Abstract
Hypoxic-ischaemic encephalopathy (HIE) resulting from perinatal asphyxia or intrapartum-related events is a common cause of neonatal brain damage death among neonatal brain injury and term infants. However, the potential mechanisms of brain injury in term infants with HIE remain to be elucidated. This study aimed to investigate the role of miR-454-3p in HIE. The protective effect of microRNA-454-3p (miR-454-3p) targeting ST18 on neonatal HIE was investigated by the HIE rat model and primary rat nerve cell oxygen and glucose deprivation (OGD) model. The expression of miR-454-3p was detected by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Cell apoptosis and viability was detected by flow cytometry and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay, respectively. TargetScan predicted the binding sites between miR-454-3p and ST18. Dual luciferase reporter system was used to validate the relationship between ST18 and miR-454-3p. We found a significant decrease in miR-454-3p expression in the brain tissue of rats with HIE, while apoptosis and S100B and neuron-specific enolase (NSE) expression were significantly increased. In addition, miR-454-3p was significantly decreased in OGD-induced neurons, while the mRNA and protein expression of ST18 was significantly increased. OGD reduced neuronal cell viability and increased cell apoptosis were significantly inhibited by miR-454-3p mimic, but all these effects were significantly reversed by over-expression of ST18 gene. In conclusion, our study suggested that miR-454-3p was down-regulated in HIE rats, and it could attenuate OGD-induced neuronal apoptosis by targeting ST18, thereby playing a protective role in HIE, which might become a new and effective therapeutic target for HIE.
Introduction
Hypoxic-ischaemic encephalopathy (HIE) resulting from perinatal asphyxia or intrapartum-related events is a common cause of neonatal brain damage death among term infants and neonatal brain damage [Citation1,Citation2]. In addition, HIE can cause serious long-term neurological diseases such as cerebral palsy, developmental delay and seizures [Citation3]. According to report, the mortality rate of infected newborns is about 15–20% in the postpartum period, plus an additional 25% of infected newborns will continue to develop into severe permanent neuropsychiatric sequelae [Citation4]. And only a small percentage of HIE infants have no disability after rehabilitation [Citation5,Citation6]. Currently, therapeutic hypothermia, as the gold standard for HIE treatment, reduces mortality and improves neurological outcomes in the clinical setting [Citation7]. However, nearly half of those who have been treated for hypothermia are still dying or suffer significant neurologic disability [Citation8]. The molecular mechanisms and pathways of brain damage in children with HIE are still unclear. Therefore, our study explores the pathogenesis of neonatal HIE injury and identifies the potential targets of HIE treatment.
MicroRNAs (miRNAs) are small class of non-coding RNAs (21–22 nucleotides in length) that bind to the 3′-untranslated regions (3′-UTR) of the target gene, which can regulate gene expression through inducing mRNA degradation and/or inhibiting translation [Citation9,Citation10]. It is worth noting that the miRNA-mediated silencing mechanism has become an important regulatory function involved in a wide range of gene regulatory pathways [Citation10,Citation11]. There is growing evidence indicated that miRNAs are involved in a variety of neurological diseases, including neonatal HIE [Citation12–14]. Therefore, miRNAs are considered to be potential therapeutic targets for neonatal hypoxic-ischaemic encephalopathy.
miR-454-3p has been reported as a cytoprotective miRNA that protects against various pathological insults, such as chondrosarcoma growth inhibition and tumour-suppressive functions [Citation15,Citation16]. In recent years, it has been reported that the level of miR-454-3p is down-regulated in HIE neonates [Citation17]. However, the mechanism of action of miR-454-3p in neonates with HIE is unclear. Therefore, this study aimed to investigate the role of miR-454-3p in the model of ischaemia and hypoxia in vivo and in vitro and further to explore whether miR-454-3p has protective effects on neonatal hypoxic-ischaemic encephalopathy, so as to provide new therapeutic strategy targets for neonatal HIE.
Materials and methods
This study was approved by the Institutional Animal Care and Use Committee of Fujian Provincial Maternity and Children’s Hospital.
Experimental animals
Pregnant Sprague-Dawley (SD) rats were purchased from Charles River Laboratories (Portage, MI, China). Animals were allowed to give birth and were then kept with their pups in a room maintained under controlled conditions at 23 ± 1 °C, 55 ± 5% humidity with a 12 h light/dark cycle, and the animals had free access to normal rat chow and filtered water. On day 7 of gestation, the male rats were randomly divided into two groups: the HIE model group (HIE, n = 5) and the sham group (Control, n = 5). We maintained a sterile environment during the surgical procedure. All animals were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Hypoxic-ischaemic model in rat
The newborn rat HIE model is a modified Rice-Vannucci model. In brief, postnatal Day 7 (P7) SD rats were anaesthetized with 2% isoflurane in oxygen. The complete anaesthesia was determined by the loss of any reaction from the animal in response to pinching the ear, the toe or the tail and the loss of the pedal withdrawal reflex of the animal. The rats underwent a surgery where the left common carotid artery was double ligated using silk surgical sutures and then transected between two ligation sites. The incision was then sutured closed, and after recovery for 1 h, pups were treated with 8% FiO2. The sham-operated rats were given anaesthesia and then the left common carotid artery was exposed but not ligated without subsequent ischaemia and occlusion. After recovery of 1 h, the rat pups were put in a hypoxic sealed chamber (8% oxygen balanced with nitrogen) at 37 °C for 2.5 h and then returned to their cage. The model was established after the third day, the rats were anaesthetized with pentobarbital (40 mg/kg, intraperitoneal injection) before sacrificed through cervical dislocation. After sacrificed, brain tissue were collected. Throughout the experiment, we tried to alleviate the pain of the rats.
Primary culture cortical neurons
Primary cultures of cortical neurons were obtained from the brain cortex of embryonic day 17 (E17) and E18 SD rat embryos. Under aseptic conditions, the primary cortical neurons were dissociated and cultured as described previously. The dissociated cerebrocortical cells were added to poly-l-lysine coated culture plates and maintained in neurobasal medium (Gibco BRL, Rockville, MD, USA) with 2% B-27 (Gibco, USA) supplement. Cytosine arabinoside (1 mM) was added to the cultures to stop the cell proliferation of astroglia and microglia, and the purity of neuronal cultures was more than 90% after the cells has been seeded for 24 h. And the medium was changed every 24 h. All cells were cultured in an incubator of 5% CO2 at 37 °C. These cells were seeded at a cell density of 2 × 105/well into 24-well plates for subsequent experimental analysis. All animal experimental procedures and protocols were in accordance with the NIH Guide for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Fujian Provincial Maternity and Children’s Hospital.
Construction of oxygen and glucose deprivation (OGD) model in vitro
OGD model was established in primary cortical neurons. Primary cerebral cortex neurons were prepared as we mentioned previously. In brief, after replacing cell cultures with DMEM without glucose, the cultures were transferred to a hypoxia modular incubator chamber (Billups-Rothenberg, USA) filled with a gas mixture of 95% N2 and 5% CO2 at 37 °C. After 4 h of OGD treatment, the neurons were cultured again in neurobasal medium supplemented with 2% B27 and returned to the 37 °C incubator of 5% CO2 for 24 h before further experiment. In addition, the neurons in the control groups were grown in the medium (neurobasal medium supplemented with 2% B27) in a humidified atmosphere.
Cell transfection
For cell transfection, 100 nmol/L miR-454-3p mimic (Guangzhou Ribobio Co., Ltd., Guangzhou, China), 100 nmol/L mimic control, 1 μg Control CRISPR Activation Plasmid (control-plasmid; Cat No. sc-437275; Santa Cruz Biotechnology, USA), 1 μg ST18 CRISPR Activation Plasmid (ST18-plasmid; Cat No. sc-407660-ACT; Santa Cruz Biotechnology, USA), or 100 nmol/L miR-454-3p mimic + 1 μg ST18-plasmid were transfected into primary cortical neurons using Lipofectamine 3000 (Invitrogen) according to the operational instructions. Primary rat neurons were transfected with miR-454-3p mimic, mimic control, miR-454-3p mimic + control-plasmid or miR-454-3p mimic + ST18-plasmid, and 48 h later, the nerve cells were subjected to OGD induction. Forty-eight hours after cell transfection, the transfection efficiency was measured using qRT-PCR. The cells were divided into five groups: Control; OGD; OGD + mimic control; OGD + miR-454-3p mimic; OGD + miR-454-3p mimic + control-plasmid; OGD + miR-454-3p mimic + ST18-plasmid. Experiment was performed in triplicate.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
After transfection, the neuronal cell viability was detected by the MTT assay. In brief, the cultured cells (5 × 103 cells/well) were seeded into 96-well plates. After 24 h of OGD exposure, the neuronal cells were washed, and 20 μL MTT (5 mg/mL; Sigma, St. Louis, MO, USA) were added to each well, followed by incubation for 4 h at 37 °C. The supernatant was carefully removed, and then 100 μL DMSO was added to solubilize the formazan salt formed. Thereafter, the absorbance was measured at 490 nm using a micro-plate reader (BioRad Laboratories, CA, USA). Experiment was performed in triplicate.
Cell apoptosis assay
Cell apoptosis rate was determined by flow cytometry analysis according to the instructions of Annexin V-fluorescein isothiocyanate (FITC)/PI Apoptosis Detection Kit (R&D systems, Abingdon, UK). Forty-eight hours after cell transfection, the neurons were subjected to OGD induction. Twenty-four hours after OGD induction, cultured neuronal cells were washed twice in phosphate buffer saline (PBS), suspended in 500 mL of binding buffer, subsequently, at room temperature treated with 5 mL Annexin V-FITC/PI for 12 min in the dark. At last, cell apoptosis rate was measured on a FACSAria flow cytometer (BD Biosciences, San Jose, CA, USA). Experiment was performed in triplicate.
Cell apoptosis in brain tissue was also detected using flow cytometry. The collected brain tissue was washed with saline and then mechanically dissociated into a single cell suspension. Thereafter, the brain tissue cell suspension was centrifuged for 10 min and the supernatant was discarded. Finally, flow cytometer (FACSCalibur, Becton Dickinson) was used to measure cell apoptosis. Experiment was performed in triplicate.
Dual luciferase reporter assay
The potential targets of miR-454-3p was predicted by bioinformatics software (http://www.targetscan.org/vert_71/). 293T cells (1 × 106) were seeded into 24-well plates. The wild-type and mutant 3′-UTR of ST18 (WT-ST18 and MUT-ST18, respectively) were cloned into a pmiR-RB-Report™ dual luciferase reporter gene plasmid vector (Guangzhou RiboBio Co., Ltd.) according to the manufacturer’s protocol. miR-454-3p mimic or mimic control were transfected into293T cells with theWT-ST18 or MUT-ST18 by using Lipofectamine® 3000 (Invitrogen) in accordance with the manufacturer’s protocol. Dual Luciferase Assay System (Promega, Madison, WI, USA) was used to measure the relative activities of firefly luciferase and Renilla luciferase in the cell lysates, and Renilla luciferase activity was used as a control to normalize the luciferase activity. Experiment was performed in triplicate.
qRT-PCR analysis
Total RNA from tissues and cells were isolated by TRIzol reagent (Invitrogen; Thermo Fisher Scientific Inc.) and quantified by NanoDrop ND 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). SoFast EvaGreen Supermix (BioRad, Hercules, CA) and SuperScript III First Strand Synthesis System kit (Invitrogen, USA) were performed to generate cDNA from mRNA, while NCode VILO miRNA cDNA Synthesis kit (Life Technologies, USA) was performed to generate cDNA from miRNA, according to the manufacturer’s protocol. qPCR of mRNA and miRNA was performed by SoFast EvaGreenH Supermix (BioRad) and EXPRESS SYBR Green ER miRNA qRT-PCR kit (Life Technologies), respectively, according to the manufacturer’s protocol. The primer sequences for PCR were as follows:
ST18, forward 5′-CGTGCCAGCTCTTATAGCTACG-3′;reverse 5′-TATTTCGGCTCCCTTGGCAT-3′;miR-454-3p, forward 5′-GCGCGCTTATAATACAACCTGA-3′;reverse 5′-GTGCAGGGTCCGAGGT-3′;
U6, forward 5′-GTGTGCTACGGAGTTCAGAGGTT-3′;reverse 5′-TGGGGTTATACATTGTGAGAGGA-3′.
GAPDH, forward 5′-TGTTGCCATCAATGACCCCTT-3′;reverse 5′-CTCCACGACGTACTCAGCG-3′.
The amount of target gene expression was normalized using U6 and glyceraldehyde phosphate dehydrogenase (GAPDH) as the reference gene. Following the initial activation step for 15 min at 95 °C, the reaction system entered into the three-step cycling which included the following steps for 40 cycles: denaturation at 94 °C for 15 s; annealing at 55 °C for 30 s and extension at 70 °C for 30 s. Gene expression was then calculated using the 2−ΔΔCt method. Experiment was performed in triplicate.
Western blot analysis
Proteins were extracted from tissue and cells using RIPA Lysis Buffer (Beyotime, Shanghai, China) with Protease inhibitor cocktail for general use (Beyotime, Shanghai, China). Proteins were separated by electrophoresis on 10% SDS-PAGE. After transferring the protein onto polyvinylidene fluoride membrane (Invitrogen), the membrane was incubated with primary antibody overnight at 4 °C and then incubated with secondary antibody for 1 h. The primary antibodies were as following: cleaved-Caspase3 (Cat No. 9664; 1:1000; Cell Signaling Technology, Inc., Danvers, MA, USA), pro-Caspase3 (Cat No. ab183179; 1:1000; Abcam, USA), ST18 (Cat No. ab86563; 1:1000; Abcam, USA) and GAPDH (Cat No. 5174; 1:1000; Cell Signaling Technology, Inc.). Finally, enhanced chemiluminescence (ECL) kit (Multisciences, Hangzhou, China) was used for the signals detection. Protein bands were quantified by densitometry using QuantityOne 4.5.0 software (Bio-Rad Laboratories Inc., USA).
Statistical analysis
The data were processed using GraphPad Prism 6.0 Software. All values were expressed as the mean ± standard deviation (SD). The statistical dissimilarities between groups were performed by Student’s t test or one-way analysis of variance (ANOVA) followed by Tukey’s test. For all comparisons, p < .05 indicated statistical significance.
Results and discussion
miRNAs have been reported to be involved in various neurological diseases, including neonatal HIE [Citation12–14], and they are considered to be potential therapeutic targets for neonatal hypoxic-ischaemic encephalopathy. This study focussed on investigating the role of miR-454-3p in the model of ischaemia and hypoxia in vivo and in vitro, so as to explore the role miR-454-3p in neonatal hypoxic-ischaemic encephalopathy.
Expression of miR-454-3p in the brain tissue of neonatal rats with hypoxic-ischaemic encephalopathy
To determine the expression of miR-454-3p in the brain tissue of HIE rats, we first established a neonatal rat HIE model and an in vitro primary cortical neuron OGD model. qRT-PCR analysis detected molecular markers associated with the development of HIE, including the expression of S100B and neuron-specific enolase (NSE). At the same time, cell apoptosis in the brain tissue of rats was analyzed. The results of the experiment showed that the mRNA expression of S100B () and NSE () was significantly increased in the HIE model group compared with the control group, and cell apoptosis in rat brain tissue was significantly increased in HIE rats (). The expression of miR-454-3p in the brain tissue of HIE rats was detected by qRT-PCR, and the data indicated that the expression of miR-454-3p was significantly reduced in the HIE model group compared with the control group ().
Figure 1. Expression of miR-454-3p in brain tissue in neonatal rats with hypoxic-ischaemic encephalopathy. (A and B) mRNA expression of S100B and NSE detected by qRT-PCR assay; (C and D) cell apoptosis in brain tissue detected by FCM method; (E): expression of miR-454-3p detected by qRT-PCR. **p<.01 versus control group. Note. On the third day after the establishment of the HIE rat model, the rats were sacrificed and brain tissue was collected.
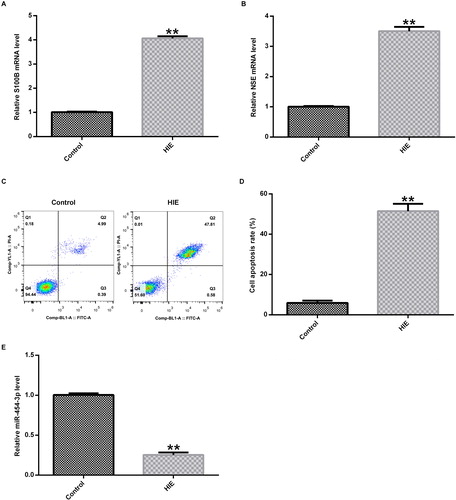
In brief, we found that hypoxia and ischaemia induced neuronal apoptosis in brain tissue. In addition, we found that the expression level of miR-454-3p was significantly reduced in the HIE model group, which is consistent with previous studies [Citation18]. It has been reported that miRNA is regulated in the expression of brain tissue in neonatal rats with HIE [Citation18].
miR-454-3p on ST18 expression in primary rat neuronal OGD model
We further predicted that ST18 contains a theoretical miR-454-3p binding site in its 3′-UTR by bioinformatics software (). The ST18 gene is thought to be both a tumour suppressor gene and an oncogene in different human cancers, but to date, there is no direct evidence for its role in tumourigenesis, mainly in the brain [Citation19]. To confirm that ST18 was a target gene of miR-454-3p, a luciferase reporter gene assay was performed, and it was shown that ST18 was a direct target gene of miR-454-3p (). In addition, we used Western blot assay and qRT-PCR to detect the expression of ST18 in rat brain tissue. The results showed that the expression of ST18 in the brain tissue of rats in the HIE model group was significantly higher than that of the control group ().
Figure 2. ST18 as the direct target of miR-454-3p. (A) Binding sites between miR-454-3p and ST18 predicted by TargetScan; (B) relationship between ST18 and miR-454-3p verified by dual luciferase reporter system; (C) protein expression of ST18 in rat brain tissue detected by Western blot assay; (D) ratio of ST18 protein level/GAPDH protein level; (E) expression of ST18 in rat brain tissue detected by qRT-PCR.**p<.01 versus mimic control group; ##p<.01 versus control group.
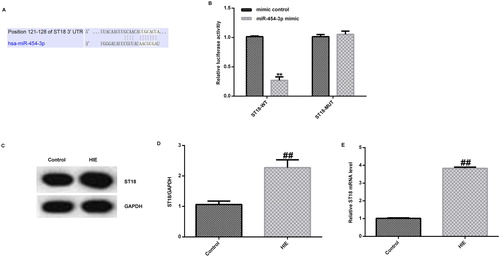
Meanwhile, to verify the function of miR-454-3p in HIE in vitro, we induced an OGD model in primary rat neurons. The expression of miR-454-3p was first detected by qRT-PCR, and the results showed that the expression of miR-454-3p was significantly decreased in the OGD group compared with the control group (). The mRNA and protein expression of ST18 in the cells were further examined by qRT-PCR and western blot assay. The results showed that the mRNA and protein expression of ST18 was significantly increased in OGD-induced neurons ().
Figure 3. Expression of miR-454-3p and ST18 in primary rat neuronal cells induced by OGD. (A) Expression of miR-454-3p detected by qRT-PCR 24 h after OGD induction; (B) mRNA expression of ST18 detected by qRT-PCR; (C) protein expression of ST18 detected by Western blot assay; (D) ratio of ST18 protein level/GAPDH protein level. **p<.01 versus control group.
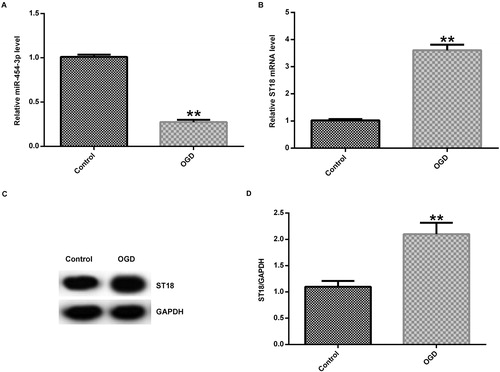
In brief, we found that increased expression of miR-454-3p could promote cell viability and reduce apoptosis in neurons in the OGD induction group. It has been reported that miR-454-3p is involved in a variety of physiological and pathological processes. For example, miR-454-3p and miR-374b-5p suppress migration and invasion of bladder cancer cells through target of ZEB2 gene [Citation20], other studies have also shown that miR-454-3p exerts tumour-suppressive functions by down-regulation of NFATc2 in Glioblastoma [Citation16], and miR-454-3p promotes proliferation and induces apoptosis in human cervical cancer cells by targeting TRIM3 [Citation21].
Effect of miR-454-3p on ST18 expression in primary cortical neurons
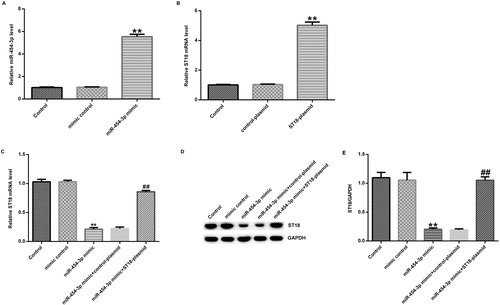
This study further investigated the biological function of miR-454-3p in primary neurons induced by OGD using miR-454-3p mimic. In previous studies, miRNAs such as miR-210 [Citation22], miR-374a [Citation18] and miR-181b [Citation21] were found to be involved in hypoxic-ischaemic injury. For example, miRNA-181b is reduced in neonatal hypoxic-ischaemic brain damage (HIBD), and its over-expression inhibits neuronal apoptosis in the OGD model [Citation23]. Therefore, we hypothesized that miR-454-3p might play an important role in neonatal hypoxic-ischaemic encephalopathy. Primary neurons were transfected with miR-454-3p mimic, mimic control, control-plasmid, ST18-plasmid, miR-454-3p mimic + control-plasmid or miR-454-3p mimic + ST18-plasmid for 48 h, and then the transfection efficiency was tested using qRT-PCR. The results showed that miR-454-3p mimic significantly increased the expression of miR-454-3p in primary neurons (), and ST18-plasmid significantly increased the expression of ST18 mRNA in primary neurons (). In addition, miR-454-3p mimic significantly reduced the expression of ST18 mRNA and protein levels, which was abolished by ST18-plasmid (). Taken together, these results again indicated that ST18 was negatively regulated by miR-454-3p in neurons.
Figure 4. Effect of miR-454-3p on ST18 expression in primary cortical neurons. Primary rat neurons were transfected with miR-454-3p mimic, mimic control, ST18-plasmid, control-plasmid; miR-454-3p mimic + control-plasmid or miR-454-3p mimic + ST18-plasmid. Transfection efficiency was measured after 48 h. (A) Level of miR-454-3p in neurons transfected with mimic control or miR-454-3p mimics for 48 h determined using qRT-PCR; (B) ST18 mRNA level in neurons transfected with control-plasmid or ST18 plasmid for 48 h determined using qRT-PCR; (C) ST18 mRNA level in neurons transfected with mimic control, miR-454-3p mimic, miR-454-3p mimic + control-plasmid or miR-454-3p mimic + ST18-plasmid for 48 h determined using qRT-PCR; (D) ST18 protein level in neurons transfected with mimic control, miR-454-3p mimic, miR-454-3p mimic + control-plasmid or miR-454-3p mimic + ST18-plasmid for 48 h determined using western blot assay; (E) ratio of ST18 protein level/GAPDH protein level. Data are expressed as mean values ± SD. **p < 0.01 versus control group; ##p<.01 versus miR-454-3p mimic group.
Effect of miR-454-3p on OGD-induced neuronal cells
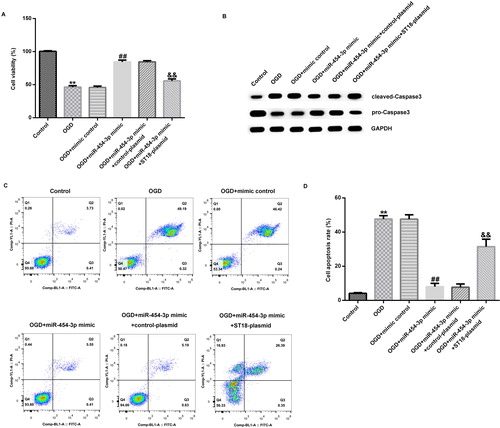
Finally, primary rat neurons were transfected with miR-454-3p mimic, mimic control, miR-454-3p mimic + control-plasmid or miR-454-3p mimic + ST18-plasmid, and 48 h later, nerve cells were subjected to OGD induction. After another 24 h, cell viability was measured by MTT, and the results showed that the cell viability of neurons in the OGD model group was significantly lower than that of the control group (). Compared with OGD induction group, miR-454-3p mimic significantly enhanced the viability of primary rat neurons, and this enhancement was reversed by ST18-plasmid (). The protein expression of cleaved-Caspase3 and pro-Caspase3 was detected by Western blot analysis to analyze cell apoptosis. Also, cell apoptosis was detected by flow cytometry. OGD-induced cleaved-Caspase3 protein increase and pro-Caspase3 protein decrease in primary rat neurons were markedly inhibited by miR-454-3p mimic, and this inhibition was reversed by ST18-plasmid (). ST18 has been reported to be significantly reduced in many different cells, including liver cancer cells and fibroblasts [Citation24,Citation25]. At the same time, we found that ST18 was significantly enhanced in the brain tissues of HIE rats and in OGD-induced neurons. All the effects of miR-454-3p mimic on OGD-induced primary neurons were markedly reversed by ST18 up-regulation. It is worth noting that, OGD-induced primary rat neuronal cell apoptosis was reduced by miR-454-3p mimic, and this decrease was significantly reversed by over-expression of the ST18 gene ().
Figure 5. Effect of miR-454-3p on OGD-induced neuronal cells. (A) Cell viability determined by MTT assay; (B) cleaved-Caspase3 and pro-Caspase3 protein level determined by western blot; (C) cell apoptosis analyzed by FCM; (D) cell apoptosis rate. Data are expressed as mean values ± SD. **p<.01 versus control group; ##p<.01 versus OGD group; &&p<.01 versus OGD + miR-454-3p mimic group.
Conclusions
In our study, down-regulation of miR-454-3p was observed in the brain tissue of HIE rats compared to the control rats. In addition, our study showed that ST18 was a direct target gene of miR-454-3p. Further results showed that miR-454-3p over-expression attenuated OGD-induced neuronal apoptosis by targeting ST18. Therefore, miR-454-3p might protect neurons from ischaemia and hypoxia by targeting ST18, and thus playing a protective role in HIE, which might be a new and effective therapeutic target for neuronal HIE treatment.
Disclosure statement
The authors reported no conflict of interest.
Additional information
Funding
References
- Smith J, Wells L, Dodd K. The continuing fall in incidence of hypoxic-ischaemic encephalopathy in term infants. BJOG 2000;107:461–466.
- Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet 2016;388(10063):3027–3035.
- Vannucci RC. Current and potentially new management strategies for perinatal hypoxic-ischemic encephalopathy. Pediatrics 1990;85:6.
- Lai MC, Yang SN. Perinatal Hypoxic-Ischemic Encephalopathy. J Biomed Biotechnol. 2010;2011:1110–7243.
- Levene ML, Kornberg J, Williams TH. The incidence and severity of post-asphyxial encephalopathy in full-term infants. Early Hum Dev. 1985;11(1):21–26.
- Cerio F, Lara-Celador I, Alvarez A, et al. Neuroprotective therapies after perinatal hypoxic-ischemic brain injury. Brain Sci. 2013;3(4):191–214.
- Davidson JO, Wassink G, van den Heuij LG, et al. Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy – where to from here? Front Neurol. 2015;6:198.
- Wood T, Osredkar D, Puchades M, et al. Treatment temperature and insult severity influence the neuroprotective effects of therapeutic hypothermia. Sci Rep. 2016;6:23430.
- Ma Q, Zhang L. Epigenetic programming of hypoxic-ischemic encephalopathy in response to fetal hypoxia. Prog Neurobiol. 2015;124:28–48.
- Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5″ UTR as in the 3″ UTR. Proc Natl Acad Sci USA 2007;14:9667–9672.
- Ambros V. microRNAs: tiny regulators with great potential. Cell 2001;107(7):823–826.
- Ma Q. Inhibition of miRNA-210 reverses nicotine-induced brain hypoxic-ischemic injury in neonatal rats. Int J Biol Sci. 2017;13:76–84.
- Ding X, Sun B, Huang J, et al. The role of miR-182 in regulating pineal CLOCK expression after hypoxia-ischemia brain injury in neonatal rats. Neurosci Lett. 2015;591:75–80.
- Ma Q, Dasgupta C, Li Y, et al. Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol Dis. 2016;89:202–212.
- Bao X, Ren T, Huang Y, et al. Knockdown of long non-coding RNA HOTAIR increases miR-454-3p by targeting Stat3 and Atg12 to inhibit chondrosarcoma growth. Cell Death Dis. 2017;8:e2605.
- Zuo J, Yu H, Xie P, et al. miR-454-3p exerts tumor-suppressive functions by down-regulation of NFATc2 in glioblastoma. Gene 2019;710:233–239.
- Azuma J, Nabatame S, Nakano S, et al. Prognostic factors for acute encephalopathy with bright tree appearance. Brain Dev. 2015;37:191–199.
- Looney AM, Walsh BH, Moloney G, et al. Downregulation of umbilical cord blood levels of miR-374a in neonatal hypoxic ischemic encephalopathy. J Pediatr. 2015;2:269.
- Jandrig B, Seitz S, Hinzmann B, et al. ST18 is a breast cancer tumor suppressor gene at human chromosome 8q11.2. Oncogene 2004;57:9295–9302.
- Wang S, Zhang G, Zheng W, et al. MiR-454-3p and miR-374b-5p suppress migration and invasion of bladder cancer cells through targetting ZEB2. Biosci Rep. 2018;38(6):BSR20181436.
- Song Y, Guo Q, Gao S, et al. miR-454-3p promotes proliferation and induces apoptosis in human cervical cancer cells by targeting TRIM3. Biochem Biophys Res Commun. 2019;516(3):872–879.
- Li B, Dasgupta C, Huang L, et al. MiRNA-210 induces microglial activation and regulates microglia-mediated neuroinflammation in neonatal hypoxic-ischemic encephalopathy. Cell Mol Immunol. 2019.
- Looney AM, O’Sullivan MP, Ahearne CE, et al. Altered expression of umbilical cord blood levels of mir-181b and its downstream target mUCH-L1 in Infants with moderate and severe neonatal hypoxic-ischaemic encephalopathy. Mol Neurobiol. 2019;5:3657–3663.
- Ravа M, D’Andrea A, Doni M, et al. Mutual epithelium-macrophage dependency in liver carcinogenesis mediated by ST18. Hepatology 2017;5:1708–1719.
- Yang J, Siqueira MF, Behl Y, et al. The transcription factor ST18 regulates proapoptotic and proinflammatory gene expression in fibroblasts. FASEB J. 2008;11:3956–3967.
