Abstract
Plant invertases comprise a family of enzymes that catalyze the irreversible hydrolysis of sucrose into glucose and fructose, which plays a crucial role in carbohydrate partitioning and regulating plant growth and development. However, currently, there is little information on the roles of invertases in the development of pear trees. In this study, we performed genome-wide identification of a total of 23 invertases from the predicted genome data of the pear plant (Pyrus bretschneideri R.). Based on phylogenetic analysis, the pear invertases could be clustered into 13 acid invertase genes and 10 alkaline/neutral invertase genes. Chromosome location showed that tandem duplication and segmental duplication have played an important role in the expansion of acid and neutral/alkaline invertases in pear plants. Evolutionary analysis of invertases from pear and two model plant species (Arabidopsis thaliana and Oryza sativa) indicated that the evolutionary divergence of invertases occurred before the common ancestor of dicots and monocots. Tissue-specific expression analysis showed that the transcripts of all the identified invertase genes were detectable in various tissues. We hypothesize that the identified invertase genes all participate in regulating pear growth and development.
Introduction
Sucrose is the major form of photosynthate for export from the source into cellular metabolism [Citation1]. In plant species, sucrose is hydrolyzed into UDP-glucose and fructose by sucrose synthase or irreversibly cleaved into glucose and fructose by invertase. It has been verified that sucrose and its components, glucose and fructose, play important roles in cellular metabolism and plant growth and development [Citation2]. The invertase family is divided into three types in most plant species: cell wall invertase (CWIN), vacuolar invertase (VIN) and alkaline/neutral invertase [Citation3]. The cell wall invertase and vacuolar invertase are referred as acid invertases due to acidic pH optima. Alkaline/neutral invertase has neutral or slightly alkaline pH optimum and is located in the cytosol, plastids and mitochondria [Citation4–6].
Acid invertase can be used to decompose sucrose and β-fructose oligosaccharides, therefore, it is also called β-fructofuranosidase. The members of the acid invertase subfamily share enzymatic and biochemical properties. The acid invertase is involved in sucrose partitioning, cell elongation and enlargement, sugar signal transduction and environmental stimuli response [Citation7,Citation8]. For example, the expression of acid invertase is regulated by sugar, pathogenic bacteria and freezing injury [Citation9–12]. In addition, acid invertase is also regulated by auxin, gibberellin, cytokinin and abscisic acid [Citation1]. They play vital roles in crop yield and quality [Citation13–15]. However, much less is known about alkaline/neutral invertases due to unstable enzymatic activity [Citation1,Citation16]. The optimum pH of neutral/alkaline invertase is 7.0; it is only found in plants and photosynthetic bacteria [Citation13], and its expression is highest in the rapid growth tissues, indicating that the neutral/alkaline invertase is associated with plant growth [Citation16–18].
Over the past few years, the acid invertase and alkaline/neutral invertase family genes have been described in several plant species, such as Arabidopsis thaliana and Oryza sativa [Citation4], Lotus japonicus[Citation16], Vitis vinifera[Citation19], Populus trichocarpa [Citation20], maize (Zea mays) [Citation21], sugarcane (Saccharum sinensis) [Citation22], pepper (Capsicum annuum) [Citation23], Brassica rapa[Citation24] and Glycine max[Citation25]. These works have opened the door for the systematic analysis of the acid invertase and alkaline/neutral invertase family genes. Pear (Pyrus bretschneideri R.), an important fruit crop, grows in the regions of China and its fruit has a rich storage of sugar. Therefore, it is important to study sucrose metabolism processes in pear source and sink organs.
The availability of whole-genome sequences provided an opportunity to identify gene families using bioinformatics methods [Citation26–29], some which have been used to elaborate their function in plant growth and development [Citation30–36] and under different abiotic stress (salt stress [Citation37–42], drought stress [Citation43–47], heat stress [Citation48–51]) and hormone treatment conditions [Citation52–55]. In the present study, we explored the acid and alkaline/neutral invertase gene family, and analyzed, motif distribution, chromosomal localization, phylogenetic relationships and expression levels by using bioinformatics and qRT-PCR (Quantitative Real Time Polymerase Chain Reaction). These results will help us understand the possible roles of acid- and alkaline/neutral invertases in sucrose metabolism in pear plants.
Materials and methods
Database searches and analyses
To identify invertase gene family genes (acid- and neutral/alkaline invertase genes) in pear, the Hidden Markov Model (HMM) profiles of the two types of invertase genes (Glyco_hydro_32N-PF00251, Glyco_hydro_32C-PF08244 and Glyco_hydro_100-PF12899) were downloaded from PFam (http://pfam.sanger.ac.uk/). Glyco_hydro_32N-PF00251, Glyco_hydro_32C-PF08244 correspond to N- and C-terminal domains of acid invertase genes; Glyco_hydro_100-PF12899 is the conserved domain of cytoplasmic invertase (CIN). The predicted sequences were downloaded from the Pear Genome Project database (http://peargenome.njau.edu.cn/) and a local database was constructed. The BlastP search was performed using the HMM profile in the local database, followed by removal of redundant sequences. Sequences with an E-value over 10−10 and query over 50% were chosen as the candidates. All sequences were further confirmed against the Pfam database (www.sanger.ac.uk/Software/Pfam/search.shtml). In addition, the published members of the invertase gene family of Arabidopsis thaliana and Oryza sativa [Citation19,Citation20] were selected for constructing a phylogenetic tree.
Chromosome location and gene duplication
The chromosome locations of invertase genes in the pear genome were determined according to their positions given in the Pear Genome Project (http://peargenome.njau.edu.cn/). The tandem gene duplications were identified based on the investigations described in rice [Citation56], which were defined as an array of two or more invertase genes with alignment e values ≤ 1 × 10−25 in the range of 100-kb distance. The segmental gene duplications were identified according to the method reported previously by Schauser et al. [Citation57].
Phylogenetic relationship and conservative motif
Multiple amino acid sequences of invertase genes were performed using the Clustal X software [Citation58]. The phylogenetic tree of invertase genes from Arabidopsis, rice and pear was constructed by MEGA5.0 software with the neighbor joining method [Citation59]. Bootstrap value was set to 1000. Conserved motifs were analyzed by using MEME (http://meme.nbcr.net/meme/cgi-bin/meme.cgi) software.
RNA extraction and cDNA synthesis
The pear trees were planted in an experimental orchard at Hubei Academy of Agricultural Sciences. We collected seven pear (Pyrus bretschneideri Rehd. var. Dangshansu) tissues including shoot, young leaf, mature leaf, old leaf and at three fruit developmental stages (small fruit stage, 52 days after flowering (DAF); enlarged fruit stage, 94 DAF; mature fruit stage, 138 DAF) for qRT-PCR analysis. Total RNA from the samples of pear shoot, leaf and fruit was extracted using the Plant Total RNA Isolation Kit Plus (FOREGENE Co. Ltd.). Then, total RNA was adjusted to the same concentration, and based on the adjusted RNA, first-strand cDNA was synthesized using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech Co. Ltd.).
Gene expression analysis based on qRT-PCR
In this study, a total of sixteen pairs of primers () were designed to amplify the seven candidate gene sequences using Primer 5.0 software. The expression levels of the candidate genes in different tissues and different stages of fruit development were detected by using fluorescence quantitative qRT-PCR. The LightCycler® 480 Instrument (Roche) was used to perform the qRT-PCR analysis. A 20 μL reaction mix system was constructed, each containing 100 ng of template cDNA, 0.5 μmol/L of each primer and 10 μL of LightCycler 480 SYBR GREEN I Master. All reactions were carried out in 96-well plates with four replicates for each cDNA sample. We set the qRT-PCR conditions as follows: first cycle for 5 min at 95 °C for pre-incubation, 55 cycles at 95 °C for 3 s, 60 °C for 10 s, and 72 °C for 30 s, and then 3 min at 72 °C for extension. Finally, the step of fluorescence signal data collection was carried out at 60 °C. Pyrus EF1α (accession No. AY338250) was used as the internal control gene. The average threshold cycle (Ct) of each cDNA sample was calculated using the running results displayed on the computer. Meanwhile, the relative expression levels of invertase genes were calculated using the 2-ΔΔCt method.
Table 1. Primers used to detect expression levels of pear invertase genes.
Results and discussion
Sequence and database search for invertase genes in pear
The availability of the complete pear genome sequences facilitated the search for invertase genes. We used bioinformatics tools to identify the potential invertases (acid invertases and neutral/alkaline invertases). The two types of invertases were searched independently since they are enzymes with unrelated structures [Citation3]. Firstly, A BLASTP search was performed using A. thaliana and O. sativa invertases as the query. Secondly, the search of the candidate invertases in pear was performed using the HMM profiles (Glyco_hydro_32N domain,-PF00251 and Glyco_hydro_32C domain-PF08244 and Glyco_hydro_100 domain-PF12899) as a query to find possible homologues. Finally, based on the above results, candidate invertases were further searched repeatedly using the BLASTP tool. The results yielded 28 predicted members of the invertase gene family (Supplemental Table S1).
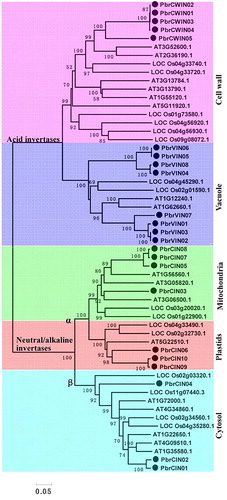
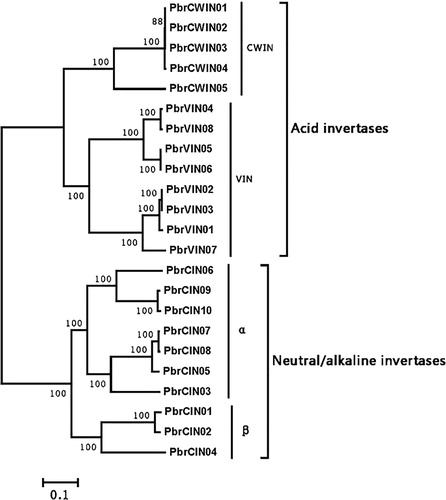
The pear invertase genes were composed of acid and neutral/alkaline invertase families based on the established criteria [Citation4] and their phylogenetic relationship (). The former contained 13 genes, five of which are predicted to target to the cell wall and eight, to the vacuoles, according to prediction analyses using PSORT (http://psort.hgc.jp/). The latter contained 15 members, which were targeted to the cytoplasm. For neutral/alkaline invertases, each of five sequences (Pbr000839.2, Pbr005836.1, Pbr028541.1, Pbr004247.1, and Pbr005837.1) missed significant portion(s) of the coding region, hence most likely encoded truncated protein (Supplemental Table S1). They were excluded from the subsequent analysis. The neutral/alkaline invertases were divided into α- and β-subgroups ().
Figure 1. Phylogenetic tree of invertase genes in pear.
Note: All genes grouped into two different groups: acid invertases and neutral/alkaline invertases. The former were separated into CWIN and VIN clades, whereas the latter was grouped into α and β clades.
shows the characteristics of the remaining 23 putative CWINs, VINs and CINs, including sizes of their open reading frames (ORF), number of exons, protein molecular weight and pI values. The length of these invertase isoenzyme genes ranged from 1671 bp (Pbr004245.1) to 2175 bp (Pbr030762.1), and the predicted molecular masses ranged from 63.41 kDa (Pbr004245.1) to 81.35 kDa (Pbr030762.1). The isoelectric point (pI) values of the pear invertases ranged from 4.58 (Pbr031750.1) to 8.98 (Pbr015353.1), which were consistent with those in other plant species [Citation1].
Table 2. The invertase genes in pear.
Multiple sequence alignment of invertase genes
To gain insights into the sequence structure of CWINs, VINs and CINs in pear, multiple sequence alignment of invertases was performed using Cluster X (Supplemental Figure S1). For CWINs and VINs, four residues (DDEC), which were proposed to be essential for the recognition and stable binding of sucrose [Citation4], were highly conserved in pear (Supplemental Figure S1). On the contrary, two key motifs (NDPN and WECP/VDF), which are essential for the catalytic activity of acid invertases [Citation60,Citation61], showed poor conservation in pear (Supplemental Figure S1).
Previously, defective and/or nonfunctional invertases were reported in plant species [Citation3]. In pear, we found that most of the acid invertase sequences lacked the NDPN motif (Supplemental Figure S1(a)), rendering them incapable of hydrolyzing sucrose; hence, they were deemed nonfunctional [Citation62,Citation63]. In the present study, these DPN were not found in the PbrVIN01, PbrVIN02, PbrVIN03 and PbrCWIN05 in pear acid invertase genes, suggesting that the nonfunctional invertases also existed. Moreover, an amino acid irregular substitution for Asp 239 was not found in the examined invertases (Supplemental Figure S1(a)), a characteristic of defective cell wall invertases (deCWINs) that are unable to hydrolyze Sucrose [Citation63].
For CINs, a recent report identified two amino acids (D188 and E414) to be catalytic residues for sucrose hydrolysis based on the crystal structures of a CIN isolated from the cyanobacterium Anabaena sp. [Citation64]. These two residues occurred in all the CIN members examined in pear (Supplemental Figure 1(b)), which suggests that the two catalytic residues of CINs are highly conserved during the evolution process from the cyanobacterium Anabaena to higher plants. In addition, an amino acid residue Ser 547 of CIN, which was recently identified as the target of calcium-dependent protein kinases for phosphorylation and subsequent interaction with 14-3-3 for activating CIN activity [Citation65], also was highly conserved in cytosol invertases (PbrCIN01, PbrCIN02, PbrCIN04) in pear (Supplemental Figure S1(b)).
Chromosomal location and gene duplication of invertase genes in pear
Out of the 23 predicted invertase genes, 17 are randomly distributed across 9 of the 17 pear chromosomes (). Among them, three members of invertases were found located at each of three chromosomes (Chr8, Chr14 and Chr15). Each of four chromosomes (Chr3, Chr4, Chr7 and Chr13) contained two invertase genes. The remaining invertases were mapped on two chromosomes (Chr1 and Chr12), each of these chromosomes contained three members.
Figure 2. Chromosomal map and duplication events of paralogous invertase gene candidates in pear.
Note: The identity of each linkage group is indicated at the top of each bar. Only the chromosomes where invertase genes were mapped are shown. Possible segment and tandem duplicated genes are connected by color lines and boxes, respectively.
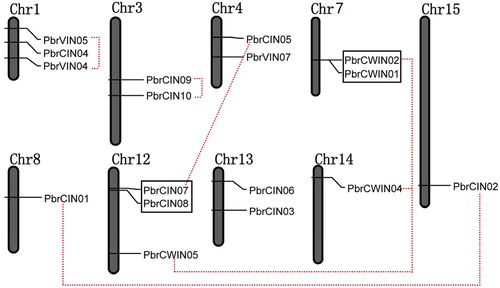
We further performed chromosome mapping to determine the gene duplication of invertase genes on the pear chromosomes. As shown in , two groups of invertase genes (PbrCWIN1/PbrCWIN2 and PbrCIN7/PbrCIN8) can be identified as tandem duplication genes. One group (PbrCWIN1/PbrCWIN2) was from the cell wall invertase subfamily and was located on chromosome 7. The other (PbrCIN7/PbrCIN8) was from the neutral/alkaline invertase subfamily and was mapped on chromosome 12, indicating that most invertase genes on these two chromosomes were mainly due to tandem duplication events.
On the other hand, four segmental duplication groups were found to scatter in six chromosomes (). A duplicated segment of the PbrCWIN4 region on chromosome 14 was present on the same location of chromosome 12, where PbrCWIN5 was located. PbrCIN1 on chromosome 15 also showed synteny to PbrCIN2 localized on a duplicated segment of chromosome 8. In addition, four invertase gene (PbrVIN4/PbrVIN5, PbrCIN9/PbrCIN10) regions showed segmental duplication and were present on chromosome 1 and 3, respectively. All these results indicated that tandem duplication and segmental duplication have played an important role in the expansion of acid and neutral/alkaline invertases in pear.
Motif distribution in pear invertases
In order to reveal the conservation of invertase genes in pear, we analyzed conserved motifs by using MEME online tools. The results showed ten different conserved motifs (Motif 1 to Motif 10) among these invertase genes. Moreover, the distributions of these conserved motifs were highly consistent in each class of invertase. showed that acid invertase genes contained six different motifs (Motif1, Motif3, Motif4, Motif5, Motif6 and Motif8), whereas neutral/alkaline invertase genes were composed of five different motifs (Motif2, Motif7, Motif8, Motif9 and Motif10). Among these motifs, Motif8 not only existed in acid invertase genes, but also existed in neutral/alkaline invertase genes. The best matching sequences of each conserved motif are shown in .
Figure 3. Motifs identified by MEME tools in pear invertases.
Note: Ten motifs (1 to 10) were identified and indicated by different colors.
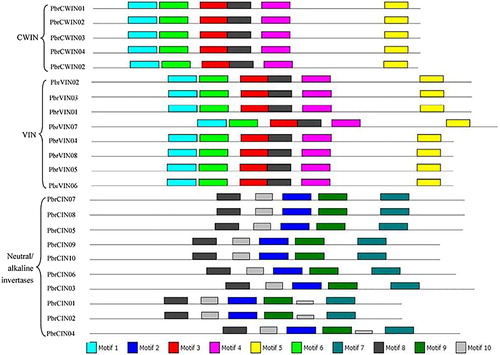
Table 3. Analysis of conserved motifs of invertase genes in pear.
Intron–extron and evolutionary analysis of invertase genes in pear
The predicted number of introns/exons were determined by alignment of genomic DNA and full-length cDNA sequences of invertase genes (). Among the acid invertases, the numbers of exons ranged from 5 to 8. Seven members (PbrCWIN01, PbrCWIN02, PbrCWIN03, PbrCWIN04, PbrVIN01, PbrVIN02 and PbrVIN03) contain five predicted exons. Four members (PbrVIN04, PbrVIN05, PbrVIN06 and PbrVIN08) have seven exons. Each of the remaining two members (PbrCWIN05 and PbrVIN07) contains six and eight exons, respectively. For neutral/alkaline invertases, each of three members (PbrCIN01, PbrCIN02 and PbrCIN04) contain four exons, whereas each of the remaining seven members (PbrCIN03, PbrCIN05, PbrCIN06, PbrCIN07, PbrCIN08, PbrCIN09 and PbrCIN10) contains six exons. These exon–intron structure results are consistent with the reported invertases in other plant species, including rice, populus, tomato and grape [Citation19,Citation20,Citation66] and also show that a complex structure has evolved in the invertase genes of pear.
Further, we attempted to elaborate the evolutionary relationship among invertase gene families in pear and other plant species. A total of 56 plant amino acid sequences of invertases, including the 23 isoforms of pear invertases together with those representing two model plant species (Arabidopsis and rice), were used to construct the phylogenetic tree (). These invertase amino acid sequences were aligned with the ClustalX program and were used to construct an unrooted tree for phylogenetic analysis using the neighbor-joining method. Bootstrap analysis (1000 replicates) was used to determine the robustness of the phylogram’s topology. The phylogenetic tree analysis revealed both relatively deep evolutionary root and the existence of more recent duplications for the invertase genes. The results showed that all these invertase genes from three different plant species were obviously divided into two classes (acid invertase and neutral/alkaline invertase), as they are supported by high bootstrap values >95%. Genes from dicot- and monocotyledonous plants were found in all the three groups, suggesting possible evolutionary divergence before the common ancestor of dicots and monocots. Acid invertases were further separated into two subclasses, which belonged to cell-wall and vacuole invertases. Similarly, neutral/alkaline invertases were divided into three subclasses, which belonged to cytosol-, plasid- and mitochondrial invertases. They could have possibly originated from a common accentor.
Gene expression analysis of invertase genes in pear
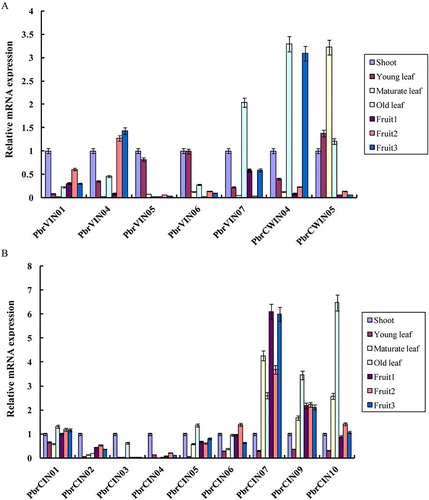
Analysis of gene expression patterns is important for understanding gene functions in plant species [Citation67–72]. In this study, to reveal the gene expression patterns of pear invertase genes in different tissues and different developmental stages of fruit, including shoot, young leaf, mature leaf, old leaf, fruit at small fruit stage, 52 DAF; enlarged fruit stage, 94 DAF; mature fruit stage, 138 DAF (fruit1, fruit2 and fruit3), qRT-PCR was carried out. Among the 23 invertases, 16 genes were used to detect the expression levels in different tissues and different developmental stages of fruit, except for seven genes (PbrCWIN01, PbrCWIN02, PbrCWIN03, PbrVIN02, PbrVIN03, PbrVIN08 and PbrCIN08), which the software failed to design primers . For acid invertases, it was found that most of the genes were differentially expressed during the development of leaves and fruits in pear. The four invertase genes (PbrVIN01, PbrVIN04, PbrVIN05 and PbrVIN06) showed low expression levels in the tested tissues (). The PbrCWIN5 gene was highly expressed in mature leaves. PbrVIN07 was expressed only in the old leaves. PbrCWIN04 was highly expressed in old leaves and mature fruit (Fruit3). For neutral/alkaline invertases, three out of nine members (PbrCIN07, PbrCIN09 and PbrCIN10) were highly expressed, whereas the remaining invertases were expressed at low levels in the tested tissues (). The expression patterns of acid invertases and neutral/alkaline invertases in various tissues reveal that these genes possibly play an important role in their respective organs and provide carbohydrates for their growth and development.
Figure 5. Relative expression levels of invertase genes in different tissues and the stage of fruit development. Expression patterns of acid invertases (a) and neutral/alkaline invertases (b).
Note: qRT-PCR analysis was performed to measure the expression levels. The x-axis represents the different tissues and the stage of fruit development and the y-axis represents the relative expression levels of the genes, shown as mean values ± SD from three replications. Pyrus EF1a (accession No. AY338250) was used as internal reference.
Conclusions
In the present study, 23 invertase members, including five truncated proteins, were identified in the pear genome. These invertase members could be classified into two groups: acid and neutral/alkaline invertases. The former was also further subdivided into cell-wall invertases and vacuole invertases based on subcellular localization, whereas the latter was separated into two clades, the α- and β-subgroups. Chromosome location found that tandem duplication and segmental duplication events occurred during evolution of invertases, suggesting that tandem duplication and segmental duplication have possibly played an important role in the expansion of acid and neutral/alkaline invertase families in pear. Moreover, qRT-PCR analysis demonstrated that all invertases were expressed in different tissues and stages of fruit development in pear. All of these data will be helpful to understand the roles of invertases in the regulation of plant development and sucrose metabolism and evolution of the invertase gene family in pear.
Author contributions
T.W., Z.L., L.Y. performed and analyzed most of the experiments in this study, with assistance from Y.C., J.T., F.Y., H.Z., X.L. Y.D., X.N. Z.Q., T.W., Z.L., L.Y. provided all financial support and critical intellectual input in this study and preparation of the manuscript. Z.Q., T.W., Z.L. designed this study and wrote the manuscript.
Supplemental Material
Download PDF (420.7 KB)Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Roitsch T, González MC. Function and regulation of plant invertases: sweet sensations. Trends Plant Sci. 2004; 9(12):606–613.
- Sturm A, Tang GQ. The sucrose-cleaving enzymes of plants are crucial for development growth and carbon partitioning. Trends Plant Sci. 1999; 4(10):401–407.
- Wan H, Wu L, Yang Y, et al. Evolution of sucrose metabolism: the dichotomy of invertases and beyond. Trends Plant Sci. 2018; 23(2):163–177.
- Ji X, Van den Ende W, Van Laere A, et al. Structure evolution and expression of the two invertase gene families of rice. J Mol Evol. 2005; 60(5):615–634.
- Murayama S, Handa H. Genes for alkaline/neutral invertase in rice: alkaline/neutral invertases are located in plant mitochondria and also in plastids. Planta. 2007; 225(5):1193–1203.
- Martín ML, Lechner L, Zabaleta EJ, et al. A mitochondrial alkaline/neutral invertase isoform (A/N-InvC) functions in developmental energy-demanding processes in Arabidopsis. Planta. 2013; 237(3):813–822.
- Chourey P, Li Q, Cevallos-Cevallos J. Pleiotropy and its dissection through a metabolic gene Miniature1 (Mn1) that encodes a cell wall invertase in developing seeds of maize. Plant Sci. 2012; 184:45–53.
- Ruan Y, Patrick J, Bouzayen M, et al. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012; 17(11):656–665.
- Wiberley-Bradford A, Busse J, Bethke P. Temperature-dependent regulation of sugar metabolism in wild-type and low-invertase transgenic chipping potatoes during and after cooling for low-temperature storage. Postharvest Biol Technol. 2016; 115:60–71.
- Chen Z, Gao K, Su X, et al. Genome-wide identification of the invertase gene family in Populus. PLoS ONE. 2015; 10(9):e0138540.
- Balk P, Boer D. Rapid stalk elongation in tulip (Tulipa gesneriana L cv Apeldoorn) and the combined action of induced invertase and the water-channel protein γTIP. Planta. 1999;209(3):346–354.
- Benhamou N, Grenier J, Chrispeels MJ. Accumulation of β-fructosidase in the cell walls of tomato roots following infection with a fungal wild pathogen. Plant Physiol. 1991;97(2):739–750.
- Morey SR, Hirose T, Hashida Y, et al. Genetic evidence for the role of a rice vacuolar invertase as a molecular sink strength determinant. Rice (N Y). 2018; 11(1):6.
- Malek J, Mathew S, Mathew L, et al. Deletion of beta-fructofuranosidase (invertase) genes is associated with sucrose content in Date Palm fruit. BioRxiv. 2019. doi:10.1101/652289
- Yan W, Wu X, Li Y, et al. Cell wall invertase 3 affects cassava productivity via regulating sugar allocation from source to sink. Front Plant Sci. 2019;10:541
- Tracey W, Jodie P, Irmtraud H, et al. A cytosolic invertase is required for normal growth and cell development in the model legume Lotus japonicas. J Exp Bot. 2009; 60(12):3353–3365.
- Yao S, Kodama R, Wang H, et al. Analysis of the rice SHORT‐ROOT5 gene revealed functional diversification of plant neutral/alkaline invertase family. Plant Sci. 2009;176(5):627–634.
- Jha A, Dubey R. Carbohydrate metabolism in growing rice seedlings under arsenic toxicity. J Plant Physiol. 2005;161(7):867–872.
- Nonis A, Ruperti B, Pierasco A, et al. Neutral invertases in grapevine and comparative analysis with Arabidopsis poplar and rice. Planta. 2008; 229(1):129–142.
- Bocock P, Morse A, Dervinis C, et al. Evolution and diversity of invertase genes in Populus trichocarpa. Planta. 2008; 227(3):565–576.
- Juárez-Colunga S, López-González C, Morales-Elías N, et al. Genome-wide analysis of the invertase gene family from maize. Plant Mol Biol. 2018; 97(4-5):385–406.
- Wang L, Zheng Y, Ding S, et al. Molecular cloning structure phylogeny and expression analysis of the invertase gene family in sugarcane. BMC Plant Biol. 2017;17(1):109.
- Shen L, Qin Y, Qi Z, et al. Genome-wide analysis expression profile and characterization of the acid invertase gene family in pepper. IJMS. 2019;20(1):15.
- Eom S, Rim Y, Hyun T. Genome-wide identification and evolutionary analysis of neutral/alkaline invertases in Brassica rapa. Biotechnol Biotechnol Equip. 2019; 33(1):1158–1163.
- Tao S, Han M, Min J, et al. Genome-wide survey of invertase encoding genes and functional characterization of an extracellular fungal pathogen-responsive invertase in Glycine max. IJMS. 2018;19(8):2395.
- Lu T, Zhang G, Sun L, et al. Genome-wide identification of CBL family and expression analysis of CBLs in response to potassium deficiency in cotton. Peer J. 2017; 5:e3653.
- Chen M, Li K, Li H, et al. The glutathione peroxidase gene family in Gossypium hirsutum: genome-wide identification classification gene expression and functional analysis. Sci Rep. 2017;7(1):44743.
- Sun Q, Wang G, Zhang X, et al. Genome-wide identification of the TIFY gene family in three cultivated Gossypium species and the expression of JAZ genes. Sci Rep. 2017;7(1):42418.
- Zhang G, Lu T, Miao W, et al. Genome-wide identification of ABA receptor PYL family and expression analysis of PYLs in response to ABA and osmotic stress in Gossypium. Peer J. 2017; 5:e4126.
- Wang PC, Du Y, Li Y, et al. Hydrogen peroxide–mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell. 2010; 22(9):2981–2998.
- Liang JY, Xia JY, Liu LL, et al. Global patterns of the responses of leaf-level photosynthesis and respiration in terrestrial plants to experimental warming. J Plant Ecol. 2013; 6(6):437–447.
- Guo S, Dai S, Singh PK, et al. A membrane-bound NAC-like transcription factor OsNTL5 represses the flowering in Oryza sativa. Front Plant Sci. 2018; 9:555.
- Zhao X, Wang YL, Qiao XR, et al. Phototropins function in high-intensity blue light-induced hypocotyl phototropism in Arabidopsis by altering cytosolic calcium. Plant Physiol. 2013; 162(3):1539–1551.
- Lü D, Wang W, Miao C. ATHK1 acts downstream of hydrogen peroxide to mediate ABA signaling through regulation of calcium channel activity in Arabidopsis guard cells. Chin Sci Bull. 2013; 58(3):336–343.
- Ma XN, Zhang XR, Yang L, et al. Hydrogen peroxide plays an important role in PERK4-mediated abscisic acid-regulated root growth in Arabidopsis. Functional Plant Biol. 2019; 46(2):165–174.
- Büttner M, Truernit E, Baier K, et al. AtSTP3 a green leaf-specific low affinity monosaccharide-H+ symporter of Arabidopsis thaliana. Plant Cell Environ. 2000;23(2):175–184.
- Xu LH, Wang WY, Guo JJ, et al. Zinc improves salt tolerance by increasing reactive oxygen species scavenging and reducing Na+ accumulation in wheat seedlings. Biol Plant. 2014; 58(4):751–757.
- Gao W, Xu FC, Guo DD, et al. Calcium-dependent protein kinases in cotton: insights into early plant responses to salt stress. BMC Plant Biol. 2018; 18(1):15.
- Li W, Zhao F, Fang W, et al. Identification of early salt stress responsive proteins in seedling roots of upland cotton (Gossypium hirsutum L) employing iTRAQ-based proteomic technique. Front Plant Sci. 2015; 11(6):732.
- Zhao X, Wang YJ, Wang YL, et al. Extracellular Ca2+ alleviates NaCl-induced stomatal opening through a pathway involving H2O2-blocked Na + influx in Vicia guard cells. J Plant Physiol. 2011; 168(9):903–910.
- Ma L, Zhang H, Sun L, et al. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J Exp Bot. 2012; 63(1):305–317.
- Zhang J, Wang F, Zhang C, et al. A novel VIGS method by agroinoculation of cotton seeds and application for elucidating functions of GhBI-1 in salt-stress response. Plant Cell Rep. 2018; 37(8):1091–1100.
- Qi J, Song CP, Wang B, et al. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol. 2018; 60(9):805–826.
- Wang TP, Liu H, Hua HJ, et al. A vacuole localized beta-glucosidase contributes to drought tolerance in Arabidopsis. Chin Sci Bull. 2011;56(33):3538–3546.
- Gao ZY, Liu H, Wang HL, et al. Generation of the genetic mutant population for the screening and characterization of the mutants in response to drought in maize. Chin Sci Bull. 2014;59(8):766–775.
- Wang DJ, Yang CL, Dong L, et al. Comparative transcriptome analyses of drought-resistant and -susceptible Brassica napus L. and development of EST-SSR markers by RNA-Seq. J Plant Biol. 2015;58(4):259–269.
- Wang P, Yang CL, Chen H, et al. Transcriptomic basis for drought-resistance in Brassica napus L. Sci Rep. 2017;7(1):40532.
- Zhao Q, Chen W, Bian J, et al. Proteomics and phosphoproteomics of heat stress-responsive mechanisms in spinach. Front Plant Sci. 2018; 9:800.
- Zhou HR, Xu M, Hou RX, et al. Thermal acclimation of photosynthesis to experimental warming is season dependent for winter wheat (Triticum aestivum L.). Environ Exper Bot. 2018; 150:249–259.
- Zhang H, Yue MX, Zheng XK, et al. The role of promoter-associated histone acetylation of haem oxygenase-1 (HO-1) and giberellic acid-stimulated like-1 (GSL-1) genes in heat-induced lateral root primordium inhibition in maize. Front Plant Sci. 2018; 9:1520
- Lin DL, Xia JY, Wan SQ. Climate warming and biomass accumulation of terrestrial plants: a meta-analysis. New Phytol. 2010;188(1):187–198.
- Li K, Yang F, Miao Y, et al. Abscisic acid signaling is involved in regulating the mitogen-activated protein kinase cascade module AIK1-MKK5-MPK6. BMC Plant Biol. 2017; 12:5.
- Hao F, Zhao S, Dong H, et al. Nia1 and Nia2 are involved in exogenous salicylic acid-induced nitric oxide generation and stomatal closure in Arabidopsis. J Integr Plant Biol. 2010; 52(3):298–307.
- Song Y, Xiang F, Zhang G, et al. Abscisic acid as an internal integrator of multiple physiological processes modulates leaf senescence onset in Arabidopsis thaliana. Front Plant Sci. 2016;7:181.
- Liu LY, Li N, Yao CP, et al. Functional analysis of the ABA-responsive protein family in ABA and stress signal transduction in Arabidopsis. Chin Sci Bull. 2013;58(31):3721–3730.
- Zhou T, Wang Y, Chen JQ, et al. Genome-wide identification of NBS genes in rice reveals significant expansion of divergent non-TIR NBS genes. Mol Genet Genomics. 2004; 271:402–415.
- Schauser L, Wieloch W, Stougaard J. Evolution of NIN-like proteins in Arabidopsis, rice and Lotus japonicas. J Mol Evol. 2005; 60(2):229–237.
- Chenna R, Sugawara H, Koike T, et al. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003; 31(13):3497–3500.
- Koichiro T, Daniel P, Nicholas P, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood evolutionary distance and maximum parsimony methods. Mol Biol Evol. 2011; 28(10):2731–2739.
- Alberto F, Bignon C, Sulzenbacher G, et al. The three-dimensional structure of invertase (beta-fructosidase) from Thermotoga maritima reveals a bimodular arrangement and an evolutionary relationship between retaining and inverting glycosidases. J Biol Chem. 2004; 279(18):18903–18910.
- Ruan Y. Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol. 2014;65(1):33–67.
- Sturm A, Chrispeels M. cDNA cloning of carrot extracellular beta-fructosidase and its expression in response to wounding and bacterial-infection. Plant Cell. 1990;2(11):1107–1119.
- Le Roy K, Vergauwen R, Struyf T, et al. Understanding the role of defective invertases in plants: tobacco Nin88 fails to degrade sucrose. Plant Physiol. 2013;161(4):1670–1681.
- Xie J, Cai K, Hu H, et al. Structural analysis of the catalytic mechanism and substrate specificity of anabaena alkaline invertase InvA reveals a novel glucosidase. J Biol Chem. 2016; 291(49):25667–25677.
- Gao J, van Kleeff P, Oecking C, et al. Light modulated activity of root alkaline/neutral invertase involves the interaction with 14-3-3 proteins. Plant J. 2014;80(5):785–796.
- Hyun T, Eom S, Rim Y, et al. Alteration of the expression and activation of tomato invertases during Botrytis cinerea infection. Plant Omics 2011; 4:413–417.
- Li L, Hou M, Cao L, et al. Glutathione S-transferases modulate Cu tolerance in Oryza sativa. Environ. Exp. Bot. 2018; 155:313–320.
- Pang Y, Li J, Qi B, et al. Aquaporin AtTIP5;1 as an essential target of gibberellins promotes hypocotyl cell elongation in Arabidopsis thaliana under excess boron stress. Functional Plant Biol. 2018; 45(3):305–314.
- Sun L, Ma L, He S, et al. AtrbohD functions downstream of ROP2 and positively regulates waterlogging response in Arabidopsis. Plant Signal Behav 2018; 13(9):1–5.
- Xu F, Liu H, Xu Y, et al. Heterogeneous expression of the cotton R2R3-MYB transcription factor GbMYB60 increases salt sensitivity in transgenic Arabidopsis. Plant Cell Tiss Organ Cult. 2018;133(1):15–25.
- Lv S, Yu D, Sun Q, et al. Activation of gibberellin 20-oxidase 2 undermines auxin-dependent root and root hair growth in NaCl-stressed arabidopsis seedlings. Plant Growth Regul. 2018;84(2):225–236.
- Shang B, Zang Y, Zhao X, et al. Functional characterization of GhPHOT2 in chloroplast avoidance of Gossypium hirsutum. Plant Physiol Biochem. 2019; 135:51–60.