Abstract
Lipoxygenases (LOXs; EC1.13.11.12) are widely involved in plant growth, development, maturation, senescence and stress resistance. LOXs have been studied in many crop species but not in Tartary buckwheat [Fagopyrum tataricum (L.) Gaertn.]. This study aimed to systematically identify and analyze LOX genes in Tartary buckwheat. Eight LOX genes, which were unevenly distributed on five chromosomes, were identified in the Pinu1 Genome database. The data indicated that during long evolution, FtLOX genes were subjected to purifying selection. The conservation of motifs within the 9-LOX and 13-LOX subfamilies supported the results of phylogenetic analyses. All FtLOX proteins contained the LOX family-specific His-(X)4-His-(X)4-His-(X)17-His-(X)8-His motif, suggesting that this motif is essential for protein function. Analyses of physical and chemical properties showed that FtLOX6 might function under both acidic and alkaline conditions. The FtLOX gene promoters contained many light-responsive elements, and transcriptome data further suggested their roles in growth regulation and the response to light. Quantitative real-time polymerase chain reaction analysis of expression differences in plants exposed to different wavelengths of light confirmed that FtLOX1 and FtLOX4–7 could respond to light. Genes co-expressed with FtLOX genes were involved in the photoreaction-related pathway. This study provides a basis for further functional research in order to better understand the biological role of individual LOX gene in Tartary buckwheat.
Introduction
Lipoxygenases (LOXs; EC1.13.11.12) are widely found in animals, plants and even prokaryotes [Citation1]. LOX was first discovered in soybean in 1932 [Citation2]. The enzyme catalyzes the peroxidation of lipids, which activates multiple downstream pathways and regulates biological activities [Citation3]. In plants, lipoxygenases, oxidized lipids and their derivatives are associated with seed development, fruit ripening, senescence and resistance to stress [Citation4–7]. LOXs mainly catalyze the conversion of linolenic acid and linoleic acid and generate 9- and 13-fatty acid hydroperoxides after catalytic oxygenation at C9 and C13 positions, respectively [Citation8]. In pear, LOX can replace aminocyclopropanecarboxylate (ACC) oxidase to convert ACC into ethylene under stress, which can lead to fruit ripening and senescence [Citation9]. In general, LOX members are divided into 9-LOX and 13-LOX, based on the order in which linoleic acid is oxidized. In addition, LOXs are divided into type I and type II based on sequence similarity; the type II LOX N-terminal region typically has a chloroplast transport peptide with 13-LOX activity [Citation10]. All type II LOX proteins identified to date belong to the 13-LOX subfamily [Citation3]. There are many factors affecting LOXs. For example, in maize, the increase in LOX activity is highly correlated with the decline of Aspergillus flavus infection [Citation7]. In tea plants, CsLOX1 can be up-regulated by the inducement of methyl jasmonate (MeJA) [Citation11].
Fagopyrum tataricum (L.) Gaertn., commonly known as Tartary buckwheat, is a traditional food and medicinal plant whose seeds have a slight bitterness. Tartary buckwheat is rich in flavonoids and starch and is regarded as an important pseudocereal crop [Citation12]. The whole plant is abundant in nutrients, which are concentrated in stems, roots, flowers, leaves and seeds. The rutin content in the whole plant of Tartary buckwheat is extremely high [Citation13]. Light is one of the essential factors for plants, not only for their photosynthesis but also as a trigger for plant growth and development and as an effector of plant regulatory processes. The expression of LOX is affected by light during growth and development; however, it varies greatly among different plants [Citation14]. Illumination was shown to regulate 13-LOX in olives at the transcriptional level [Citation15]. Red light is the main inducer of the defense response in Arabidopsis against pathogen infection, and the transcription and activity of LOX are upregulated [Citation16]. However, in young leaves of wheat, light treatment reduces the LOX activity, which leads to the membrane lipid peroxidation [Citation17]. In pea bean seedlings, light promotes the stem and leaf growth, but the increase in LOX activity under light conditions may be entirely due to the increased contribution of leaf tissue to the total activity [Citation18]. However, in Tartary buckwheat, the effects of light on LOX genes and their functional properties have not been fully investigated.
In this study, we identified the Tartary buckwheat LOX genes and systematically predicted the gene structure, protein properties and phylogenetic relationships among the family members. Furthermore, we evaluated the roles of LOXs in different tissues and light treatments. Multiple evidence suggested that the Tartary buckwheat LOX genes respond to different wavelengths of light. Our results provide useful insights into the role of these LOX genes in buckwheat light responses.
Materials and methods
Data search and sequence acquisition
To identify LOX gene family members in Tartary buckwheat, whole genome and protein data were obtained from a genome database (http://www.mbkbase.org/Pinku1/). The hidden Markov model (HMM) for the LOX gene family (PF00305) was downloaded from the Pfam database (http://pfam.xfam.org). RNA sequencing (RNA-seq) data for Tartary buckwheat were acquired from the National Center of Biotechnology Information Sequence Read Archive database (https://www.ncbi.nlm.nih.gov/sra). All samples are listed in Supplemental Table S1. The LOX protein sequences of other species were downloaded from the UniProt database (https://www.uniprot.org/).
Identification of LOX proteins in tartary buckwheat
HMM search [Citation19] was performed locally in the Tartary buckwheat protein database using the HMM profile of the PF00305 domain. We rejected protein sequences lacking the PLAT (polycystin-1, lipoxygenase, alpha-toxin) or LH2 (lipoxygenase homolog) domain [Citation20]. Domains of the protein sequences were verified using the Simple Modular Architecture Research Tool for further analysis (http://smart.embl-heidelberg.de/).
Phylogenetic analyses
To investigate the phylogenetic relationships among LOX proteins, LOX amino acid sequences from 14 species were analyzed using ClustalW2 [Citation21]. A multiple sequence alignment of the sequences of 69 LOX proteins was obtained and adjusted manually with DNAMAN [Citation22]. Phylogenetic trees were generated using the neighbor-joining (NJ) and maximum-likelihood (ML) methods using MEGAX; bootstrap values were obtained from 1,000 replicates [Citation23].
Analyses of chromosomal locations, gene duplication, gene structure and conserved motifs
The locations of LOX genes on the Tartary buckwheat chromosomes were obtained from the genome structure annotation file (V2). Based on the location of each LOX gene on the chromosome, the distribution of all FtLOX genes in the genome was plotted using the MapInspect online tool (http://mg2c.iask.in/mg2c_v2.0/). Duplicated genes were analyzed based on the following criteria: (a) the length of the alignable sequence covered >75% of the longer gene and (b) the similarity in the aligned region was >75% [Citation24,Citation25]. MCScanX was used with default parameters to analyze collinearity events between species [Citation26]. To reveal the synteny relationships between LOX genes of Tartary buckwheat and Arabidopsis thaliana, syntenic analysis maps were constructed using the Circos software [Citation27]. The Ka/Ks ratio was calculated using the PAL2NAL online tool (http://www.bork.embl.de/pal2nal/). Gene structures were visualized using the GSDS 2.0 online tool (http://gsds.cbi.pku.edu.cn/). Conserved structural motifs were identified using MEME (maximum number of motifs = 7, optimum motif width = 6–200) [Citation28]. A motif plot was generated using TBtools (https://github.com/CJ-Chen/TBtools).
Codon usage patterns of FtLOX genes
The sequences were evaluated with respect to the total GC content of the coding sequence (CDS) and the GC content at the 3rd position (GC3s) of the codon for the eight FtLOX gene family members using CodonW (http://codonw.sourceforge.net/).
Analysis of physical and chemical properties of FtLOXs
The protein length (number of amino acids), molecular weight, isoelectric point (pI) and other physical and chemical properties of the FtLOX proteins were analyzed using ProtParam (http://web.expasy.org/protparam/). Signal peptides were analyzed using the SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/) and TargetP 1.1 (http://www.cbs.dtu.dk/services/TargetP/) servers. Subcellular localization of FtLOXs was predicted using Plant-mPLoc [Citation29].
Analysis of cis-acting elements in FtLOX promoters
Cis-acting elements were predicted in the promoter region (2,000 bp upstream of the start codon) and were identified using plantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Plant materials and treatments
Tartary buckwheat seeds were sowed in soil in the darkness for 4 days at 25 °C, followed by various continuous light treatments for 2 days. The wavelengths of the light-emitting diodes were as follows: red light (670 nm), blue light (470 nm) and far-red light (735 nm). The light intensity was 50 ± 5 µmol/(m2·s) in all treatments. After light treatments, samples were ground into powder and stored in liquid nitrogen. One gram of each sample was used to extract RNA for sequencing. Each treatment comprised three biological replicates. For transcriptome sequencing at different developmental stages, seeds were grown naturally in the soil at room temperature.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
The total RNA of plant was isolated using an RNA Extraction Kit (Tiangen, Beijing, China) according to the instructions. Next, first-strand cDNA was synthesized using oligo d(T) by reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The gene primers were designed using Primer Premier 3.0 (Supplemental Table S2). FtLOX gene expression was confirmed by qRT-PCR using a Rotor-GeneQ instrument (Qiagen, Hilden, Germany). cDNA (diluted 1:5) was used as a template and mixed with the corresponding primers and TransStart Green qPCR SuperMix UDG (TransGen Biotech, Beijing, China). Relative quantification of the target genes was performed using actin as the internal reference, the ratio of the FtLOX genes to the copy number of actin mRNA was estimated as the relative transcription level of the gene. For qPCR, there were three biological replicates used for each sample. The qPCR reaction mixture was as follows: each 20 μL of the reaction mixture contained 10 μL of qPCR SuperMixUDG TaqTM, 0.4 μL of each primer (10 μmol/L), 0.4 μL of LROX Reference Dye (50*), 1.5 μL of diluted cDNA, and 7.7 μL of double distilled water. The amplification procedure was 94 °C pre-denaturation for 10 min, then 94 °C denaturation for 10 s, 60 °C annealing for 15 s, 72 °C extension for 15 s as a cycle, 40 cycles, and fluorescence signals were recorded at the end of each cycle from 72 °C to 95 °C and with a hold of 5 s every 1 °C.
Analysis of FtLOX gene expression
The RNA-seq data were aligned to the genome using STAR (version 2.5.1b) [Citation30]. Gene expression levels were calculated based on fragments per kilobase of transcript per million mapped reads (FPKM) values using HTseq version 0.9.0 [Citation31]. A heatmap of LOX genes was obtained using the R package “pheatmap”. Co-expressed genes were analyzed using a Perl script based on FPKM data [Citation32]. Gene enrichment was analyzed using clusterProfiler [Citation33].
Statistical analysis
The SPSS statistical software package (Windows version 22.0; SPSS, Chicago, IL, USA) was used for statistical analysis of the effective codon number (ENC), relative synonymous codon usage (RSCU), clustering and codon preference. Parametric one-way analysis of variance was used to analyze the transcription level of each gene under different light conditions. The level of significance was set to p < 0.05 when comparing the means among groups.
Results and discussion
Genome-wide identification and characterization of LOX genes in F. tataricum
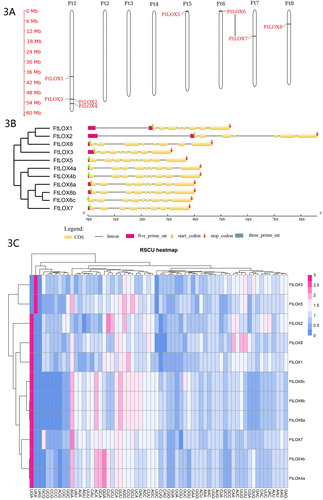
To identify LOX gene family members in F. tataricum, HMM (Pfam: PF00305) was used to search against the F. tataricum protein database. In addition, the HMMER web server was used to verify the presence of domains. Eight LOX genes were identified by retaining sequences that contained the LOX and PLAT/LH domains. The genes were named FtLOX1–8 according to their locations on chromosomes (). The number of LOX genes in the genome vary substantially among species. Only one LOX gene has been detected in lentil, six in A. thaliana and 23 in tobacco [Citation34–36]. Interestingly, FtLOX4 encoded two alternative splicing isoforms, and FtLOX6 encoded three isoforms. Alternative splicing (AS) is an important mechanism underlying the response to stress [Citation37]. Previous studies, except those on tea trees, have not reported AS in LOX genes. Our results suggest that AS events in FtLOX4 and FtLOX6 may increase protein diversity to meet functional demands.
Table 1. Overview of the LOX gene family in the F. tataricum genome.
LOX proteins have been reported in various plants, and their sequences were used to uncover phylogenetic relationships with FtLOXs. LOXs from 14 species (rice, tomato, soybean, etc.) were used to build NJ and ML trees by aligning the sequences of six AtLOXs from A. thaliana, seven GmLOXs from Glycine max, and other LOX members. The tree topologies generated by the two methods were highly concordant, which agrees with those obtained in previous studies [Citation3]. Consistent with previous research, the 69 LOXs compared were divided into two major clades (9-LOXs and 13-LOXs). Specific valine residue at a certain position was shown to be a characteristic amino acid of 9-LOXs, based on the sequence alignment () [Citation38]. The properties of the FtLOX proteins are shown in . Among these, FtLOX4, FtLOX6 and FtLOX7 belonged to the 9-LOX group, and the others belonged to the type II 13-LOX group. In the 9-LOX subfamily, FtLOX4 was closely related to AtLOX1. FtLOX6 and FtLOX7 were closely related to AtLOX5, StLOX2 and CsLOX4, and this subfamily was most closely related to that of Arabidopsis LOXs. No FtLOX belonged to type I 13-LOX, similar to Arabidopsis LOXs [Citation34]. Type I 13-LOXs are monocotyledon-specific proteins. Type II 13-LOXs show a distribution in both monocots and dicots, indicating that FtLOXs evolved before monocots and dicots differentiation.
Figure 1. Multiple alignment of LOX amino acid sequences from F. tataricum and other plants (A–C). Note: Black dots represent the characteristic amino acids of 9-LOXs.
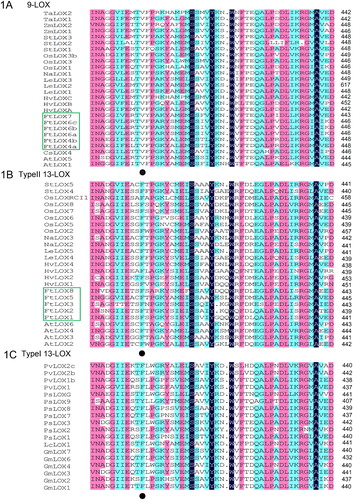
Figure 2. Phylogenetic analysis of LOX members from 14 species. Note: Evolutionary relationships were inferred using the neighbor-joining method (with 1,000 bootstrap replicates). Branches corresponding to partitions that were reproduced in less than 50% of the bootstrap replicates were collapsed.
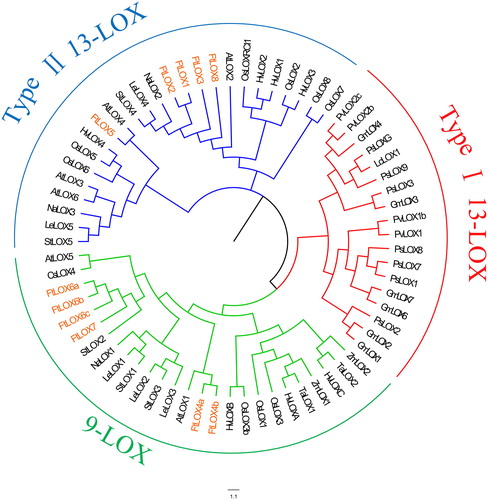
Evolutionarily similar genes tend to have similar functions. Based on the phylogenetic tree, different functions can be assigned to the 9/13 LOX group in Tartary buckwheat. For example, the TypeII − 13 LOX group includes five FtLOX genes that are clustered together with rice OsLOX2, which is known to participate in aging processes that delay decomposition during storage. We, thus, speculate that these five genes may have similar functions [Citation39].
Chromosomal distribution and collinearity of FtLOX genes
The results of chromosomal distribution analysis showed that the eight Tartary buckwheat LOX genes were unevenly distributed on five chromosomes ( and ), including four LOX genes on chromosome 1, one each on chromosomes 5–8, and no LOX genes on chromosomes 2–4. But AtLOXs are only distributed on two Arabidopsis chromosomes, suggesting that the FtLOX gene family has been expanded. Based on the gene duplication analysis, only one segmental duplication event was identified between FtLOX6 and FtLOX7, which both belonged to the type II 13-LOX group, suggesting that they share similar functions and expression patterns. In plants, gene duplication is a major force driving the increase in gene family size and functional diversity [Citation40]. Thus, it was inferred that they can play different roles in different environments. The Ka value for the pair was 0.123769, Ks was 0.707433, and Ka/Ks was <1, consistent with purifying selection. To further infer the phylogenetic relationships in the LOX family, we conducted comparative collinearity analysis of Tartary buckwheat and Arabidopsis genes (Supplemental Figure S1). Among these, two FtLOX genes showed collinearity with Arabidopsis genes. FtLOX5 is collinear with AtLOX6 and FtLOX6 is collinear with AtLOX5. Thus, two pairs of LOX paralogous genes (FtLOX8-FtLOX1 pair and FtLOX8-FtLOX2 pair) were shown to have a collinear relationship.
Gene structure analysis
Based on GSDS analysis of the structures of the eight FtLOX gene family members (), there were mainly between seven and nine exons and seven to eight introns in the 9-LOX subfamily. FtLOX4, FtLOX6 and FtLOX7 (type II 13-LOX subfamily) all had nine exons and eight introns.
Codon usage patterns in FtLOX genes
ENC was used as an index of individual codon usage, which ranges from 20 to 61 [Citation41]. Genes with higher ENC have weaker codon usage bias. The ENC values varied for the FtLOX genes from 53.68 to 58.61, with an average of 55.29, indicating that the codon usage in the FtLOX genes is very weak. The average A3s and T3s contents in the FtLOX genes were 0.3386 and 0.3605, respectively, which were significantly higher than those of G3s and C3s. The average GC content was 45.7%, and the average GC3s content was 44%, indicating that FtLOX genes prefer to use A- or T-ending codons.
A total of six codons, including CUU for leucine, AGG for arginine, GCU for alanine, GGA for glycine and CCU and CCA for proline, had RSCU values of greater than 1.00 in the FtLOX genes, indicating that these codons are used more frequently than other synonymous codons.
The FtLOX1, FtLOX2, FtLOX3, FtLOX5 and FtLOX8 genes in the 13-LOX type II subclass clustered together. The correlation in RSCU values between FtLOX1 and FtLOX2 was the most significant (0.767), followed by those for FtLOX1 and FtLOX8 (0.764) and FtLOX3 and FtLOX5 (0.630). The correlations between FtLOX6a and FtLOX6b, FtLOX6a and FtLOX6c, FtLOX4b and FtLOX7, and FtLOX4a and FtLOX7 in the 9-LOX subgroup were also high (1.000, 0.979, 0.830 and 0.825 respectively). The clustering method yielded similar results to those of the phylogenetic analysis of CDSs by the NJ method, which can reflect the homology between genes, to some extent ().
Functional analysis of FtLOXs
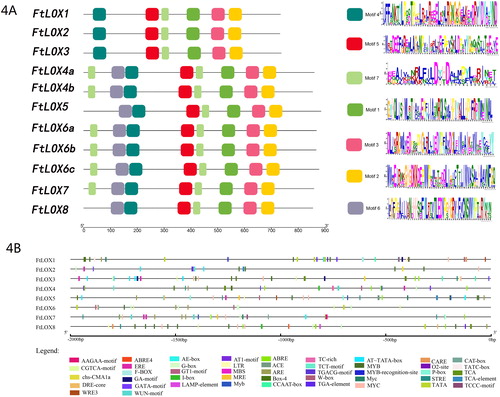
Putative conserved motifs were predicted by MEME, and seven motifs were identified in the Tartary buckwheat LOX proteins (). As expected, most of the LOX members had common motif compositions, suggesting that LOX subfamily proteins share similar functions. Notably, FtLOX4, FtLOX6 and FtLOX7, belonging to the 9-LOX subfamily, had two sequences of motif 7, and FtLOX1–3 lacked motif 6. All FtLOX proteins contained the LOX family-specific motif 1, which includes a representative 38-residue sequence with a highly conserved structure, His-(X)4-His-(X)4-His-(X)17-His-(X)8-His (Supplemental Figure S2) [Citation42], suggesting that this motif is a reliable target sequence for subsequent functional studies. These motifs were detected in LOXs using Pfam, and different groups of motifs may lead to functional differentiation among LOX members. During long-term evolution, due to the dual influence of environmental conditions and selection pressure, species adaptation can result in a specific codon usage pattern, with distinct characteristics. We detected an obvious codon usage for FtLOX mRNAs ending in A or T, which was consistent with that in dicotyledons. Moreover, the exon/intron structure, protein motif compositions, conserved threonine/valine motifs, collinearity blocks and codon usage of FtLOXs support their observed phylogenetic relationships. With respect to evolutionary relationships, clustering based on codon usage is more suitable as a supplement to traditional clustering methods [Citation10].
Figure 4. Analysis of FtLOXs. (A) Analysis of functional domains of FtLOXs. The motif analysis was performed using TBtools. LOX proteins are listed on the left. Squares in different colors represent different motifs in each LOX sequence. (B) Distribution of cis-elements in the promoter regions (−2,000 bp) of the eight FtLOX genes. Different colors represent different elements.
Gene Ontology (GO) annotation further suggested the functional roles for the LOX proteins (Supplemental Table S3). All members were associated with cytoplasmic components and were involved in lipid metabolism (biological process) and oxidoreductase activity (molecular function). In particular, FtLOX4, FtLOX6 and FtLOX7, belonging to the 9-LOX subclass, were predicted to play a role in anatomical structural development. It is expected that seven LOX family members, excluding FtLOX5, are able to respond to stress.
Physical and chemical properties of tartary buckwheat LOX proteins
The open reading frames of the FtLOX genes were 2,199–2,658 bp in length (), and the predicted LOX proteins contained 732–885 amino acids, with FtLOX2 being the shortest. The molecular weights of the proteins were 83.08–100.4 kDa. The theoretical isoelectric points were 5.55–7.12, and only the FtLOX6-encoded protein had an isoelectric point in the alkaline range, while the rest were acidic, suggesting that FtLOX6 responds to different pressures. The instability indexes were 35.18–50.4, and FtLOX1, FtLOX2, FtLOX3 and FtLOX5 were unstable. All FtLOXs were hydrophilic proteins, and most were predicted to be translocated to the cytoplasm. FtLOX1 was predicted to be distributed in chloroplasts, and FtLOX3 and FtLOX5 were predicted to be distributed in chloroplasts and the cytoplasm. FtLOX2 had a signal peptide structure, whereas FtLOX3 contained a putative chloroplast transit peptide and FtLOX5 contained a targeting peptide. Similar to those in other species, the FtLOX proteins were hydrophilic. In general, 13-LOX proteins contain transit peptides [Citation43]. In this study, FtLOX2, FtLOX3 and FtLOX5 had signal peptide sequences. Among them, FtLOX3 had a putative chloroplast transit peptide sequence, suggesting that the enzyme is mainly localized in chloroplasts. LOX functions in Tartary buckwheat are still unclear, and subcellular localization of the key proteins can guide future research. FtLOX2 was localized in the cytoplasm, while FtLOX3 and FtLOX5 in the cytoplasm and chloroplasts, and the others were localized in chloroplasts. In the soybean cytoplasm, LOXs may be involved in the transport of lipids from lipid bodies to glyoxysomes [Citation44]. All LOX pathways that are differentially localized within chloroplasts may be associated with thylakoid membranes [Citation45,Citation46]. In addition, a chloroplast LOX is required for wound-induced jasmonic acid (JA) production [Citation47,Citation48]. Moreover, a chloroplast LOX may be related to leaf senescence [Citation6,Citation49,Citation50]. Localization patterns of FtLOXs were mostly consistent with those of Arabidopsis LOXs, suggesting that the subcellular targeting mechanisms are conserved.
Table 2. Physical and chemical properties of Tartary buckwheat LOX proteins.
Analysis of cis-elements in promoters of FtLOX genes
To further understand FtLOX functions and regulatory patterns, cis-elements were predicted by analyzing sequences 2,000 bp upstream of the start codons of the FtLOX genes. As shown in Supplemental Table S4 and , in addition to large numbers of CAAT and TATA boxes, several cis-elements were identified in FtLOX promoters, with 1–5 copies.
The largest class included 14 types of light-responsive elements, of which FtLOX4 contained five types, including four copies of Box-4 and G-box each. FtLOX6 only had Box-4 and G-box light-response elements. The light-response elements of FtLOX2 and FtLOX5 had the smallest number of copies (four). All of the FtLOX promoters contained at least two types of cis-elements involved in light responses, indicating that their expression can be affected by a variety of light sources.
The FtLOX genes also had a large number of hormone-related cis-elements in their promoters, with a total of nine types identified. MeJA-responsive elements (TGACG- and CGTCA-motifs), the abscisic acid response element (ABRE), and the salicylic acid-responsive element (TCA-element) were detected in six FtLOX genes. Five members contained the ethylene-responsive element (ERE), and a few carried the auxin response element (TGA-element) and gibberellin response elements (W-box, TATC-box, and P-box). These results suggested that FtLOXs play important roles in defense mechanisms. Both the JA and salicylic acid pathways can respond to the invasion of pathogenic bacteria, and these two pathways show synergistic effects [Citation51]. Many previous studies have shown that LOX genes are regulated by jasmonic acid and other hormones in species, such as A. thaliana, H.vulgare and C. jubata [Citation52–54]. These reports also support the possible role of LOX genes in the defense mechanism of Tartary buckwheat.
In addition, many cis-elements involved in the response to stress were found, including the dehydration-responsive element (DRE-core), drought-inducible element (MBS), anaerobic induction element (ARE), O2-site element (involved in the regulation of zein metabolism), low-temperature-responsive (LTR) element, stress response elements (STRE and TC-rich repeats) and wound response elements (WRE3 and WUN-motif). MYB and MYC components are also found in many LOX genes, and all FtLOX genes contained MYB components. Thus, FtLOX promoters may be involved in various stress response mechanisms via these elements. Linolenic acid and linoleic acid are substrates of LOXs, and LOX activity is increased under low-temperature stress, resulting in a decreased content of linolenic acid and linoleic acid. Aghdam et al. [Citation14] found that the LOX activity increases when tomatoes are chilled. In tomato, chilling can improve the integrity of the cell membrane and enhance cold tolerance by reducing the activity of LOX [Citation14]. LOXs can contribute to the degradation of storage lipids during seed germination [Citation39].
Expression profiles of FtLOX genes in different organs and tissues and at different developmental stages
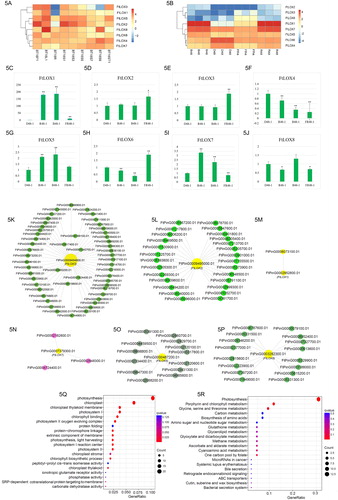
To better understand the function of LOX family members in different tissues, a comprehensive expression analysis was performed using publicly available RNA-seq data for Tartary buckwheat (Supplemental Table S1). shows a graphical representation of the expression pattern of each FtLOX, in which the genes were arranged based on hierarchical clustering analysis. All FtLOX members were expressed in flowers and leaves. In the root, only two FtLOX genes were highly expressed, whereas three were expressed at very low levels. In the stem, all FtLOX genes were expressed, but the overall expression levels were not as high as those in the leaves. FtLOX1 showed the highest expression in leaves and no expression in flowers. FtLOX2 was highly expressed in flowers and seeds, and its expression gradually decreased during seed development. FtLOX3 was highly expressed in flowers, stems and leaves but exhibited almost no expression in the mature and dry-stage seeds. The expression pattern of FtLOX5 was similar to that of FtLOX3, but FtLOX5 was expressed in all samples. FtLOX8 was highly expressed during leaf and seed desiccation. FtLOX4, FtLOX6 and FtLOX7 were all classified as belonging to the 9-LOX type, and their expression patterns were relatively similar.
Figure 5. Expression profiles and enrichment analysis of FtLOX genes. The scale bar represents log2 FPKM values. (A) Heatmap of LOX gene expression in various buckwheat tissues. BT18F1: flower; BT18L1: leaf; BT18R: root; BT18ST1: stem; BT18S1: seed at the cell division stage; BT18S3: seed at the cell enlargement stage; BT18S5: seed at the reserve deposition stage; BT18S7: seed at the maturation stage; BT18S9: seed at the desiccation stage. (B) Heatmap of FtLOX gene expression with various light treatments. B: blue; D: dark; FR: far red; R: red. (C–J) qRT-PCR analysis of LOX gene expression in Tartary buckwheat exposed to different light wavelengths. Vertical bars indicate standard deviation. Asterisks indicate significantly up- or downregulated genes compared with their expression in the untreated control (*p < 0.05, **p < 0.01; Student’s t-test). (K–P) Co-expression network of FtLOX genes, obtained using Cytoscape v3.7.0. (Q) GO enrichment analysis of co-expressed genes. (R) KEGG enrichment analysis of co-expressed genes.
We can understand the biological function of LOX genes in Tartary Buckwheat further based on their homology with LOX genes from another species [Citation55]. For example, FtLOX5 is highly expressed in the leaves and seeds of Tartary buckwheat, and its orthologous gene AtLOX4 plays an important role in the development of Arabidopsis seeds [Citation56]. The expression of AtLOX4 in leaves increases significantly during leaf senescence [Citation57]. Therefore, it can be inferred that FtLOX5 may have similar functions in buckwheat. FtLOX6 and FtLOX7 are segmental duplication, indicating that the functions of LOX have diverged. FtLOX7 was mainly expressed in seeds, whereas FtLOX6 was mainly expressed in roots and flowers. Their homologous AtLOX5 gene regulates the synthesis of JA in A. thaliana in response to pathogen attack and participates in lateral root development [Citation58]. Therefore, we speculate that FtLOX6 and FtLOX7 may have similar functions and participate in lateral root development. FtLOX4 is highly expressed in flowers, seeds and leaves. Interestingly, its orthologous gene AtLOX1 is also simultaneously expressed in the roots, leaves and flowers of A. thaliana [Citation58–60], indicating that they may have similar functions.
Expression patterns of FtLOX genes in response to light
RNA-seq data for seedlings exposed to different wavelengths of light were obtained [Citation61] (Supplemental Table S1). As shown in , the eight FtLOX genes were grouped into two clusters. The expression levels of FtLOX2, FtLOX3 and FtLOX8 have a low level of expression observed after far-red irradiation, and hardly any expression in others conditions. FtLOX1 was not expressed in the control group, but its expression was upregulated after 48 h of exposure to red and blue light. FtLOX7 was expressed in seedlings treated with different wavelengths of light, being significantly upregulated after treatment with red and blue light and slightly upregulated after treatment with far-red light. The expression levels of FtLOX4 and FtLOX6 were the highest in the control group and were downregulated under different light conditions. The FtLOX5 expression was the lowest in the dark and increased after light treatment. Red and blue light induced the expression of FtLOX1 and FtLOX7, whereas FtLOX4 and FtLOX6 were inhibited by light. The FtLOX5–7 expression levels were the highest under far-red light, whereas other genes were barely expressed. To confirm the gene expression results in response to light, the expression levels of the eight FtLOX genes were analyzed by qRT-PCR. Single-peak melting curves were obtained for all qRT-PCR amplifications (Supplemental Figure S3). Interestingly, FtLOX1 was strongly induced by red and blue light (p < 0.01). The FtLOX genes were expressed at an early stage of seed development and at a late stage of tissue development. The qRT-PCR results showed a correlation with the RNA-seq results ().
Previous studies on LOX have shown that its activity is affected by dark and light conditions [Citation16,Citation46,Citation62]. LOX gene expression can be affected by a variety of light sources. Some valuable clues were provided for the study of gene function in Tartary buckwheat under different wavelength light irradiation. AtLOX1 showed a higher expression under light than under dark conditions, with the mRNA levels four-fold greater in light-grown seedlings [Citation63]. In runner bean, chloroplast LOX can be induced by dark chilling and subsequent photoactivation [Citation46]. In this study, transcriptome data were used to analyze the expression of FtLOX genes under different light conditions. For example, FtLOX4 was highly expressed under red light and blue light, whereas its ortholog in Arabidopsis, AtLOX1, could interact with PPCK2, encoding calcium-dependent kinases involved in light-dependent phosphorylation [Citation64]. FtLOX4 in Tartary buckwheat may share similar functions. Interestingly, FtLOX6 was more highly expressed in the dark than in light, implying that it has a differentiation function. The genes co-expressed with LOX were enriched in light- and chlorophyll-related pathways, supporting the important role of LOX genes in the photo-responses of Tartary buckwheat. Overall, we deduced that FtLOXs play important roles in light responses of Tartary buckwheat.
Global identification of co-expressed genes
LOXs play important roles in response to stress. To determine FtLOX functions and their regulation, the eight FtLOX genes were used as guide genes, and a total of 273 genes were found to be co-expressed with the FtLOX genes (r > 0.9, p < 0.05; ). GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were applied to functionally annotate the co-expressed genes. The GO analysis revealed several key biological processes involved in photosynthesis. The most overrepresented GO term in the main molecular functions were ion binding and kinase activity (Supplemental Table S5). The GO and KEGG enrichment results showed that the co-expressed genes were involved in the photoreaction-related pathway, which was consistent with the large number of photo-responsive components in the FtLOX promoter regions (). These findings provide a basis for further exploring the potential function of LOX genes in Tartary buckwheat.
Conclusions
As far as we know, LOXs are found in a wide range of taxa. Considering the important roles of LOX genes at all stages of plant development, they have been a major focus of research. There has not been a comprehensive analysis of the LOX gene family in Tartary buckwheat. In our study, eight LOX genes were identified and divided into two main groups: 9-LOX and type II 13-LOX. All FtLOX proteins contain conserved LOX family specific motifs. The homology analysis of LOX genes and the phylogenetic comparison of different plant species provide valuable clues for the evolution of LOX in Tartary buckwheat. Moreover, based on findings of previous reports, we explored the role of LOX in the light responses of Tartary buckwheat. Promoters of FtLOX genes contained many light-responsive elements, and transcriptome data further suggested the roles of these genes in the response to light. qRT-PCR analysis of the differences in the expression of FtLOX genes in plants exposed to different wavelengths of light confirmed that FtLOX1 and FtLOX4–7 could respond to light. Moreover, genes co-expressed with FtLOX genes were involved in the photoreaction-related pathway. This comprehensive analysis of FtLOXs provides a basis for further functional and mechanical research on these proteins, in order to better understand the biological role of individual LOX genes in Tartary buckwheat.
Supplemental Material
Download PDF (1.4 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Liu S, Lu X, Zhou J, et al. Research advance on the structure, molecular modification, and fermentation of lipoxygenases. Biotechnology Bulletin 2015;31(12):31–41.
- Regdel D, Schewe T, Kühn H. Comparative characteristics of lipoxygenase isoenzymes from green pea seeds. Biochemistry 1995;60(6):715–721.
- Chen Z, Chen X, Yan H, et al. The lipoxygenase gene family in poplar: identification, classification, and expression in response to MeJA treatment. PLoS One. 2015;10(4):e0125526.
- Bailly C, Bogatek-Leszczynska R, Côme D, et al. Changes in activities of antioxidant enzymes and lipoxygenase during growth of sunflower seedlings from seeds of different vigour. Seed Sci Res. 2002;12(1):47–55.
- Zhang B, Yin XR, Li X, et al. Lipoxygenase gene expression in ripening kiwifruit in relation to ethylene and aroma production. J Agric Food Chem. 2009;57(7):2875–2881.
- Springer A, Kang C, Rustgi S, et al. Programmed chloroplast destruction during leaf senescence involves 13-lipoxygenase (13-LOX). Proc Natl Acad Sci USA. 2016;113(12):3383–3388.
- Ogunola OF, Hawkins LK, Mylroie E, et al. Characterization of the maize lipoxygenase gene family in relation to aflatoxin accumulation resistance. PLoS One. 2017;12(7):e0181265.
- Nuñez A, Savary BJ, Foglia TA, et al. Purification of lipoxygenase from Chlorella: production of 9- and 13-hydroperoxide derivatives of linoleic acid. Lipids 2002;37(11):1027–1032.
- Kacperska A, Kubacka-Zgbalska M. Is lipoxygenase involved in the formation of ethylene from ACC? Physiol Plant. 1985;64(3):333–338.
- Zhou Z, Chang X, You F, et al. Analysis of molecular evolution and codon bias of lipoxygenase(LOX) gene family in tea tree. J Agric Sci Technol 2017;19(12):43–51.
- Liu H, He R, Zhang H, et al. Analysis of synonymous codon usage Bias in Maize. Mol Biol Rep. 2010;37(2):677–684.
- Zhang L, Li X, Ma B, et al. The tartary buckwheat genome provides insights into rutin biosynthesis and abiotic stress tolerance. Mol Plant. 2017;10(9):1224–1237.
- Jiang P, Burczynski F, Campbell C, et al. Rutin and flavonoid contents in three buckwheat species Fagopyrum esculentum, F. tataricum, and F. homotropicum and their protective effects against lipid peroxidation. Food Res Int. 2007;40(3):356–364.
- Aghdam MS, Asghari M, Khorsandi O, et al. Alleviation of postharvest chilling injury of tomato fruit by salicylic acid treatment. J Food Sci Technol. 2014;51(10):2815–2820.
- Padilla MN, Hernandez ML, Sanz C, et al. Stress-dependent regulation of 13-lipoxygenases and 13-hydroperoxide lyase in olive fruit mesocarp. Phytochemistry 2014;102:80–88.
- Zhao Y, Zhou J, Xing D. Phytochrome B-mediated activation of lipoxygenase modulates an excess red light-induced defence response in Arabidopsis. J Exp Bot. 2014;65(17):4907–4918.
- Zhang R, Wang Z. Effects of light on lipoxygenase activity and membrane lipid peroxidation in young leaves of wheat. Acta Bot Boreal-Occid Sin. 1994;14(3):198–202.
- Anstis PJP, Friend J. The effect of light on lipoxygenase activity in dwarf pea seedlings. Phytochemistry 1974;13(12):2709–2712.
- Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic acids Res. 2011;39(suppl):W29–W37.
- Li M, Li L, Dunwell JM, et al. Characterization of the lipoxygenase (LOX) gene family in the Chinese white pear (Pyrus bretschneideri) and comparison with other members of the Rosaceae. BMC Genomics. 2014;15(1):1–12.
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007;23(21):2947–2948.
- Ding S, Xin F, Du H, et al. Genome-wide analysis of maize OSCA family members and their involvement in drought stress. PeerJ. 2019;7:e6765.
- Kumar S, Stecher G, Li M, et al. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549.
- Gu Z, Cavalcanti A, Chen FC, et al. Extent of gene duplication in the genomes of drosophila, nematode, and yeast. Mol Biol Evol. 2002;19(3):256–262.
- Lozano R, Hamblin M, Prochnik S, et al. Identification and distribution of the NBS-LRR gene family in the Cassava genome. BMC Genomics 2015;16(1):360.
- Wang Y, Tang H, Debarry JD, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49–e49.
- Krzywinski M, Schein J, Birol I, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645.
- Bailey TL, Johnson J, Grant CE, et al. The MEME suite. Nucleic Acids Res. 2015;43(W1):W39–W49.
- Chou KC, Shen HB. Plant-mPLoc: a top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS One. 2010;5(6):e11335.
- Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21.
- Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 2015;31(2):166–169.
- Zhang T, Song C, Li S, et al. RNA sequencing and coexpression analysis reveal key genes involved in α-linolenic acid biosynthesis in perillafrutescens seed. Int J Mol Sci. 2017;18(11):2433.
- Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287.
- Umate P. Genome-wide analysis of lipoxygenase gene family in Arabidopsis and rice. Plant Signal Behav. 2011;6(3):335–338.
- Liu SQ, Liu XH, Jiang LW. Genome-wide identification, phylogeny and expression analysis of the lipoxygenase gene family in cucumber. Genet Mol Res. 2011;10(4):2613.
- Hilbers M, Rossi A, Finazzi Agrò A, et al. The primary structure of a lipoxygenase from the shoots of etiolated lentil seedlings derived from its cDNA. Biochim Biophys Acta. 1994;1211(2):239–242.
- Stamm S, Benari S, Rafalska I, et al. Function of alternative splicing. Gene 2013;514(1):1–30.
- Li J, Song Y, Wang Z. Cloning and expression pattern of Salvia miltiorrhiza lipoxygenase gene (SmLOX). Shaanxi J Agric Sci. 2013;59(4):19–23.
- Huang J, Cai M, Long Q, et al. OsLOX2, a rice type I lipoxygenase, confers opposite effects on seed germination and longevity. Transgenic Res. 2014;23(4):643–655.
- Magadum S, Banerjee U, Murugan P, et al. Gene duplication as a major force in evolution. J Genet. 2013;92(1):155–161.
- Wright F. The effective number of codons used in a gene. Gene 1990;87(1):23–29.
- Zhang B, Chen K, Bowen J, et al. Differential expression within the LOX gene family in ripening kiwifruit. J Exp Bot. 2006;57(14):3825–3836.
- Andreou A, Feussner I, Hause B, et al. Lipoxygenases - structure and reaction mechanism. Phytochemistry 2009;69(13):1504–1510.
- Song Y, Love MH, Murphy P. Subcellular localization of lipoxygenase‐1 and‐2 in germinating soybean seeds and seedlings. J Am Oil Chem Soc. 1990;67(12):961–965.
- Farmaki T, Sanmartin M, Jimenez P, et al. Differential distribution of the lipoxygenase pathway enzymes within potato chloroplasts. J Exp Bot. 2007;58(3):555–568.
- Mazur R, Trzcinska-Danielewicz J, Kozlowski P, et al. Dark-chilling and subsequent photo-activation modulate expression and induce reversible association of chloroplast lipoxygenase with thylakoid membrane in runner bean (Phaseolus coccineus L.). Plant Physiol Biochem. 2018;122:102.
- Bell E, Creelman RA, Mullet JE. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc Natl Acad Sci USA. 1995;92(19):8675–8679.
- Zheng SJ, van Dijk JP, Bruinsma M, et al. Sensitivity and speed of induced defense of cabbage (Brassica oleracea L.): dynamics of BoLOX expression patterns during insect and pathogen attack. Mol Plant Microbe Interact. 2007;20(11):1332–1345.
- Rangel M, Machado OL, Da CM, et al. Accumulation of chloroplast-targeted lipoxygenase in passion fruit leaves in response to methyl jasmonate. Phytochemistry. 2002;60(6):619–625.
- Wang YX, Lin ZF, Guo JY, et al. The lipoxygenase activity of pea chloroplasts in relation to leaf senescence and membrane lipid peroxidation. Acta Phytophysiol Sin 1990;16(1):57–62.
- Mur LAJ, Kenton P, Atzorn R, et al. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 2006;140(1):249–262.
- Bell E, Mullet JE. Characterization of an Arabidopsis lipoxygenase gene responsive to methyl jasmonate and wounding. Plant Physiol. 1993;103(4):1133–1137.
- Bhardwaj PK, Kaur J, Sobti RC, et al. Lipoxygenase in Caragana jubata responds to low temperature, abscisic acid, methyl jasmonate and salicylic acid. Gene 2011;483(1-2):49–53.
- Losvik A, Beste L, Glinwood R, et al. Overexpression and down-regulation of barley lipoxygenase LOX2.2 affects jasmonate-regulated genes and aphid fecundity. Int J Mol Sci. 2017;18(12):2765.
- Dossa K, Wei X, Li D, et al. Insight into the AP2/ERF transcription factor superfamily in sesame and expression profiling of DREB subfamily under drought stress. Bmc Plant Biol. 2016;16(1):171.
- Caldelari D, Wang G, Farmer EE, et al. Arabidopsis lox3 lox4 double mutants are male sterile and defective in global proliferative arrest. Plant Mol Biol. 2011;75(1-2):25–33.
- He Y, Fukushige H, Hildebrand DF, et al. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002;128(3):876–884.
- Vellosillo T, Martínez M, López MA, et al. Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell. 2007;19(3):831–846.
- Kaye Peterman T, Rattigan E, Enriquez A, et al. Immunological characterization of Arabidopsis thaliana lipoxygenase: expression of the LOX1 gene product in Escherichia coli and polyclonal antibody production. Plant Physiol Biochem. 1994;32(3):443–450.
- Melan MA, Dong X, Endara ME, et al. An Arabidopsis thaliana lipoxygenase gene can be induced by pathogens, abscisic acid, and methyl jasmonate. Plant Physiol. 1993;101(2):441–450.
- Zhang D, Jiang C, Huang C, et al. The light‐induced transcription factor FtMYB116 promotes accumulation of rutin in Fagopyrum tataricum. Plant Cell Environ. 2019;42(4):1340–1351.
- Anstis PJP, Friend J, Gardner DCJ. The role of xanthoxin in the inhibition of pea seedling growth by red light. Phytochemistry 1975;14(1):31–35.
- Melan MA, Enriquez A, Peterman TK. The LOX1 gene of arabidopsis is temporally and spatially regulated in germinating seedlings. Plant Physiol. 1994;105(1):385–393.
- Fontaine V, Hartwell J, Jenkins G, et al. Arabidopsis thaliana contains two phosphoenolpyruvate carboxylase kinase genes with different expression patterns. Plant Cell Environ. 2002;25(1):115–122.