Abstract
The aim of the present study was to determine the expression of MCP-1 in injured cartilage tissues, peripheral blood and synovial fluid from patients with cartilage injury of elbow joint of upper limbs, and try to understand its relationship with microRNA (miRNA or miR)-421. Twenty-eight patients and 26 healthy subjects were included. Injured cartilage tissues and peripheral blood were collected from all 28 patients. Synovial fluid was collected from 12 patients with elbow effusion. The expression of mRNA and miR-421 was determined by quantitative real-time polymerase chain reaction (qRT-PCR); the expression of MCP-1 protein in tissues, by western blotting; MCP-1 in serum or synovial fluid, by enzyme-linked immunosorbent assay (ELISA). Dual luciferase reporter assay was used to identify direct interaction between miR-421 and MCP-1. The migration of THP-1 cells was determined by transwell assay. The results showed that MCP-1 was elevated in patients with cartilage injury of elbow joint of upper limbs. By contrast, miR-421 was decreased. miR-421 could bind with the 3′-UTR seed region of MCP-1 mRNA to regulate its expression. Overexpression of miR-421 inhibited the expression of MCP-1 in HC-a cells, and the chemotaxis of THP-1 monocytes. This study demonstrates that the up-regulation of MCP-1 in injured cartilage tissues, serum and synovial fluid from patients with cartilage injury of elbow joint of upper limbs was related to the down-regulation of miR-421. In addition, miR-421 may affect the chemotaxis of monocytes via MCP-1, and regulate the local immune responses in injured cartilage site of elbow joint of upper limbs.
Introduction
Chondrocytes are the only type of cells in cartilage tissues, accounting for 2% of the volume of cartilage tissues [Citation1]. According to the damage degrees of cartilage and subchondral bone, three types of defects can be classified: partial cartilage defects, full-thickness cartilage defects and osteochondral defects [Citation2]. Partial cartilage defects are common, such as abrasive wear during intense exercise, which generally involves only the injury of hyaline cartilage on the surface. Because hyaline cartilage has no blood vessels or nerves, its self-regeneration is difficult when the defect area is large. This type of defect will worsen gradually. The swelling of cartilage and the decline of mechanical properties will stimulate the excessive secretion of synovial fluid around the cartilage, deepen the defect and cause full-thickness cartilage defects [Citation3].
Cartilage defects are often accompanied by inflammation. Chemokines are important mediators involved in the recruitment, maturation and activation of immune cells, and their chemical constituents are secretory proteins with small molecular weight [Citation4]. MCP-1 is a classical protein marker for detecting chemotaxis and metastasis of cells [Citation5]. It is reported that chemokine MCP-1 plays important roles in the generation and maintenance of pain [Citation6, Citation7]. In addition, myocardial tissues can also secrete MCP-1 during injury and immune responses [Citation8]. MCP-1 not only leads to chemotaxis of monocytes, but also promotes the protein expression and release by monocytes. It binds to cellular receptors and mediates the development of inflammation [Citation9].
Cartilage injury of upper limbs is accompanied by various changes in the expression of microRNAs (miRNAs or miR) and proteins, suggesting that miRNAs may play important roles in the regulation of proteins related to cartilage injury of upper limbs [Citation10, Citation11]. It is reported that miR-421 is abnormally expressed in various diseases, such as epilepsy, gastric cancer, glioma and breast cancer [Citation12–15], suggesting its special role in pathological environment. The regulation mechanism of MCP-1 in cartilage injury of upper limbs and the regulation of MCP-1 by upstream microRNA are still unclear.
In the present study, we determined the expression of MCP-1 mRNA and protein in injured cartilage tissues, peripheral blood and synovial fluid from patients with cartilage injury of elbow joint of upper limbs. In addition, we investigated the relationship between MCP-1 and miR-421.
Subjects and methods
Subjects
A total of 28 patients with cartilage injury of elbow joint of upper limbs who underwent surgery at our hospital between January 2016 and January 2019 were included in the experimental group (16 males and 12 females; age range, 16–56 years). Injured cartilage tissues and peripheral blood were collected from all 28 patients. Synovial fluid was collected from 12 patients with elbow effusion among the 28 patients with cartilage injury of elbow joint of upper limbs. In addition, normal tissues adjacent to injured cartilage tissues were collected from all 28 patients as controls for injured cartilage tissues. Peripheral blood and synovial fluid were also collected from 26 healthy subjects who underwent physical examinations at our hospital (15 males and 11 females; age range, 15 − 58 years) as controls. Neither the patients nor the healthy subjects had tuberculosis, tumors, joint deformities or systemic diseases such as infectious arthritis, rheumatoid arthritis, ankylosing spondylitis, gout, psoriasis or rheumatic fever.
Ethics statement
All procedures performed in this study were approved by the Ethics Committee of Ningbo No. 6 Hospital. Written informed consent was obtained from all patients or their families.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Tissues (100 mg) were ground into powder in liquid nitrogen, and lyzed using 1 mL TRIzol reagent following the manufacturer’s manual (Thermo Fisher Scientific, Waltham, MA, USA). Liquid samples (100 μL) were directly lyzed using 1 mL TRIzol reagent following the manufacturer’s manual (Thermo Fisher Scientific, Waltham, MA, USA). Total RNA was extracted using phenol chloroform method. The concentration and quality of RNA was measured using ultraviolet spectrophotometry (Nanodrop ND2000, Thermo Scientific, Waltham, MA, USA). Then, cDNA was obtained by reverse transcription from 1 μg RNA and stored at −20 °C. Reverse transcription of mRNA was performed using TIANScript II cDNA First Strand Synthesis Kit (Tiangen, Beijing, China), and reverse transcription of miRNA was carried out using miRcute miRNA cDNA First Strand Synthesis Kit (Tiangen, Beijing, China).
SuperReal PreMix (SYBR Green) qRT-PCR kit (Tiangen, Beijing, China) was used to detect mRNA expression of human MCP-1, using human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as internal reference. The primer sequences for MCP-1 were 5′-AGAATCACCAGCAGCAAGTGTCC-3′ (forward) and 5′-TCCTGAACCCACTTCTGCTTGG-3′ (reverse). The primer sequences for GAPDH were 5′-AAGGCTGTGGGCAAGG-3′ (forward) and 5′-TGGAGGAGTGGGTGTCG-3′ (reverse). The reaction system (20 μL) was composed of 10 μL SYBR Premix EXTaq, 0.5 μL upstream primer (10 μmol/L), 0.5 μL downstream primer (10 μmol/L), 2 μL cDNA and 7 μL ddH2O. The PCR program was: initial denaturation at 95 °C for 30 s; denaturation at 95 °C for 5 s and annealing at 60 °C for 20 s (39 cycles); elongation at 72 °C for 30 s (iQ5; Bio-Rad, Hercules, CA, USA). The 2−ΔΔCt method [Citation16] was used to calculate the relative expression of MCP-1 mRNA against GAPDH. Each sample was tested in triplicate.
The expression of miR-421 was determined by miRcute miRNA RT-PCR Kit (Tiangen, Beijing, China), using U6 as internal reference. The sequences of miR-421 primers were 5′-TTCACAGTGCCTAATCCGG-3′ (forward), and 5′-GGCGCCCAATTAATGTCTG-3′ (reverse). The sequences of the U6 primers were 5′-CTCGCTTCGGCAGCACA-3′ (forward) and 5′-AACGCTTCACGAATTTGCGT-3′ (reverse). The reaction system (25 μL) contained 12.5 μL qRT-PCR-Mix, 0.5 μL upstream primer (10 μmol/L), 0.5 μL downstream primer (10 μmol/L), 1 μL cDNA and 10.5 μL ddH2O. The reaction protocol was: initial denaturation at 95 °C for 5 min; 95 °C for 15 s and 60 °C for 30 s (40 cycles); elongation at 72 °C for 30 s (iQ5; Bio-Rad, Hercules, CA, USA). The 2−ΔΔCt method [Citation16] was used to calculate the relative expression of miR-421 against U6. Each sample was tested in triplicate.
Western blotting
Before lysis, tissues (100 mg) were ground into powder. Then, tissue samples were lyzed with pre-cooled Radio-Immunoprecipitation Assay (RIPA) lysis buffer (600 μL; 50 mmol/L Tris-base, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate, 1% TritonX-100, 1% sodium deoxycholate; Beyotime Institute of Biotechnology, Shanghai, China) for 30 min on ice. The mixture was centrifuged at 10,000 g and 4 °C for 10 min. The supernatant was used to determine protein concentration by a bicinchoninic acid (BCA) protein concentration determination kit (RTP7102, Real-Times Biotechnology Co., Ltd., Beijing, China). The samples were then mixed with 5× sodium dodecyl sulfate loading buffer before denaturation in a boiling water bath for 5 min. Afterwards, the samples (20 µg) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 100 V. The resolved proteins were transferred to polyvinylidene difluoride membranes on ice (100 V, 2 h) and blocked with 5% skimmed milk at room temperature for 1 h. Then, the membranes were incubated with rabbit anti-human MCP-1 (1:1000; ab9669; Abcam, Cambridge, UK) or β-actin (1:5000; ab129348; Abcam, Cambridge, UK) polyclonal primary antibodies at 4 °C overnight. After extensive washing with phosphate-buffered saline with Tween 20 (3 times for 15 min), the membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:10,000; ab6721; Abcam, Cambridge, UK) for 1 h at room temperature before washing with phosphate-buffered saline with Tween 20 (3 times for 15 min). Then, the membrane was developed with an enhanced chemiluminescence detection kit (ab65623; Abcam, Cambridge, UK) for imaging. Image lab v3.0 software (Bio-Rad, Hercules, CA, USA) was used to acquire and analyze the imaging signals. The relative content of the target protein was expressed against β-actin.
Enzyme-linked immunosorbent assay (ELISA)
The contents of MCP-1 in serum and synovial fluid were tested by ELISA using an MCP-1 ELISA kit (ab100589; Abcam, Cambridge, UK). In microplates, standards (50 μL) and samples (10 μL serum and 40 μL diluent) were added into predefined wells, whereas blank wells were left empty. In the wells for standards and samples, horseradish peroxidase-labelled conjugates (100 μL) were added before sealing the plates for incubation at 37 °C for 1 h. After washing the plates 5 times, substrates A (50 μL) and B (50 μL) were added into each well. After incubation at 37 °C for 15 min, stop solution (50 μL) was added into each well, and the absorbance of each well was measured at 450 nm within 15 min.
Bioinformatics
Bioinformatics prediction is a powerful tool for the study of the functions of miRNAs. We used miRanda (http://34.236.212.39/microrna/home.do) to predict genes that might regulate MCP-1.
Cells
To transfect HC-a cells (Cell Bank, Chinese Academy of Sciences, Shanghai, China) with agomiR-421, the cells (3 × 105) in logarithmic growth were seeded onto 24-well plates one day before transfection, and cultured in antibiotics-free F12/DMEM (Dulbecco’s Modified Eagle Medium) supplemented with 10% fetal bovine serum until reaching 70% confluency. In the first vial, 1.5 µL agomiR-negative control (20 pmol/µL; agomiR-NC group; forward, 5′-UUCUCCGAACGUGUCACGUTT-3′; reverse, 3′-TTAAGAGGCUUGCACAGUGCA-5′) or agomiR-421 (20 pmol/µL; agomiR-421 group; forward, 5′-AUCAACAGACAUUAAUUGGGCGC-3′; reverse, 3′-UAGUUGUCUGUAAUUAACCCGCG-5′) (Sangon Biotech, Shanghai, China) was mixed with 50 µL Opti Mem medium (Thermo Fisher Scientific, Waltham, MA, USA). In the second vial, 1 µL Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) was mixed with 50 µL Opti Mem medium. After standing still for 5 min, the two vials were combined for additional incubation at room temperature for 20 min. Then, the mixtures were added onto cells in respective groups. Six hours later, the medium was replaced with F12/DMEM containing 10% fetal bovine serum. After cultivation for 48 h, the culture supernatants of HC-a cells were used to stimulate THP-1 cells (Cell Bank, Chinese Academy of Sciences, Shanghai, China).
Dual luciferase reporter assay
According to bioinformatics results, wild-type (WT) and mutant seed regions of miR-421 in the 3′-UTR (untranslated region) of the MCP-1 gene were chemically synthesized in vitro. Then, their two ends were attached with Spe-1 and HindIII restriction sites, and then cloned into pMIR-REPORT luciferase reporter plasmids. Plasmids (0.8 μg) with WT or mutant 3′-UTR sequences were co-transfected with agomiR-421 (100 nmol/L; forward, 5′-AUCAACAGACAUUAAUUGGGCGC-3′; reverse, 3′-UAGUUGUCUGUAAUUAACCCGCG-5′; Sangon Biotech, Shanghai, China) into 293 T cells. For control, 293 T cells were transfected with agomiR-negative control (NC; forward, 5′-UUCUCCGAACGUGUCACGUTT-3′; reverse, 3′-TTAAGAGGCUUGCACAGUGCA-5′; Sangon Biotech, Shanghai, China). After cultivation for 24 h, the cells were lyzed using dual luciferase reporter assay kit (E1980; Promega, Fitchburg, WI, USA) according to the manufacturer’s manual, and luminescence intensity was measured using GloMax 20/20 luminometer (Promega, Fitchburg, WI, USA). Using renilla luminescence activity as internal reference, the luminescence values of each group of cells were measured.
Transwell assay
Transwell chambers (Corning Inc., Corning, NY, USA) were used to evaluate the migration ability of THP-1 cells. Transfected cells were collected by trypsin digestion, and resuspended to a density of 5 × 105 cells/mL using DMEM containing 0.1% bovine serum albumin. The cell suspension (200 μL) was added into the migration chamber. In the lower chamber, 500 μL DMEM medium supplemented with 20% serum was added. After 24 h culture under 5% CO2 and 37 °C, the cells in migration chamber were wiped by cotton swab. Cells that moved to the other side of the chamber were fixed with 100% methanol for 30 min. After being stained with 0.1% crystal violet, the number of cells in 5 randomly selected fields was counted under a microscope (magnification, 200×).
Statistical analysis
The results were analyzed using SPSS 18.0 statistical software (IBM, Armonk, NY, USA). The data were expressed as means values with standard deviations (±SD). Data were tested for normality. Multigroup measurement data were analyzed using one-way analysis of variance (ANOVA). In case of homogeneity of variance, Least Significant Difference and Student-Newman-Keuls methods were used; in case of heterogeneity of variance, Tamhane’s T2 or Dunnett’s T3 method was used. Comparison between two groups was carried out using Student’s t-test. Differences were considered statistically significant at a level of p < 0.05.
Results and discussion
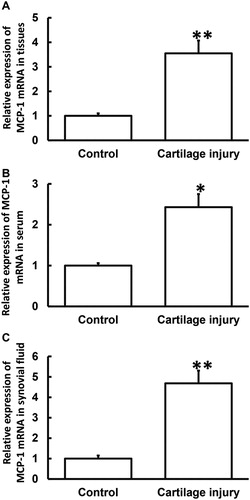
MCP-1 mRNA expression was elevated in patients with cartilage injury of elbow joint of upper limbs
Cartilage matrix is a viscoelastic material because of its biphasic matrix. Cartilage has viscoelasticity under compressive stress. These characteristics ensure cartilage functions such as load-bearing, shock absorption and stress dispersion [Citation17]. When articular cartilage is damaged, inflammation occurs at the site of the injury. The expression of some inflammatory factors such as IL-1 or TNF-α increases, and proinflammatory cytokines induce inflammatory cells to release many chemokines such as IL-8, MIP-3, SDF-la and MCP-1 [Citation18]. Expression of chemokine receptors on the surface of BMSCs is increased after stimulation by inflammation, such as MCP-1 (CCR2 and CRR4). These factors then recruit circulating BMSCs to the damaged sites to induce articular cartilage repair [Citation19]. As an important member of inflammatory factors, MCP-1 has obvious chemotactic effect on monocytes [Citation20], promotes the release of inflammatory factors such as IL-1, IL-6 and ICAM-1, activates NF-κB, mediates cytokine transcription, and causes a wider range of inflammatory reactions, resulting in a vicious circle [Citation21]. Normally, apoptosis occurs in only a few chondrocytes in healthy cartilage tissues, and small amount of chondrocyte apoptosis is a necessary physiological process to maintain the growth and development of cartilage and the stability of internal environment. Once the apoptotic rate of chondrocytes increases, cartilage tissue tends to develop pathological changes leading to osteoarthritis [Citation22]. Inflammatory factors can also trigger cascade reactions in cells, leading to osteoarthritis [Citation23–25]. To determine the expression of MCP-1 mRNA, qRT-PCR was performed. The data showed that the level of MCP-1 mRNA in injured cartilage tissues from the patients was significantly higher than that from adjacent normal tissues (p < 0.01) (). Moreover, the levels of MCP-1 mRNA in serum and synovial fluid from the patients were significantly higher than those from healthy subjects (p < 0.05 for both) (). The results suggest that MCP-1 mRNA expression was elevated in patients with cartilage injury of elbow joint of upper limbs.
Figure 1. Relative expression of MCP-1 mRNA in cartilage tissues (A), serum (B) and synovial fluid (C) from patients with cartilage injury of elbow joint of upper limbs. Note: qRT-PCR was used to determine the expression of mRNA. GAPDH was used as internal reference, and expression of target gene in each group was divided by that of GAPDH. Then, the value of experimental group was normalized to control group. *p < 0.05 and **p < 0.01 compared with control group.
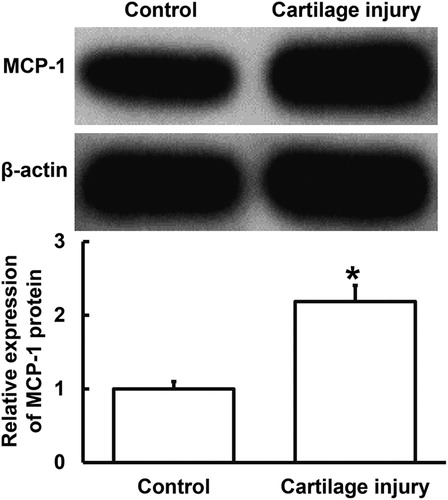
MCP-1 protein expression was increased in injured cartilage tissues from patients with cartilage injury of elbow joint of upper limbs
To determine the expression of MCP-1 protein in cartilage tissues, Western blotting was carried out. The data showed that MCP-1 protein expression in injured cartilage tissues was significantly higher than that in adjacent normal tissues (p < 0.05) (). The result indicates that MCP-1 protein expression was increased in injured cartilage tissues from patients with cartilage injury of elbow joint of upper limbs.
Figure 2. Relative expression of MCP-1 protein in cartilage tissues from patients with cartilage injury of elbow joint of upper limbs. Note: Western blotting was used to determine the expression of protein in the tissues. β-actin was used as internal reference, and expression of target gene in each group was divided by that of β-actin. Then, the value of experimental group was normalized to control group. *p < 0.05 compared with control group.
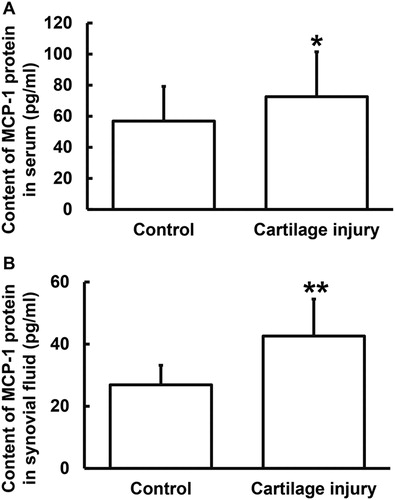
Secretion of MCP-1 protein to blood and synovial fluid was increased in patients with cartilage injury of elbow joint of upper limbs
To determine the contents of MCP-1 protein in serum and synovial fluid, ELISA was used. The data showed that the MCP-1 protein content in serum and synovial fluid from the patients were significantly elevated as compared to those from the healthy subjects (p < 0.05 for both) (). These results suggest that the secretion of MCP-1 protein to blood and synovial fluid was increased in patients with cartilage injury of elbow joint of upper limbs.
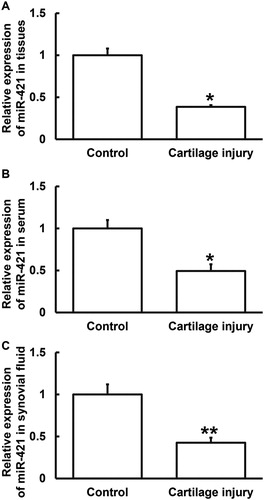
miR-421 expression was decreased in patients with cartilage injury of elbow joint of upper limbs
To determine the expression of miR-421, qRT-PCR was performed. The data showed that the level of miR-421 in injured cartilage tissues from the patients was significantly lower than that in adjacent normal tissues (p < 0.05) (). In addition, the levels of miR-421 in serum and synovial fluid from the patients were lower than those from the healthy subjects (p < 0.05 for both) (). These results suggest that miR-421 expression was decreased in patients with cartilage injury of elbow joint of upper limbs.
Figure 4. Relative expression of miR-421 in cartilage tissues (A), serum (B) and synovial fluid (C) from patients with cartilage injury of elbow joint of upper limbs. Note: qRT-PCR was used to determine the expression of miR-421. U6 was used as internal reference, and expression of target gene in each group was divided by that of U6. Then, the value of experimental group was normalized to control group. *p < 0.05 and **p < 0.01 compared with control group.
miR-421 can bind with the 3′-UTR seed region of MCP-1 mRNA to regulate its expression
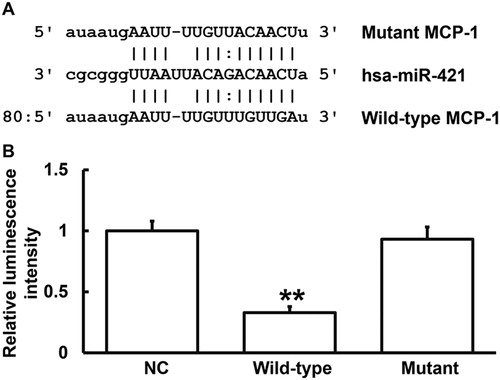
miRanda prediction of genes that might regulate MCP-1 showed that miR-421 was a potential gene that might regulate MCP-1, and UGUUGA was the potential seed region of miR-421 (). It is widely known that miRNA could cut the mRNA of MCP-1 and inhibit its translation [Citation26]. In this way, miRNAs alter the expression of mRNA to regulate the activity of proteins, playing important roles in the occurrence and development of diseases [Citation27, Citation28]. The expression of miR-421 in gastric cancer has been studied before [Citation29]. In addition, up-regulated expression of miR-421 in neuroblastoma, pancreatic cancer and prostate cancer is also reported [Citation30–32]. Of note, miR-421 is closely related to cell growth. For example, the expression of miR-421 in gastric cancer tissues is higher than that in normal tissues, confirming that miR-421 plays an important role in the early growth of gastric cancer [Citation33]. It is also reported that inhibition of miR-421 expression in animals suppressed tumor growth [Citation34]. These studies suggest that miR-421 is closely related to the occurrence and development of human diseases. Of note, the levels of miR-421 and MCP-1 in serum can reflect inflammatory responses and tissue damage in upper limb cartilage to a certain degree. To identify the direct interaction between miR-421 and the 3′-UTR of MCP-1 mRNA, dual luciferase reporter assay was performed. The luminescence value of cells co-transfected with agomiR-421 and pMIR-REPORT-WT luciferase reporter plasmids was significantly lower than that in the negative control group (p < 0.01). By contrast, the luminescence value of cells co-transfected with agomiR-421 and pMIR-REPORT-mutant luciferase reporter plasmids was not significantly different from that in the negative control group (p > 0.05) (). The results indicate that miR-421 can bind with the 3′-UTR seed region of MCP-1 mRNA to regulate its expression.
Figure 5. Identification of interaction between miR-421 and MCP-1 mRNA. (A) Bioinformatics prediction (miRanda) of genes that might regulate MCP-1. (B) Dual luciferase reporter assay. Note: Plasmids (0.8 μg) with wild-type or mutant 3′-UTR sequences were co-transfected with agomiR-421 into 293T cells. For control, 293T cells were transfected with agomiR-negative control (NC). Renilla luminescence activity was used as internal reference to determine the luminescence values of each group of cells. **p < 0.01 compared with NC group.
miR-421 overexpression inhibited the expression of MCP-1 and the chemotaxis of THP-1 monocytes
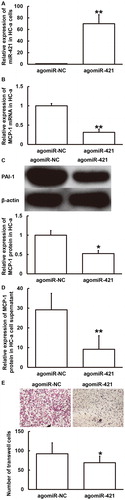
To examine how miR-421 regulates the release of MCP-1 by HC-a cells and affects the migration of THP-1 cells, HC-a cells were transfected with agomiR-421 or agomiR-NC and their culture supernatants were used to stimulate THP-1 cells. qRT-PCR showed that the expression of miR-421 in HC-a cells transfected with agomiR-421 was significantly higher than that in the agomiR-NC group (p < 0.01) (). In addition, the expression of MCP-1 mRNA and protein in HC-a cells and the secretion of MCP-1 protein by HC-a cells in the agomiR-421 group was significantly lower than that in the agomiR-NC group (p < 0.05) (). Transwell assay showed that the number of migrated THP-1 cells treated with supernatant of HC-a cells that were transfected with agomiR-421 was significantly lower than that in the agomiR-NC group (p < 0.05) (). The results suggest that miR-421 overexpression could inhibit the expression of MCP-1 and the chemotaxis of THP-1 monocytes.
Figure 6. Effect of miR-421 overexpression on the expression of MCP-1 in HC-a cells (A–D) and the migration of THP-1 cells (E). (A) Expression of miR-421 in HC-a cells after transfection with agomiR-NC or agomiR-421. **p < 0.01 compared with agomiR-NC group. (B, C) Expression of MCP-1 mRNA (B) and protein (C) in HC-a cells after transfection with agomiR-NC or agomiR-421. *p < 0.05 and **p < 0.01 compared with agomiR-NC group. (D) Content of MCP-1 protein in supernatant of HC-a cells after transfection with agomiR-NC or agomiR-421. **p < 0.01 compared with agomiR-NC group. (E) Number of migrated THP-1 cells after stimulation by culture supernatant of HC-a cells transfected with agomiR-NC or agomiR-421. Transwell assay was used to determine cell migration. *p < 0.05 compared with agomiR-NC group.
Conclusions
The present study demonstrates that reduced expression of miR-421 in injured cartilage tissues, blood and synovial fluid results in increased expression of MCP-1. In addition, miR-421 affects the chemotaxis of monocytes via MCP-1 and regulates local immune responses at injured sites of elbow joint cartilage of upper limbs.
Author contributions
FZ and JX contributed to the design of the study. FZ, JY and JL performed the experiments. FZ, JY and JX analyzed the data. FZ and JX interpreted results and prepared the manuscript. The final version of the manuscript has been read and approved by all authors.
Ethical approval and consent to participate
All procedures performed in this study were approved by the Ethics Committee of Ningbo No. 6 Hospital. Written informed consent was obtained from all patients or their families.
Consent for publication
Written informed consent forms for publication of any associated data and accompanying images were obtained from all patients or their parents, guardians or next of kin.
Acknowledgements
The authors wish to thank Professor Hong Chen from the Department of Hand Surgery, Ningbo No. 6 Hospital.
Disclosure statement
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request.
References
- Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science (New York, NY). 2012;338(6109):917–921.
- Zhang W, Chen J, Tao J, et al. The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair. Biomaterials. 2013;34(3):713–723.
- Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng B Rev. 2010;16(3):305–329.
- Laing KJ, Secombes CJ. Chemokines. Dev Comp Immunol. 2004;28(5):443–460.
- Wang ZW, Wang JJ, Zhang JZ, et al. Thrombolysis of deep vein thrombosis and inhibiting chemotaxis of macrophage by MCP-1 blockage. Eur Rev Med Pharmacol Sci. 2017;21(7):1695–1701.
- Thacker MA, Clark AK, Bishop T, et al. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain (London, England). 2009;13(3):263–272.
- Abbadie C, Lindia JA, Cumiskey AM, et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Am Sci USA. 2003;100(13):7947–7952.
- Hayashidani S, Tsutsui H, Shiomi T, et al. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 2003;108(17):2134–2140.
- Ridiandries A, Tan JTM, Bursill CA. The role of chemokines in wound healing. Int J Mol Sci. 2018;19(10):3217.
- Liu N, Zhang T, Cao BR, et al. Icariin possesses chondroprotective efficacy in a rat model of dexamethasone-induced cartilage injury through the activation of miR-206 targeting of cathepsin K. Int J Mol Med. 2018;41(2):1039–1047.
- Qiang Y, Hao-Peng L, Xiong G. [The mechanism advance of microRNA in cartilage injury and degeneration]. Zhongguo gu Shang. 2012;25(6):530–534
- Wen X, Han XR, Wang YJ, et al. MicroRNA-421 suppresses the apoptosis and autophagy of hippocampal neurons in epilepsy mice model by inhibition of the TLR/MYD88 pathway. J Cell Physiol. 2018;233(9):7022–7034. PubMed PMID: 29380367; eng.
- Hu TB, Chen HS, Cao MQ, et al. MicroRNA-421 inhibits caspase-10 expression and promotes breast cancer progression. Neoplasma. 2018;65(01):49–54.
- Yang P, Zhang M, Liu X, et al. MicroRNA-421 promotes the proliferation and metastasis of gastric cancer cells by targeting claudin-11. Exp Ther Med. 2017;14(3):2625–2632.
- Liu L, Cui S, Zhang R, et al. MiR-421 inhibits the malignant phenotype in glioma by directly targeting MEF2D. Am J Cancer Res. 2017;7(4):857–868.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif). 2001;25(4):402–408. PubMed PMID: 11846609; eng.
- Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17(5):377–394.
- Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1825–1834.
- Park MS, Kim YH, Jung Y, et al. In situ recruitment of human bone marrow-derived mesenchymal stem cells using chemokines for articular cartilage regeneration. Cell Transplant. 2015;24(6):1067–1083.
- Yoshimura T. The production of monocyte chemoattractant protein-1 (MCP-1)/CCL2 in tumor microenvironments. Cytokine. 2017;98:71–78.
- Yeo ES, Hwang JY, Park JE, et al. Tumor necrosis factor (TNF-alpha) and C-reactive protein (CRP) are positively associated with the risk of chronic kidney disease in patients with type 2 diabetes. Yonsei Med J. 2010;51(4):519–525.
- Taniguchi N, Carames B, Ronfani L, et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Am Sci USA. 2009;106(4):1181–1186.
- Wang X, Li F, Fan C, et al. Effects and relationship of ERK1 and ERK2 in interleukin-1beta-induced alterations in MMP3, MMP13, type II collagen and aggrecan expression in human chondrocytes. Int J Mol Med. 2011;27(4):583–589.
- Marks PH, Donaldson ML. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy. 2005;21(11):1342–1347.
- Aigner T, McKenna L, Zien A, et al. Gene expression profiling of serum- and interleukin-1 beta-stimulated primary human adult articular chondrocytes–a molecular analysis based on chondrocytes isolated from one donor. Cytokine. 2005;31(3):227–240.
- Lu JB, Yao XX, Xiu JC, et al. MicroRNA-125b-5p attenuates lipopolysaccharide-induced monocyte chemoattractant protein-1 production by targeting inhibiting LACTB in THP-1 macrophages. Arch Biochem Biophys. 2016;590:64–71. PubMed PMID: 26603571; eng.
- Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8(2):93–103. PubMed PMID: 17230196; eng.
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20.
- Chureau C, Chantalat S, Romito A, et al. Ftx is a non-coding RNA which affects Xist expression and chromatin structure within the X-inactivation center region. Hum Mol Genet. 2011;20(4):705–718. PubMed PMID: 21118898; eng.
- Hao J, Zhang S, Zhou Y, et al. MicroRNA 421 suppresses DPC4/Smad4 in pancreatic cancer. Biochem Biophys Res Commun. 2011;406(4):552–557.
- Ostling P, Leivonen SK, Aakula A, et al. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 2011;71(5):1956–1967.
- Hu H, Du L, Nagabayashi G, et al. ATM is down-regulated by N-Myc-regulated microRNA-421. Proc Natl Am Sci USA. 2010;107(4):1506–1511.
- Jiang Z, Guo J, Xiao B, et al. Increased expression of miR-421 in human gastric carcinoma and its clinical association. J Gastroenterol. 2010;45(1):17–23.
- Zhou H, Xiao B, Zhou F, et al. MiR-421 is a functional marker of circulating tumor cells in gastric cancer patients. Biomarkers. 2012;17(2):104–110.