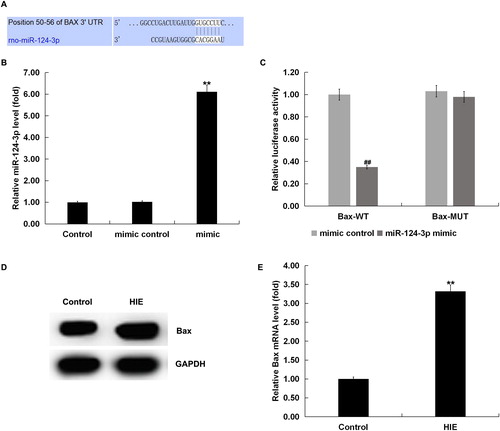
Then we detected the Bax expression in HIE using western blot assay and qRT-PCR. We found that Bax was significantly up-regulated in the brain tissues of rats with HIE compared with the control group ().
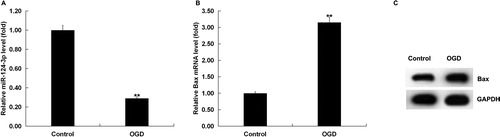
The effect of miR-124-3p over-expression on OGD-induced neuronal injury
In order to investigate whether miR-124-3p was involved in regulating neuronal damage induced by OGD, we firstly examined the expression of miR-124-3p in neurons with the treatment of OGD. Results displayed that the expression of miR-124-3p was significantly down-regulated and the expression of Bax was significantly up-regulated in OGD-treated neurons ().
Figure 3. miR-124-3p and Bax expression in OGD-induced neurons. After 48 h of OGD induction, the expression of miR-124-3p and the mRNA and protein expression of Bax in the neurons were detected. (A) miR-124-3p expression in OGD-induced neurons. (B and C) Bax mRNA and protein expression in OGD-induced neurons.
Note: Data are mean values ± SD. **p < 0.01 vs. control group.
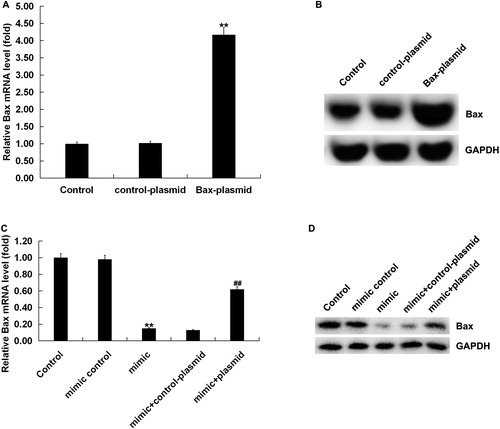
There are many studies indicating that miRNAs are enriched and serve important roles in the nervous system, and it has been confirmed that miRNAs are involved in cell proliferation, differentiation, apoptosis and other important cellular events [Citation8,Citation32]. So, we next performed the functional gain experiments of miR-124-3p in order to explore the exact function of miR-124-3p in regulating OGD-induced neuronal injury. qRT-PCR analysis revealed that the transfection of miR-124-3p mimic significantly up-regulated the expression of miR-124-3p in neurons (), the transfection of Bax-plasmid significantly up-regulated Bax expression (), and the transfection of miR-124-3p mimic significantly down-regulated Bax expression, which was reversed by Bax-plasmid ().
Figure 4. Effect of miR-124-3p on Bax expression in neurons. Neurons were transfected with miR-124-3p mimic, mimic control, Bax-plasmid, control-plasmid, miR-124-3p mimic + control-plasmid or miR-124-3p mimic + Bax-plasmid, respectively. (A and B) mRNA and protein expression of Bax in neurons transfected with Bax-plasmid or control-plasmid. (C and D) mRNA and protein expression of Bax in neurons transfected with miR-124-3p mimic, mimic control, miR-124-3p mimic + control-plasmid or miR-124-3p mimic + Bax-plasmid.
Note: Data are mean values ± SD. **p < 0.01 vs. control group. ##p < 0.01 vs. mimic group.
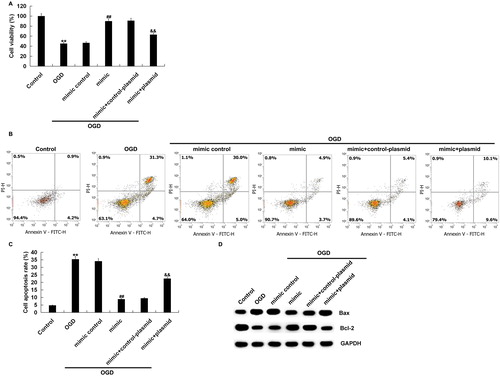
Functional experiments showed that over-expression of miR-124-3p induced the damaged cell viability in neurons exposed to OGD (). Moreover, over-expression of miR-124-3p significantly reduced the apoptosis of neurons induced by OGD treatment (). Besides, miR-124-3p over-expression down-regulated Bax expression and up-regulated Bcl-2 expression significantly in OGD-exposed neurons (). In summary, these results suggested that over-expression of miR-124-3p protected neurons from OGD-induced injury.
Figure 5. Effect of miR-124-3p on OGD-induced neuronal injury. Neurons were transfected with miR-124-3p mimic, mimic control, miR-124-3p mimic + control-plasmid or miR-124-3p mimic + Bax-plasmid. After 48 h, the cells were subjected to OGD induction for 48 h. Then, cell viability (A), cell apoptosis (B and C), and the expression of Bcl-2 and Bax in neurons were detected (D).
Note: Data are mean values ± SD. **p < 0.01 vs. control group. ##p < 0.01 vs. OGD group. &&p < 0.01 vs. mimic + OGD group.
Conclusion
Our results suggested that miR-124-3p might protect neurons from ischaemia and hypoxia-induced injury by targeting Bax, thus playing a protective role in HIE. This might be a new and effective therapeutic target for HIE. These findings provide new ideas for miRNA-mediated neuroprotective studies of neonatal hypoxia-ischaemia brain damage.
Disclosure statement
None of the authors declared any financial competitive interest.
References
- Lv H, Wang Q, Wu S, et al. Neonatal hypoxic ischemic encephalopathy-related biomarkers in serum and cerebrospinal fluid. Clin Chim Acta. 2015;450:282–297.
- Silveira RC, Procianoy RS. Hypothermia therapy for newborns with hypoxic ischemic encephalopathy. J Pediatr. 2015;91(6):S78–S83.
- Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 2015;169(4):397–403.
- Vannucci RC, Connor JR, Mauger DT, et al. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999;55(2):158–163.
- Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28(9):1353–1365.
- Li L, Klebe D, Doycheva D, et al. G-cSF ameliorates neuronal apoptosis through GSK-3β inhibition in neonatal hypoxia-ischemia in rats. Exp Neurol. 2015;263:141–149.
- Mortezaei Z, Lanjanian H, Masoudi-Nejad A. Candidate novel long noncoding RNAs, microRNAs and putative drugs for Parkinson’s disease using a robust and efficient genome-wide association study. Genomics. 2017;109(3–4):158–164.
- Li N, Pan X, Zhang J, et al. Plasma levels of mir-137 and mir-124 are associated with Parkinson’s disease but not with Parkinson’s disease with depression. Neurol Sci. 2017;38(5):761–767.
- Ding X, Sun B, Huang J, et al. The role of miR-182 in regulating pineal CLOCK expression after hypoxia-ischemia brain injury in neonatal rats. Neurosci Lett. 2015;591:75–80.
- Ma Q, Dasgupta C, Li Y, et al. Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol Dis. 2016;89:202–212.
- Wang L, Ke J, Li Y, et al. Inhibition of miRNA-210 reverses nicotine-induced brain hypoxic-ischemic injury in neonatal rats. Int J Biol Sci. 2017;13(1):76–84.
- Xiong L, Zhou H, Zhao Q, et al. Overexpression of miR-124 protects against neurological dysfunction induced by neonatal hypoxic–ischemic brain injury. Cell Mol Neurobiol. 2020;1:1–14.
- Cho KHT, Xu B, Blenkiron C, et al. Emerging roles of miRNAs in brain development and perinatal brain injury. Front Physiol. 2019;10:227.
- Diana A, Gaido G, Murtas D. MicroRNA signature in human normal and tumoral neural stem cells. Int J Mol Sci. 2019;20(17):4123.
- Lee MR, Kim JS, Kim KS. MiR-124a is important for migratory cell fate transition during gastrulation of human embryonic stem cells. Stem Cells. 2010;28(9):1550–1559.
- Cheng LC, Pastrana E, Tavazoie M, et al. MiR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12(4):399–408.
- Wang Y, Chen L, Wu Z, et al. MiR-124-3p functions as a tumor suppressor in breast cancer by targeting CBL. BMC Cancer. 2016;16(1):826.
- Zhang F, Wang B, Long H, et al. Decreased miR-124-3p expression prompted breast cancer cell progression mainly by targeting beclin-1. Clin Lab. 2016;62(6):1139–1145.
- Zheng F, Liao YJ, Cai MY, et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut. 2012;61(2):278–289.
- Kang Q, Xiang Y, Li D, et al. MiR-124-3p attenuates hyperphosphorylation of Tau protein-induced apoptosis via caveolin-1-PI3K/Akt/GSK3β pathway in N2a/APP695swe cells. Oncotarget. 2017;8(15):24314–24316.
- Liu S, Qiu J, Tang X, et al. LncRNA-H19 regulates cell proliferation and invasion of ectopic endometrium by targeting ITGB3 via modulating miR-124-3p. Exp Cell Res. 2019;381(2):215–222.
- Laferriere NR, Kurata WE, Grayson CT 3rd, et al. Inhibition of microRNA-124-3p as a novel therapeutic strategy for the treatment of Gulf War Illness: evaluation in a rat model. Neurotoxicology. 2019;71:16–30.
- Rice J III, Vannucci R, Brierley JB. The influence of immaturity on hypoxic ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–141.
- Birch D, Britt BC, Dukes SC, et al. MicroRNAs participate in the murine oligodendroglial response to perinatal hypoxia-ischemia. Pediatr Res. 2014;76(4):334–340.
- Li Y, Zhang Z, Liu X, et al. MiR-124 functions as a tumor suppressor in the endometrial carcinoma cell line HEC-1B partly by suppressing STAT3. Mol Cell Biochem. 2014;388(1–2):219–231.
- Dong P, Ihira K, Xiong Y, et al. Reactivation of epigenetically silenced miR-124 reverses the epithelial-to-mesenchymal transition and inhibits invasion in endometrial cancer cells via the direct repression of IQGAP1 expression. Oncotarget. 2016;7(15):20260–20270.
- Huang S, Ge X, Yu J, et al. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018;32(1):512–528.
- Dong RF, Zhang B, Tai LW, et al. The neuroprotective role of MiR-124-3p in a 6-hydroxydopamine-induced cell model of Parkinson’s disease via the regulation of ANAX5. J Cell Biochem. 2018;119(1):269–277.
- Shao Q, Jiang W, Jin Y. MiR-124 effect in neurons apoptosis in newborn rat with thyroid hypofunction. Int J Clin Exp Pathol. 2015;8(11):14465–14471.
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74(4):609–619.
- Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986;47(1):19–28.
- Chen R, Wang M, Fu S, et al. MicroRNA-204 may participate in the pathogenesis of hypoxic-ischemic encephalopathy through targeting KLLN. Exp Ther Med. 2019;18(5):3299–3306.
