Abstract
Gamma-decalactone is an interesting flavuring compound with an intense oily-peachy aroma. It is commonly used in food and cosmetic industry. Biotechnological methods of its synthesis are based on the processes of fatty acid degradation, mainly ricinoleic acid, which is the main component of castor oil. The aim of this study was to compare the productivity of gamma-decalactone in batch cultures of two strains of Yarrowia lipolytica, wild strain W29 and its derived mutant MTLY40-2p, grown in flasks and a bioreactor. We analyzed the concentration of gamma-decalactone in the aqueous and oil phase, the optical density of the medium, the yield of yeast dry matter and the particle size distribution in the particular media. The modified strain had a higher aroma synthesis capacity. This strain grown in flasks produced thrice as much gamma-decalactone (5.5 ± 0.16 g/L) as compared to the wild-type strain (1.8 ± 0.03 g/L). During the 7-day biotransformation, the mutant strain did not have the ability to degrade gamma-decalactone, whereas in the biotransformation using the wild-type strain, reutilization was observed after the third day. Regardless of the type of culture, larger amounts of gamma-decalactone were accumulated in the lipid phase. Cultivation in a bioreactor resulted in a lower biomass yield and a lower concentration of lactone compared to flask culture, regardless of the strain used. The MTLY40-2p strain grew in mycelium form and tended to aggregate cells.
Introduction
Gamma-decalactone (C10H18O2) is an intramolecular 4-hydroxydecanoic acid ester. It is an oily colorless liquid and is characterized by an intense creamy-peach note, already perceptible at very low concentration - about 88 µg/L in aqueous solutions. The main application of gamma-decalactone is to create fragrance compositions of peach, mango, strawberry and chocolate [Citation1].
Although gamma-decalactone is a natural component of the fragrance note of many types of fruit, extraction or distillation from natural sources is not profitable due to its low content in fruit. Therefore, the current production of gamma-decalactone is based mainly on chemical [Citation2–7] or biotechnological methods [Citation8–10]. The disadvantage of chemical methods is often their multi-stage nature and lack of enantioselectivity. Biotechnological methods, despite their low efficiency, enable the synthesis of the desired enantiomer (R) and respond to consumer demand for the use of natural ingredients in food.
Biotechnological methods for the synthesis of gamma-decalactone are mainly based on the processes of oxidative degradation of fatty acids occurring in yeast peroxisomes [Citation11]. Most research is devoted to the β-oxidation of ricinoleic acid which is present at an amount of about 80% in castor oil (lat. Oleum ricini) obtained from seeds of castor oil plant (Ricinus communis). The conversion of this acid to gamma-decalactone requires cleavage of an eight-carbonic fragment from the carboxyl acid end, which occurs as a result of four cycles of β-oxidation. The reactions catalyze the enzymes of the β-oxidation pathway: acyl-CoA oxidase, 2-enol-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase and ketoacyl-CoA thiolase. From the 18-carbon chain, a 10-carbon compound (4-hydroxydecanoic acid) is finally obtained, which is then cyclized to gamma-decalactone [Citation12].
Previous studies on gamma-decalactone biosynthesis have enabled the selection of several yeast species capable of β-oxidation and reduction of unsaturated binding in ricinoleic acid. In the group of microorganisms synthesizing gamma-decalactone from ricinoleic acid or its esters, the following yeast species are distinguished: Candida [Citation9], Pichia [Citation13], Sporobolus [Citation14–16], Rhodotorula [Citation17] and Yarrowia [Citation18,Citation19].
Yarrowia lipolytica is characterized by the highest efficiency in the production of gamma-decalactone and has the largest number of identified genes encoding enzymes that specialize in the degradation of hydrophobic substrates [Citation11]. There are six acyl-CoA oxidases (Aox1 to Aox6 encoded by the POX1 to POX6 genes) in Y. lipolytica cells. These enzymes have different preferences for the length of the degraded carbon chain. Aox2 oxidase has specificity for long chain substrates, whereas Aox3 is active for short chain compounds. It has been proven that the other three enzymes - Aox1, Aox4 and Aox5 – have very weak activity towards most of the available substrates [Citation19]. Therefore, all genetic modifications are based mainly on manipulation within POX2 and POX3 genes.
In this study, an attempt was made to compare the efficiency of gamma-decalactone synthesis by two strains of Yarrowia lipolytica yeast: wild strain W29 and modified strain MTLY40-2p propagated in flasks and bioreactor. The modification of MTLY40-2p strain consisted of the removal of three (out of five) active acyl-CoA oxidases - Aox3, Aox4 and Aox5. In this modification, it was especially important to limit the activity of Aox3, which is responsible for the oxidation process to continue beyond the ten-carbon fragment, and thus contributes to the degradation of the produced gamma-decalactone.
Materials and methods
Materials
The materials used for preparing the media YPG and YPGA (yeast extract, peptone, glucose, agar-agar) were obtained from BTL Łódź (Poland). Sodium chloride, ethyl alcohol 99.8% were from POCH (Poland). Castor oil was from Carl ROTH. Tween 80 was purchased from ACROS ORGANICS and diethyl ether (99.5%) from CHEMPUR (Poland). γ-decalactone and γ-undecalactone 98% were obtained from SIGMA-ALDRICH.
The following two strains of Yarrowia lipolytica were used in this study: MTLY40-2p and W29 (ATCC20460). The strains were obtained from the culture collection of UMR PAM AgroSup-University de Bourgogne in France. Stock cultures were maintained in 20% glycerol (w/v) at −80 °C.
Culture conditions
Y. lipolytica strains were cultured for 48 h on Petri dishes with YPGA medium (yeast extract 10 g/L, peptone 20 g/L, glucose 20 g/L, agar 20 g/L) at 27˚C and were used to inoculate a 250 mL baffled Erlenmeyer flasks containing 50 mL YPG medium (yeast extract 10 g/L, peptone 20 g/L, glucose 20 g/L,). Flasks were shaken at 140 rpm and 27˚C for 24 h. This medium was used to inoculate the biotransformation medium.
Biotransformation conditions
For gamma-decalactone production, the cells in logarithmic growth phase (24 h) were transferred to the biotransformation medium at an initial concentration corresponding to optical density (OD600) of 0.25. This medium was composed of castor oil (100 g/L), peptone (20 g/L) and Tween 80 (5 g/L). The biotransformation was then conducted in 500-mL Erlenmeyer flasks or a bioreactor BIOFLO 3000 (New Brunswick Scientific Edison, N.I., USA) containing a working volume of 3 L; 0.2 vvm (volume air per cultivation volume and minute) at 27˚C and 140 rpm for 7 days. Biotransformation reactions (both in flasks and bioreactor) were carried out in triplicate.
Gamma-decalactone extraction and quantification
For the quantification of γ-decalactone, 1.5 mL samples were collected regularly from the culture medium for 7 days. In order to stop the metabolism and to achieve the complete lactonisation of 4-hydroxydecanoic acids, 10 µL of HCl was added to the samples. Then γ-undecalactone was introduced as an internal standard. The samples were extracted with 1.5 mL of diethyl ether. After 5 min, the ether phase was separated and analyzed by gas chromatography (GC). All assays were performed in triplicate.
The content of γ-decalactone was monitored by gas chromatography (YL 6100 Young Lin Instrument) using a capillary column BPX (30 m × 0.25 mm) and a flame ionisation detector (280 °C), with azote as a gas vector, at a flow rate of 1.1 mL/min. The reaction mixture (1 µL) was injected by using a split injector (280 °C). After injection of samples, the temperature of the column oven was kept at 165 °C, then increased to 180 °C (3 °C/min) and finally, increased to 230 °C at a rate of 5 °C/min. In these conditions, the reaction times for γ-decalactone and γ-undecalactone were 11.5 min and 12.4 min.
Optical density measurement
The samples were taken two times a day, and 1 mL of these was centrifuged using an Eppendorf microcentrifuge (5418, ROTH). The supernatant was decanted and the cells were suspended in 1 mL of distilled water. For the prepared sample, after its appropriate dilution (OD between 0.7 and 1.3), the optical density was measured at a wavelength of 600 nm, using a spectrophotometer UV-VIS THERMO Scientific Helios γ.
Dry mass measurement
In case of both yeast culture conducted in flasks and in a bioreactor, biomass yield was determined after the elimination of the fatty fraction of culture medium. Then, 20 mL of medium was centrifuged at 5000 rpm for 10 min (Centrifuge MPW-223). The supernatant was discarded, and the biomass was washed with a mixture of acetone/ethanol (10:10, v/v) followed by a second washing with distilled water. Biomass was oven-dried (SML 32/250 Zelmet) until it reached a constant weight. The results of biomass yield were given with respect to 1 L of medium (g d.w. of cells/L).
Medium particle size determination
The droplet size distribution in the medium was evaluated by Malvern Mastersizer 2000 laser granulometry measurements. Emulsions were analyzed without cells. Medium was shaken for 1 h prior to analysis. Separately, a cellular suspension prepared in sterile water was also analyzed, in order to determine the size of the yeast cells.
Microscopy observations
Yeast cells from the biotransformation medium were observed with Carl Zeiss Axioplan 2 Microscope.
Results and discussion
Comparison of gamma-decalactone biosynthesis by two yeast strains of Y. lipolytica, wild-type strain W29 and its derived mutant disrupted for genes coding for acyl-CoA oxidases (POX) - MTLY40-2p (Δ pox3, pox4, pox5), was done by batch culture in flasks and a bioreactor. An oil-in-water medium was used in these cultures, using also castor oil as the lipid substrate. The content of γ-decalactone was analyzed in the aqueous and in the lipid phase.
Growth and catalytic activity in biotransformation reactions of the Y. lipolytica strains grown in flasks
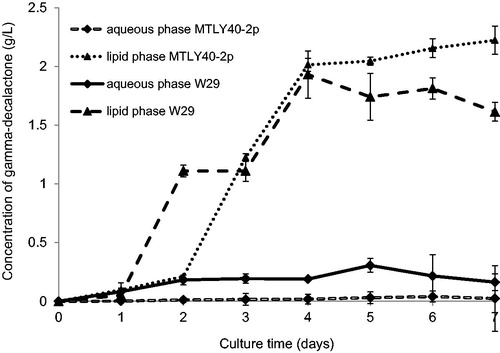
presents data on batch culture carried out in flasks for strain W29 and MTLY 40-2p. The results showed that both tested Yarrowia yeast strains synthesized gamma-decalactone in batch culture conducted in flasks. With the application of 10% additive of castor oil in the medium (100 g/L), wild strain Y. lipolytica W29 produced even approximately 1.8 g per liter of medium (total score of aroma content in aqueous and lipid phase). The maximum efficiency of biotransformation was reached on the third day of culture. After this point, the γ-decalactone content in both fractions decreased gradually: on the last day of culture the total content was only 0.3 g/L. The ability of the yeast Y. lipolytica strain W29 to produce γ-decalactone is in agreement with previous studies of Waché et al. [Citation19], Groguenin et al. [Citation20] and Gomes et al. [Citation21] or Try et al. [Citation22].
Figure 1. Accumulation of γ-decalactone in aqueous (♦) and lipid (▲) phase during growth of Yarrowia lipolytica W29 and MTLY40-2p strains in Erlenmeyer flasks.
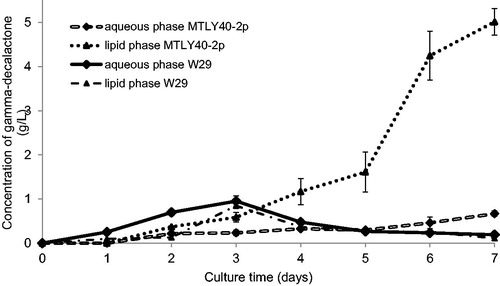
In 2002, Waché et al. [Citation19] conducted research on the synthesis of lactones, using methyl ricinoleate as the only carbon source (3.5 g/L). After 4 days of biotransformation, they reported that strain W29 produced approximately 0.5 g of lactones per liter. Among the lactones they identified: γ-decalactone, 3-hydroxy-γ-decalactone, dec-2-en-4-olide and dec-3-en-4-oilde. γ-decalactone was produced to the maximum amount of 0.07 g/L at 10 h of culture. After 24 h, a five-fold decrease in its content (up to 0.014 g/L) was observed. A similar result was obtained in our experiments. Between day 3 and day 7 of culture there was about 4.5-fold decrease in the concentration of γ-decalactone (). The decrease in the aroma in the medium was also observed by the team of Groguenin et al. [Citation20] and Gomes et al. [Citation21]. In the first case, using methyl ricinoleate in the medium at 5 g/L, the team obtained 0.15 g of lactone per liter after 24 h of culture. After 40 h the concentration of γ-decalactone decreased to 0.05–0.06 g/L and in the final stage there was only 0.02 g/L (7.5-fold decrease). By the introduction to the medium of 10-fold more amount of substrate (50 g/L of methyl ricinoleate) by Gomes et al. [Citation21], 1 g/L was reached at the 5th day of culture, with a subsequent 10-fold decrease in the subsequent days of culture. During solid-state fermentation with a Luffa sponge as an internal support and castor seeds as a substrate, there was a decrease in gamma-decalactone in the medium from a value of approx. 0.19 g/L obtained at the 28th hour of cultivation to a value of 0.06 g/L at the 90th hour of fermentation [Citation22].
There are several theories to explain the decline in γ-decalactone concentration. Yeast cells are capable of degrading γ-decalactone. It is also possible that high concentration of aroma compounds reached during biotransformation may lead to toxic effects towards the producing microorganism [Citation23]. Therefore, the gamma-decalactone concentration in the medium results from the difference between is the amount produced and is the amount degraded.
Although the re-utilization pathway is not precisely known, there are some aspects related to this degradation that can be mentioned. Several pathways of degradation are possible. According to Braga and Belo [Citation24], the most probable one includes the opening of the cyclic form trough gamma-lactonase activity, followed by the activation of the CoA esters and β-oxidation. Another possible pathway involves the oxidation of the lactone to yield, after delactonisation, a dicarboxylic acid. According to Waché et al. [Citation19] thedecline of γ-decalactone can be attributed to entry of the lactone into the cell and, prior to or after this step, hydrolysis of the lactone cycle. The difference in pH may also have an impact on the re-utilization of γ-decalactone.
The gamma-decalactone production profile for the derived mutant MTLY40-2p disrupted for genes coding for acyl-CoA oxidases (POX) was very different (). By using a genetically modified strain, it was possible to produce up to 5.5 g/L - about three times more than with strain W29. γ-decalactone was accumulated in this case mainly in e lipid phase and its content within 7 days of culture increased gradually. In the case of the aqueous phase, the amount of lactone did not exceed 0.5 g/L, which resulted from the solubility of this compound. Unlike the wild-type strain W29, the constructed strain produced gamma-decalactone slowly but gradually without utilizing the produced compound. Groguenin et al. [Citation20] working with a mutant strain (pox2–pox5) observed four times higher production of γ-decalactone compared to the wild-type strain (400 mg vs. 100 mg/L) without degradation of this aroma compound.
Production of gamma-decalactone from non-hydroxylated fatty acids by engineered yeast cells of Y. lipolytica in a single-host fermentation process is also possible. The modified strain produced δ-decalactone from linoleic acid in fed-batch culture and through metabolic engineering, the yield of was improved 4-fold [Citation25].
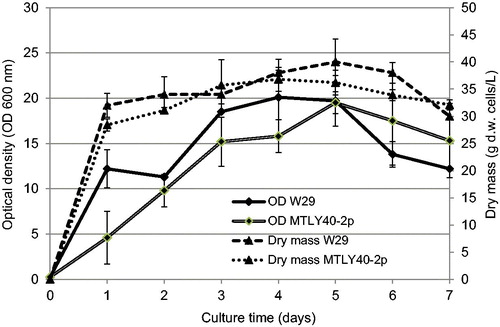
Since the synthesis of γ-decalactone is also connected with the growth phase of microorganisms, in this work we determined the optical density, dry mass of cells and the amount of living cells at day 7 of culture, both for the strains W29 and MTLY 40-2p. The growth of Y. lipolytica W29 was fairly intensive until day 3 of culture: OD value increased from 0.25 to 18.5 and the dry matter content reached up to about 34 g/L (). The yeast culture was in logarithmic growth phase, as shown also by the number of living cells: at day 3 of culture, the cell count reached the maximum of 33.0 ± 2.8 × 1011 CFU/mL. After 72 h, the yeast culture probably entered the stationary phase. Thus, the maximum production of γ-decalactone by the wild-type strain W29 occurred in the logarithmic phase.
According to Groguenin et al. [Citation20], the production of γ-decalactone occurs in the logarithmic phase with a small delay after growth, corresponding probably to the time necessary for the fatty acids to undergo four β-oxidation cycles. Also the research by Moradi et al. [Citation26] on the synthesis of gamma-decalactone by fed-batch cultivation of Yarrowia lipolytica DSM 3286 strain confirms that growth of biomass was concomitant with accumulation of gamma-decalactone in the medium which reached its maximum almost at the end of the exponential growth phase. The lactone concentration decreased when cells entered the stationary phase of growth.
Comparing the value of OD, dry mass and the viable cell counts for Y. lipolytica MTLY40-2p, a clear difference in the rate of multiplication of microorganisms was observed. In the first two days, MTLY40-2p cells proliferated at a much slower rate (). After 24 h of culture, the OD for the genetically modified strain was 4.6, whereas that of W29 at the same time was 12.2. A similar trend was observed for the dry matter content and the cell counts. They were more than 100-fold lower in the mutant culture, and the maximum was 27 × 109 CFU/mL.
The difference in the rate of multiplication of the modified strain and the wild-type one was also reported by Groguenin et al. [Citation20]. Researchers have suggested that a lower rate of yeast growth of mutants (Y. lipolytica 40-2p and 36-2p) compared with wild-type strain (W29) can be explained by the lack of Aox3 and Aox5.
Growth and catalytic activity in biotransformation reactions of the Yarrowia lipolytica strains grown in bioreactor
Due to the fact that Y. lipolytica belongs to aerobic microorganisms, we analyzed the effects of oxygen in the medium on the cell growth and production of γ-decalactone by conducting culture in a bioreactor.
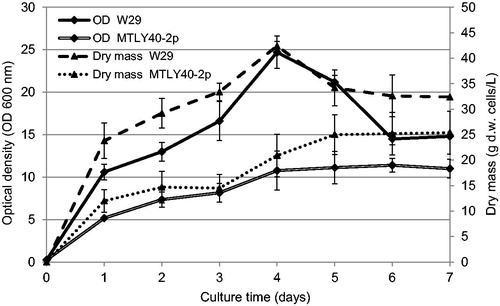
The wild-type strain W29 cultured in a bioreactor produced a slightly higher level of aroma in comparison to the culture in flasks (). The maximum was about 2.1 g/L (maximum total value of the aqueous and lipid phase). In contrast to the culture in flasks, the culture in the bioreactor resulted in higher accumulation of γ-decalactone in the lipid phase. After day 2 of culture, the aroma content in the lipid phase was over five times higher in comparison with the aqueous phase (1.1 g/L vs. 0.18 g/L at day 2) and this trend continued until the last day of culture (at day 7, the concentration in the lipid phase was 1.61 g/L vs. 0.16 g/L in the aqueous phase). Compared to the flask culture, the Y. lipolytica W29 strain showed gamma-decalactone degradation in the final days of the bioreactor culture. The maximum concentration of lactone was achieved at the turn of day 4 and day 5, after which its content in the medium decreased, both in the aqueous and lipid phase.
Figure 3. Accumulation of γ-decalactone in aqueous (♦) and lipid (▲) phase during growth of Yarrowia lipolytica W29 and MTLY40-2p strains in bioreactor.
The growth curve (based on OD-values and dry mass) for this culture () showed that the exponential growth phase lasted until day 4. At that time, the OD value increased from 0.25 at the start of cultivation to 25 on day 4 of culture. The dry mass increased to about 42.4 ± 4.9 g/L. The largest number of living cells, reaching levels of 28 × 1010 CFU/mL, was however observed on day 3. After day 4, began a slow process of cell death. Thus, the maximum production of gamma-decalactone in this culture most probably occurred in the final stage of logarithmic growth and the beginning of the stationary phase.
In contrast, the genetically modified strain MTLY40-2p showed different behavior from that of the wild-type strain W29. The biotransformation of ricinoleic acid to gamma-decalactone during cultivation in bioreactor was approximately 2-fold lower than that during cultivation in flasks ().
Taking into account the period of maximum productivity, the level of gamma-decalactone obtained in the bioreactor was 2.2 g/L, which is one half of the concentration of lactone obtained in flask-culture. The results indicate that the aeration of media with intensive mixing does not facilitate the efficient synthesis of gamma-decalactone. According to previous reports on gamma-decalactone biosynthesis, oxygen is an factor intervening in the metabolic pathway of β-oxidation and participates in the production and re-consumption reactions of γ-decalactone [Citation27,Citation28]. Aguedo et al. [Citation29] analyzing the β-oxidation pathway in culture of Y. lipolytica W29 on a medium with the addition of methyl ricinoleate at an increased pressure (5 bar) observed a decrease in the concentration of gamma-decalactone in the medium, compared to standard conditions. Higher oxygen concentration in the medium favored the accumulation of decenolide compounds in the metabolic pathway. Escamilla-Garcı ́a et al. [Citation30] conducting research on the effects of pH and oxygenation on the synthesis of lactones by yeast Yarrowia lipolytica noticed that under low aeration the concentration of gamma-decalactone is much higher than in media with intensive aeration. According to the authors, this is due to the limited activity of selected β-oxidation pathway enzymes. Very low oxygen concentration in the substrate inhibits acyl-CoA oxidase activity, whereas high oxygen concentration inhibits 3-hydroxy-acyl CoA dehydrogenase activity, which results in an increase in the concentration of 3-hydroxy-γ-decalactone, dec-2-en-4-olide and dec-3-en-4-olide [Citation31]. Aguedo [Citation32] observed also that not only the high oxygen concentration, but also the global redox environment may influence the crucial step leading to the formation of other C10-lactones from 4-hydroxydecanoic acid, the direct precursor of gamma-decalactone.
Conducting further comparison of both cultures (in bioreactor and flasks), there was a similar trend for accumulation of gamma-decalactone mostly in the lipid phase (from the second day of culture 90–95% of the aroma compound was in the lipid phase). Just as in the case of biotransformation carried out in flasks, γ-decalatone was not degraded. The amount of lactone increased within 7 days, with the highest rate of biosynthesis between day 2 and day 4 of culture (the amount of lactone increased from 0.25 g/L to 2 g/L, i.e. almost 8 times).
On the basis of the growth curves () the multiplication of the mutant yeast strain in the bioreactor proceeded much more slowly in comparison with the culture in flasks. In this experiment the OD value did not exceed 10, whereas for the culture in flasks, it was nearly 20. It is assumed that such less intense multiplication of yeast cells may be due to slightly different culture conditions. Despite the fact that there was some amount of castor oil (100 g/L) added both in flasks and bioreactor, the dispersion of the lipid phase in the bioreactor was different. This is due to different mixing systems (despite the same agitation speed 140 rpm). The castor oil in flasks was mostly found on the surface of the medium, whereas in the bioreactor it was clearly dispersed. Although it might seem that under the influence of dispersion of the lipid phase there is an increase of in the contact surface of the substrate with the enzyme, in this case it was not associated with an increase in the biotransformation productivity.
The lower rate of multiplication was also confirmed based on the biomass yield and the number of living cells per 1 mL medium. The biomass yield in the culture of strain MTLY 40-2p did not exceed 25 g/L, whereas that in the flask culture reached 35 g/L. The number of living cells observed in the medium was also lower and reached a maximum level of 13.7 × 108 CFU/mL.
According to Pereira de Andrade et al. [Citation28] the cultivation of yeast in bioreactor and the higher rates of agitation associated with it may cause metabolic changes and hydrodynamic stress in the cells, which may lead to changes in their morphology, metabolism and viability. Furthermore, research by Waché et al. [Citation19] on the optimization of Yarrowia lipolytica β-oxidation pathway for γ-decalactone production confirms that the optimized strain Yarrowia lipolytica MTLY 40-2p was not growing as rapidly as the wild type in medium with methyl esters of fatty acids.
Granulometric characterization of biotransformation media
In biotransformation of ricinoleic acid into γ-decalactone there was used an oil-in-water emulsion stabilized by nonionic surfactant Tween 80. In this type of culture the contact surface of lipid droplets with cells of propagated microorganisms and the surface between two liquid phases are determining factors in the degradation of a hydrophobic substrate, and thus cell growth and aroma production by these cells [Citation33,Citation34]. The size of fat droplets in the medium depends on the physical and physicochemical properties of the medium (pH, ionic strength, presence of surfactants) and the amount of inoculum introduced into the culture medium and the surface properties of propagated cells [Citation34]. The aim of this part of the study was to characterize emulsions obtained after 7-day propagation both in flasks and the bioreactor.
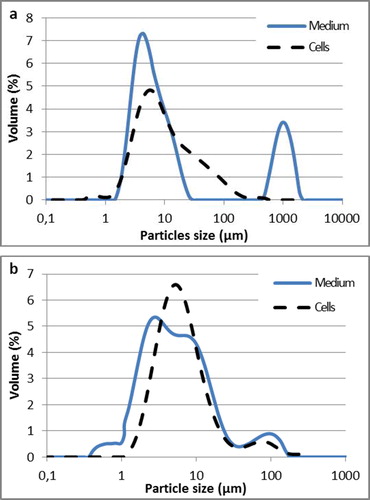
The granulometric analysis of biotransformation media with Y. lipolytica W29 cells grown in flask cultures indicated the existence of two distinct droplet populations (). The first population reached the level of 78% and contained drops with the size of 1.5 μm to 26 μm. In the second population (22%), the mean droplet diameter increased from 550 μm to 2000 μm. It is assumed that the second population is an outcome of the presence of quite big fat droplets in the medium and partial adhesion of the cells to the oil surface. Within the first population, there were yeast cells for which a separate granulometric measurement was taken as well as tiny fat droplets from the medium. Slightly different results were achieved for the emulsions after biotransformation with the use of the genetically modified Y. lipolytica MTLY40-2p strain also propagated in flasks (). In that case, four distinct populations were observed: 0.5–1 μm (4%), 1–6.5 μm (54%), 6.5–13 μm (29%) and 13–180 μm (13%). Smaller sizes of particles in the emulsions, in comparison with emulsion W29, were reflected in the higher stability. It is assumed that the population with the largest droplet size (13–180 μm) in this distribution constituted cells of yeasts that had undergone aggregation. A population similar in size was achieved while measuring only the cells of MTLY40-2p (). According to Phan The et al. [Citation35], this phenomenon could be avoided by adding solution of Sodium Dodecyl Sulfate (0.1%).
Figure 5. Castor oil droplets and cell size distribution in emulsions obtained in Yarrowia lipolytica W29 (a) and MTLY40-2p (b) grown in Erlenmeyer flask.
Next, we performed granulometric analysis in the case of yeast W29 () and MTLY40-2p () cultures in the bioreactor. Since the method of mixing the emulsions in the reactor is different from the one in the flask cultures, it was assumed that it might influence the properties of emulsions and in consequence the efficiency of biotransformation.
Figure 6. Castor oil droplets and cells size distribution in emulsions obtained in Yarrowia lipolytica W29 (a) and MTLY40-2p (b) grown in bioreactor.
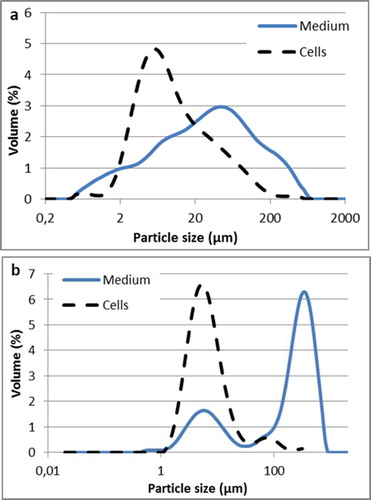
The granulometric charts for both strains () were very different and distinct from the ones obtained from the flask cultures (). The wild-type culture in the bioreactor contained four populations of particles without clear demarcation. There were particles in the range of: 0.5–3 μm (about 10%), 3–11 μm (24%), 11–150 μm (46%), and the remaining part comprised particles of big sizes, up to 700 μm. Y. lipolytica MTLY40-2p medium () was characterized by two populations: the first one consisted of particles of 1–11 μm (21%) and the second one of particles of sizes up to 800 μm.
Gomes et al. [Citation34] observed that larger oil droplets the aroma production, suggesting that in this case the access of cells to the substrate occurs by their adhesion around larger fat droplets. In our study, such dependence was difficult to establish. Nevertheless, it can be suggested that the size of the particles of the lipid substrate has an influence on the efficiency of biotransformation.
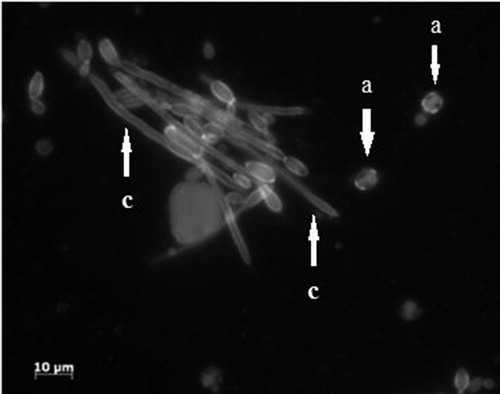
In order to confirm this during the biotransformation of ricinoleic acid by microorganism cells we made microscopic observations of both strains. Because of direct contact with the hydrophobic substrate, some cells were adhered to the surface of the lipid substrate, and some of the oil droplets were adsorbed on the surface of the microorganism cells. and show the confocal micrograph respectively for Yarrowia lipolytica W29 (7) and MTLY 40-2p (8) after 7 days of culture in medium with castor oil.
Figure 7. Confocal micrograph of Y. lipolytica W29. Yeast-like form (a); elongated yeast-like form (b).
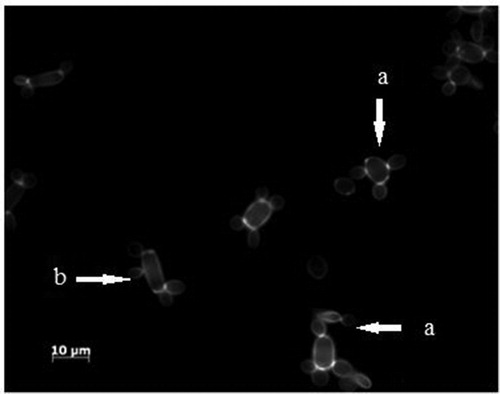
In both micrographs it is visible that fat droplets were adsorbed to the surface of the cells, which were both in the yeast-like and mycelia forms. In the case of yeasts MTLY40-2p, the cells underwent strong aggregation. In contrast to the wild-type strain, this strain produced mycelial forms.
Conclusions
Studies on gamma-decalactone biosynthesis by two strains of Yarrowia lipolytica grown in flask cultures and in a bioreactor, showed that both strains were capable of lactone synthesis, with the MTLY40-2P strain showing higher biotransformation efficiency and not degrading the lactone. Biosynthesis using the wild-type strain W29 required continuous verification of lactone concentration in the medium and stopping of biotransformation before its degradation stage. The attempt to upscale the process requires optimizing the culture conditions, including oxygenation of the substrate, mixing intensity, concentration of the introduced inoculum and concentration of the substrate, because in the conditions used in this study, with oxygenation at the level of 0.2 vvm and mixing speed of 140 rpm, the bioreactor culture resulted in lower lactone yield and lower ability to multiply cells. In further work on the biotechnological synthesis of gamma-decalactone it is also necessary to develop an efficient method of separating lactone from the biotransformation medium, due to its accumulation in the lipid phase.
Disclosure statement
No potential conflicts of interest was reported by the authors.
References
- Lee SL, Cheng HY, Chen WC, Chou CC. Production of gamma-decalactone from ricinoleic acid by immobilized cells of Sporidiobolus salmonicolor. Process Biochem. 1998;33(4):453–459.
- Inokuchi T, Matsumoto S, Nishiyama T, et al. A selective and efficient method for alcohol oxidations mediated by N-oxoammonium salts in combination with sodium bromite. J Org Chem. 1990;55(2):462–466.
- Cossy J, Bargiggia F, BouzBouz S. Tandem cross-metathesis/hydrogenation/cyclization reactions by using compatible catalysts. Org Lett. 2003;5(4):459–462.
- Wang L, Floreancig PE. Synthesis of the C16 − C35 fragment of integramycin using olefin hydroesterification as a linchpin reaction. Org Lett. 2004;6(4):569–572.
- Zope DD, Patnekar SG, Kanetkar VR. Novel synthesis of flavour quality γ-lactones. Flavour Fragr J. 2006;21(3):395–399.
- Huang L, Jiang H, Qi C, et al. Copper-catalyzed intermolecular oxidative [3 + 2] cycloaddition between alkenes and anhydrides: a new synthetic approach to γ-lactones. J Am Chem Soc. 2010;132(50):17652–17654.
- Reddy MS, Kumar YK, Thirupathi N. A new synthesis of γ-butyrolactones via AuCl(3)- or Hg(II)-catalyzed intramolecular hydroalkoxylation of 4-bromo-3-yn-1-ols. Org Lett. 2012;14(3):824–827.
- Okui S, Uchiyama M, Mizugaki M. Metabolism of hydroxy fatty acids. I. Metabolic conversion of ricinoleic acid by a certain microorganism to 8-D-hydroxy tetradec-cis-5-enoic acid. J Biochem. 1963; 53:265–270.
- Okui S, Uchiyama M, Mizugaki M. Metabolism of hydroxy fatty acids. II. intermediates of the oxidative breakdown of ricinoleic acid by genus Candida. J Biochem. 1963;54(6):536–540.
- Boratyński F, Dancewicz K, Paprocka M, Gabryś B, Wawrzeńczyk C. Chemo-enzymatic synthesis of optically active γ- and δ-decalactones and their effect on aphid probing, feeding and settling behavior. PLoS One. 2016; 11:1–18.
- Fickers P, Benetti PH, Waché Y, et al. Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res. 2005;5(6–7):527–543.
- Waché Y, Aguedo M, Nicaud JM, et al. Catabolism of hydroxyacids and biotechnological production of lactones by Yarrowia lipolytica. Appl Microbiol Biotechnol. 2003;61(5–6):393–404.
- Endrizzi A, Awadé AC, Belin JM. Presumptive involvement of methyl ricinoleate β-oxidation in the production of γ-decalactone by the yeast Pichia guilliermondii. FEMS Microbiol Lett. 1993;114(2):153–159.
- Lee SL, Lin SJ, Chou CC. Production of γ-decalactone by Sporobolomyces odorus in jar fermentors as affected by pH, aeration and fed-batch technique. J Ferment Bioeng. 1995;80(2):195–199.
- Lee SL, Cheng HY, Chen WC, Chou CC. Effect of physical factors on the production of γ-decalactone by immobilized cells of Sporidiobolus salmonicolor. Process Biochem. 1999;34(8):845–850.
- Blin-Perrin C, Molle D, Duffose L, et al. Metabolism of ricinoleic acid into γ-decalactone: β-oxidation and long chain acyl intermediates of ricinoleic acid in the genus Sporidiobolus sp. FEMS Microbiol Lett. 2000;188(1):69–74.
- Alchihab M, Destain J, Aguedo M, et al. Production of γ-decalactone by a psychrophilic and mesophilic strain of the yeast Rhodotorula aurantica. Appl Biochem Biotechnol. 2009;158(1):41–50.
- Waché Y, Aguedo M, Choquet A, et al. Role of β-oxidation enzymes in γ-decalactone production by the yeast Yarrowia lipolytica. Appl Environ Microb. 2001;67(12):5700–5704.
- Waché Y, Aguedo M, LeDall MT, Nicuad JM, Belin JM. Optimization of Yarrowia lipolytica’s β-oxidation pathway for γ-decalactone production. J Mol Catal B – Enzym. 2002; 19:347–351.
- Groguenin A, Waché Y, Garcia EE, et al. Genetic engineering of the β-oxidation pathway in the yeast Yarrowia lipolytica to increase the production of aroma compounds. J Mol Catal B: Enzym. 2004;28(2–3):75–79.
- Gomes N, Teixeira JA, Belo I. The use of methyl ricinoleate in lactone production by Yarrowia lipolytica: Aspects of bioprocess operation that influence the overall performance. Biocatal Biotransform. 2010; 28(4):227–234.
- Try S, De-Coninck J, Voilley A, et al. Solid state fermentation for the production of γ-decalactones by Yarrowia lipolytica. Process Biochem. 2018; 64:9–15.
- Aguedo M, Waché Y, Coste F, et al. Impact of surfactants on the biotransformation of methyl ricinoleate into y-decalactone by Yarrowia lipolytica. J Mol Catal B: Enzym. 2004;29(1–6):31–36.
- Braga A, Belo I. Biotechnological production of gamma-decalactone, a peach like aroma, by Yarrowia lipolytica. World J Microbiol Biotechnol. 2016; 32(10):1–8.
- Roy Marella E, Dahlin J, Inger Dam M, et al. A single-host fermentation process for the production of flavor lactones from non-hydroxylated fatty acids. Metab Eng. 2019; doi:10.1016/j.ymben.2019.08.009
- Moradi H, Asadollahi MA, Nahvi I. Improved γ-decalactone production from castor oil by fed-batch cultivation of Yarrowia lipolytica. Biocatal Agric Biotechnol. 2013;2(1):64–68.
- Gomes N, Aguedo M, Teixeira J, et al. Oxygen mass transfer in a biphasic medium: Influence on the biotransformation of methyl ricinoleate into gamma-decalactone by the yeast Yarrowia lipolytica. Biochem Eng J. 2007;35(3):380–386.
- Pereira de Andrade D, Carvalho BF, Schwan RF, Dias DR. Production of γ-decalactone by yeast strains under different conditions. Food Technol Biotechnol. 2017; 55(2):225–230.
- Aguedo M, Gomes N, Escamilla Garcia E, et al. Decalactone production by Yarrowia lipolytica under increased O2 transfer rates. Biotechnol Lett. 2005;27(20):1617–1621.
- García EE, Belin J-M, Waché Y. Use of a Doehlertfactorial design to investigate the effects of pH and aeration on the accumulation of lactones by Yarrowia lipolytica. J Appl Microbiol. 2007;103(5):1508–1515.
- García EE, Aguedo M, Gomes N, et al. Production of 3-hydroxy-γ-decalactone, the precursor of two decenolides with flavouring properties, by the yeast Yarrowia lipolytica. J Mol Catal B Enzym. 2009;57(1–4):22–26.
- Aguedo M. Production of aroma compounds from lipids. Food Technol Biotechnol. 2004; 42(4):327–336.
- Aguedo M, Wache Y, Belin J-M, et al. Surface properties of Yarrowia lipolytica and their relevance to γ-decalactone formation from methyl ricinoleate. Biotechnol Lett. 2005;27(6):417–422.
- Gomes N, Waché Y, Teixeira JA, et al. Oil-in-water emulsions characterization by laser granulometry and impact on γ-decalactone production in Yarrowia lipolytica. Biotechnol Lett. 2011;33(8):1601–1606.
- Phan The D, Péroval C, Debeaufort F, et al. Arabinoxylan-lipids-based edible films and coatings. 2.Influence of sucroester nature on the emulsion structure and film properties. J Agric Food Chem. 2002;50(2):266–272.