Abstract
The aim of this study was to investigate the association between expressions of five periodontal pathogens (Actinobacillus actinomycetemcomitans (Aa), Porphyromonas gingivalis (Pg), Tannerella forsythensis (Tf), Treponema denticola (Td) and Prevotella intermedia (Pi) and the periodontal pocket depth (PD) in people suffering from periodontitis. A study of 1164 plaque samples from individuals with periodontitis were conducted by multiplex real-time quantitative fluorescence-polymerase chain reaction to evaluate the PD and loads of five periodontal pathogens. The total bacterial number did not differ significantly between people with different subtypes of periodontitis. However, expression of specific pathogens (Pg, Td, Tf, Pi) showed a significant increase with the development of periodontitis. Expression of Pg, Td and Tf showed close correlation between them as well as with PD. Analyses of receiver operating characteristic curves showed that expression of Pg, Td, Tf and Pi had good potential for grading periodontitis severity. We found a close correlation between the relative expression of Pg, Td and Tf and PD. Measuring expression of target bacteria in the oral cavity could facilitate the understanding of the role of bacteria in periodontitis. Taking the appropriate steps to eliminate these pathogens might aid the prevention of periodontitis development.
Introduction
Periodontitis is inflammation of the gum and deeper periodontal structures. Periodontitis is caused by infection by pathogenic microflora that can lead to loosening and eventual loss of teeth [Citation1]. It is a common oral-health condition but can be prevented because it is the consequence of poor oral hygiene. The general symptoms of periodontitis are similar to those of gingivitis: swollen, reddish, tender and readily bleeding gums which pull away from periodontal pockets to generate an elongated appearance of the tooth. In addition, pus develops with halitosis [Citation2, Citation3]. Periodontitis can be divided into several subtypes, and the chronic form is the most common subtype. Plaque buildup causes destruction of the gum and bone if left untreated. Aggressive periodontitis occurs in childhood or early adulthood. However, it tends to cause rapid destruction of teeth and palatine bones. In certain conditions, periodontitis can lead to severe complications, such as necrotizing periodontal disease in immunosuppressed individuals [Citation4, Citation5]. Plaque formation on teeth epitomizes extensive accumulation of bacteria in the oral cavity. Bacterial colonization along with plaque formation are dependent on the extent of salivation, mastication and malocclusion with the type and amount of food consumed [Citation6]. Consequentially, complex flora are formed because plaque comprises about 50% of Gram-negative bacteria [Citation7]. In general, most periodontal plaques comprise normal flora. Only those which have the potential to cause damage to the periodontium and interfere with its immune system with specific virulence through multiple mechanisms are called “periodontal pathogens” [Citation8]. In 1998, Socransky et al. evaluated 13,261 plaque samples in 185 participants (meanage, 51 ± 16 years) with or without periodontitis. Bacteroides forsythus (Bf; no Bacteroides forsythus known as T. forsythia), Porphyromonas gingivalis (Pg), Treponema denticola (Td), Fusohaderium nucleatum (Fn), Prevotella intermedia (Pi), Prevotella nigrescens (Pn) and Peptostreptococcus micros (Pm) were found to be potential important pathogens [Citation9].
A periodontal pocket is a deepened gingival sulcus around a tooth at its gingival margin. “Pocket” describes the space between the detached gingiva and the tooth. It is considered to be clinically healthy if the depth of a gingival sulcus is <0.5 mm [Citation10]. In 1996, Genco classified the extent of periodontitis according to the probing depth (PD), with PD >6 mm, 4–6 mm and ≤3 mm indicating “severe”, “moderate” and “mild” conditions, respectively [Citation8]. The severity of periodontitis has a direct association with the depth of the periodontal pocket because a deep periodontal pocket allows more flora to settle and proliferate. We investigated the association between bacterial infections and depth of the periodontal pocket in patients with different severities of periodontitis.
Materials and methods
Ethical approval of the study protocol
The study protocol was approved by the ethics committee of Shenzhen Second People’s Hospital (Shenzhen, China). We conducted clinical procedures in accordance with the Declaration of Helsinki. Experimental procedures were undertaken with the understanding and written consent of each patient.
Inclusion criteria
In order to be included, patients had to fulfil the criteria for a clinical diagnosis of generalized periodontitis (stage III or IV, also known as aggressive periodontitis or severe chronic periodontitis, respectively [Citation11]) defined by the classification scheme for periodontal and peri‐implant diseases and conditions in 2017 [Citation12]. This scheme classifies periodontitis according to stage based on the: (i) severity and complexity of management; (ii) distribution of the flora and grades based on speed of progression; (iii) response to treatment.
Patients
A total of 240 adults (mean age 43 years; 50% males) participated in our study between 2017 and 2018. Patients with periodontitis and age- and sex-matched healthy control were recruited at the Division of Stomatology of six hospitals in Shenzhen, China. Both patients and healthy controls should be with good oral hygiene, with no systemic diseases or reception of antibiotics for 3 months. Patients were classified the extent of periodontitis according to the probing depth (PD) with PD >6 mm, 4–6 mm, indicating “severe” and “moderate” conditions while healthy controls were classified as PD ≤3 mm.
Clinical examinations
Periodontal parameters were assessed from five sites in the oral cavity: mesial buccal sites of 16, 26 and 46 and distal labial sites of 21 and 41. If an assigned site was missing or with a dental defect, the neighbouring healthy tooth was assessed instead. The depth of periodontal pocketing was measured to the nearest millimetre with a Williams Probe (Hu-Friedy, Chicago, IL, USA). Bleeding on the probe was recorded as the Sulcus Bleeding Index with signs of bleeding 30 s after probing. In addition, gingival withdrawal (mm), suppuration, boot bifurcation, loose degrees and Plaque Index were also assessed [Citation13]. In total, 1164 samples that involved further investigation were collected from the patients.
Microbiological examination
Samples of subgingival plaque were collected from the periodontium in five sites from each patient by a sterile test paper of diameter 0.5 mm. Clinicians inserted the paper tip into the periodontal pocket and removed it after 30 s. The genomic DNA of subgingival-plaque samples was exacted using a Magnetic Beads Bacterial and Mycoplasma DNA Extraction kit (Enriching Biotechnology, Shanghai, China).
Afterwards, multiplex quantitative fluorescence-polymerase chain reaction (QF-PCR) was employed to measure the population of five selected oral pathogens. Primers of human ribonuclease P (Hum P), total bacterial 16S rDNA (Bact 16S), Aggregatibacter actinomycetemcomitans (Aa), Pg, Pi, Td and Tannerella forsythia (Tf) were purchased from Invitrogen (Carlsbad, CA, USA) (). PCR reagents were obtained from Takara Biotechnology (Dalian, China) (see Supplemental Tables S1–S4).
Table 1. Primers for Hum P, Bact16S, Aa, Pg, Pi, Td and Tf.
QF-PCR was conducted using a fluorescence probe in a real-time PCR system (ABI PRISM 7500; Applied Biosystems, Foster City, CA, USA). Briefly, we labelled different probes with specific reporter dyes and quencher dyes (HEX, ROX, FAM and CY5). We undertook PCR twice with different primers and probes. The PCR procedure involved four stages. First, samples were denatured by incubation at 95 °C for 15 s for a total of 10 min. Then, samples were annealed by decreasing the temperature to 62 °C for 1 min. This was followed by elongation for 45 cycles. Finally, we recorded the cycle threshold (Ct;i.e. the number of cycles required for the fluorescence signal to cross the threshold in each sample).
Statistical analyses
We used the QF-PCR data of five periodontal pathogens in patients and classified the PD into three grades: healthy (<4 mm), moderate (4–6 mm) and severe (>6 mm). We eliminated all points corresponding to the Ct value at 45 (which represented the absence of the investigated periodontal pathogen in samples). Expression of Bact 16S was normalized to that of Hum P. Relative expression of specific bacteria was normalized to that of Bact 16S. The fold-change was calculated using the 2−ddCt method of relative quantification. Data are the mean ± SEM. Statistical significance was evaluated through one-way analysis of variance (ANOVA) and set as p < 0.05. The Newman–Keuls comparison was used to determine the source of significant differences. Analyses of correlation and significance were conducted using the software Prism 5 (GraphPad, San Diego, CA, USA). p < 0.05 was considered significant. Analyses of receiver operating characteristic (ROC) curves were done for selected pathogens. The area under the ROC (AUC) and 95% confidence intervals were calculated to evaluate the specificity and sensitivity for detecting periodontitis.
Results and discussion
Relative expression of pathogens
We analysed selected periodontal pathogens by multiplex QF-PCR. According to PD, we classified the severity of periodontitis into three grades: healthy, moderate and severe. Considering that expression of Bact 16S showed no significant change between any subtype of periodontitis after normalization based on human genome, we used Bact 16S to normalize the expression of specific pathogens. Aa, Pg, Td, Tf and Pi were selected in the present study. Aa is a facultative anaerobe. It occurs in aggressive, but also in chronic, periodontitis. The potential virulence factors of this organism are leukotoxin, lipopolysaccharide, cell surface-associated materials and enzymes, as well as less well-defined virulence factors that modulate the activity of host defences [Citation14]. Pg is a Gram-negative anaerobe involved in the pathogenesis of periodontitis and is a member of >500 bacterial species that live in the oral cavity [Citation15]. The most important virulence factors of Pg are its cysteine proteinases, which can lead to the development of edoema, neutrophil infiltration and bleeding after probing [Citation16]. Td is a short, strictly anaerobic spirochaete. Together with other proteolytic Gram-negative bacteria present in high numbers in periodontal pockets, Td may play an important part in the progression of periodontal disease [Citation17]. Td produces tissue-destroying proteases, hyaluronidases, phosphatases and phospholipases. Tf is a Gram-negative anaerobe that inhabits mature biofilms present at sites expressing progressive periodontitis [Citation18]. BspA protein and prtH-encoded cysteine proteases play vital parts in Tf virulence. Pi is a well-known pathogen causing periodontal diseases [Citation19]. Pi is isolated frequently from dental plaque in patients with periodontal diseases and is classified as an “orange complex”. Relative expression of Pg, Td, Tf and Pi was increased significantly in severe and moderate groups compared with that in HCs (). Furthermore, relative expression of Pg, Td, Tf and Pi increased significantly in the severe group compared with that in the moderate group. Relative expression of Aa was increased significantly in the moderate group compared with that in HCs (). However, relative expression of Aa was decreased significantly in the severe group compared with that in HCs. There was no significant difference in Bact 16S expression between any group ().
Figure 1. Relative expression of Pg (A), Td (B), Tf (C), Pi (D), Aa (E) and total bacteria (F) in the periodontium of patients.
Note: Data are the mean ± SEM (healthy controls, n = 426; moderate periodontitis, n = 602; severe periodontitis, n = 36). **P < 0.01 vs. healthy controls, ##P < 0.01 vs. moderate-periodontitis group.
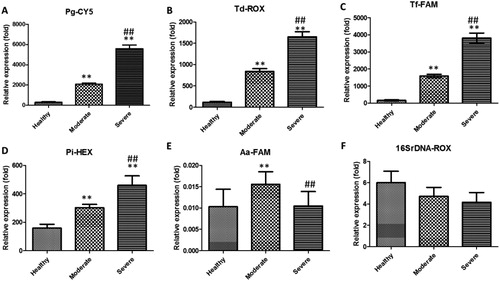
These results suggested that the total bacterial number showed no significant difference between the different subtypes of periodontitis. However, some specific pathogens (Pg, Td, Tf and Pi) showed a significant increase in expression with the development of periodontitis and might serve as biomarkers or for elucidation of the aetiology of periodontitis.
Correlation analyses
We conducted correlation analyses between different pathogens. Pg, Td, and Tf had a significant correlation between any two groups (). PD was an important marker for periodontitis severity: the higher the PD, the greater was the damage caused by periodontitis.
Figure 2. Correlation analyses among different periodontal pathogens (A–C) or between periodontal pathogens and probing depth (D–E). n = 1164, **p < 0.01.
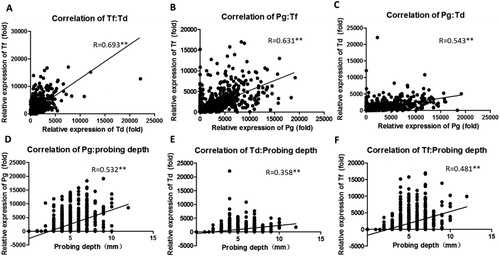
Next, we conducted correlation analyses between PD and pathogens. Expression of Pg, Td and Tf increased significantly with the severity of periodontitis and showed a close correlation with PD (). Pg, Td and Tf constitute the “red complex”, a prototype polybacterial pathogenic “consortium” in periodontitis. We attempted to use a combination of PD, Sulcus Bleeding Index and gingival withdrawal with PD alone. However, no better correlation was observed (data not shown). Although Pi expression showed a significant correlation with PD, it was very weak ().
Table 2. Correlation coefficient for different periodontal pathogens or probing depth.
Diagnostic potential of pathogens
We created ROC curves for pathogens (). The AUC for expression of Pg, Td, Tf and Pi was 0.6847 (p < 0.01), 0.7113 (p < 0.01), 0.7973 (p < 0.01) and 0.6437 (p < 0.01), respectively. Hence, these pathogens might have good diagnostic potential. ROC curves indicated that Tf expression showed the best accuracy for PD.
Figure 3. ROC curves were constructed to evaluate the diagnostic potential of periodontal pathogens for periodontitis (A–D).
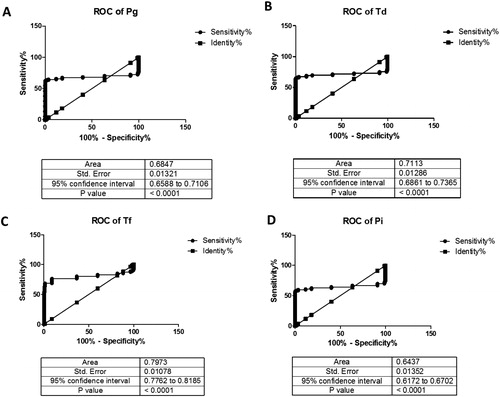
Taken together, our study may provide a possible mechanism for the pathophysiology of periodontitis. It may also help dental clinicians to evaluate periodontitis and aid management planning. Analyses of the critical level of each bacterial pathogen might facilitate further investigation of the role of each pathogen in the underlying pathogenesis and progression of periodontitis.
In addition, although most pathogens have been eliminated at the superficial dental calculus, the discovery of the pathogens remains a challenge [Citation20]. The total bacterial number in the severe-periodontitis group and moderate-periodontitis group was not increased more than that observed in HCs. However, we found that the red complex increased with the development of periodontitis. Hence, specific pathogens have an important role in the progression of periodontitis and also indicates they may be developed as important biomarkers for periodontitis.
In this study, we first reported the pathogens associated with periodontitis in patients at Shenzhen, China. Since different cultures and life styles may affect oral microbes and diseases, our results also indicated that the pathogens associated with periodontitis in patients at Shenzhen, China did not show specific regional disparity. Our data further enrich clinical data for oral microbes and periodontitis in the word since the bacterial study of periodontitis is still a challenge.
Conclusions
We found a close correlation between relative expression of Pg, Td and Tf and PD. Measuring expression of target bacteria in the oral cavity could facilitate understanding of the role of bacteria in periodontitis. Taking the appropriate steps to eliminate these pathogens might aid prevention of the development of periodontitis.
| Abbreviations | ||
| Aa | = | Actinobacillus actinomycetemcomitans |
| Ct | = | Cycle threshold |
| Pg | = | Porphyromonas gingivalis |
| Pi | = | Prevotella intermedia |
| Tf | = | Tannerella forsythensis |
| Td | = | Treponema denticola |
| Hum P | = | Human ribonuclease P |
| Bact 16S | = | Total bacterial 16S rDNA |
| HCs | = | Healthy controls |
| PD | = | Probing depth |
Supplemental Material
Download PDF (154.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Armitage GC. Periodontal diseases: diagnosis. Ann Periodontol. 1996;1(1):37–215.
- Mazza JE, Newman MG, Sims TN. Clinical and antimicrobial effect of stannous fluoride on periodontitis. J Clin Periodontol. 1981;8(3):203–212.
- Renvert S, Persson GR. Patient-based assessments of clinical periodontal conditions in relation to alveolar bone loss. J Clin Periodontol. 2004;31(3):208–213. [pii].
- Bjorn H, Holmberg K. Radiographic determination of periodontal bone destruction in epidemiological research. Odontol Revy. 1966;17(3):232–250.
- Hirschfeld L, Wasserman B. A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol. 1978;49(5):225–237.
- Lovegrove JM. Dental plaque revisited: bacteria associated with periodontal disease. J N Z Soc Periodontol. 2004;87:7–21.
- Löe H. The role of bacteria in periodontal diseases. Bull World Health Organ. 1981;59(6):821–825.
- Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67(10):1041–1049.
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–144.
- Bosshardt DD. The periodontal pocket: pathogenesis, histopathology and consequences. Periodontol 2000. 2018;76(1):43–50.
- Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34(1):9–21.. [pii].
- Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions –introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45(suppl 20):S1–S8.
- Silness J, Loe H. Periodontal disease in pregnancy. ii. correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22(1):121–135.
- Gholizadeh P, Pormohammad A, Eslami H, et al. Oral pathogenesis of Aggregatibacter actinomycetemcomitans. Microbial Pathogenesis. 2017;113:303–311.
- Mysak J, Podzimek S, Sommerova P, et al. Porphyromonas gingivalis: major periodontopathic pathogen overview. J Immunol Res. 2014;2014:1–8.
- Travis J, Pike R, Imamura T, et al. Porphyromonas gingivalis proteinases as virulence factors in the development of periodontitis. J Periodontal Res. 1997;32(1):120–125.
- Sela MN. Role of Treponema denticola in periodontal diseases. Crit Rev Oral Biol Med. 2001;12(5):399–413.
- Mahalakshmi K, Krishnan P, Chandrasekaran SC. Detection of Tannerella forsythia bspA and prtH genotypes among periodontitis patients and healthy subjects – A case–control study. Arch Oral Biol. 2018;96:178–181.
- Nomura Y, Takeuchi H, Okamoto M, et al. Chair-side detection of Prevotella intermedia in mature dental plaque by its fluorescence. Photodiagn Photodyn Ther. 2017;18:335–341.
- Mombelli A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol 2000. 2018;76(1):85–96.
