Abstract
Reducing the Cd content in crop plants is an important process for ensuring the safety of food made from the crop. In this study, the CMV△2b vector was used to express the PvSR2 gene to reduce the Cd content of Nicotiana benthamiana plants. The infectious clone plasmid pCB301-CMVFny209△2b-PvSR2 was successfully constructed. N. benthamiana plants at six leaf stages were inoculated with plasmid-Agrobacterium (GV3101) containing pCB301-CMVFny209△2b-PvSR2, pCB301-CMVFny109 and pCB301-CMVFny309 by agroinfiltration. After the virus had been detected in the non-inoculated leaves of the plant inoculated with plasmid-agrobacterium corresponding to each treatment, the plant of each treatments in the experiment were inoculated with sap of plant leaves containing the virus. After 8 days, the plants were treated with Cd++ solution. The Cd content of the plant samples was analyzed via inductively coupled plasma atomic emission spectrometry. The results show that the plants expressing the PvSR2 gene had greatly reduced Cd++ content than the control and grow normally. These results indicated that using the attenuated recombinant virus CMV△2b containing the PvSR2 gene significantly enhanced the resistance of the plant to Cd++. This is a new approach to protect tobacco plants from Cd contamination by using the attenuated recombinant virus CMV△2b containing the PvSR2 gene.
Introduction
Heavy metal pollution is a serious problem worldwide [Citation1,Citation2]. Heavy metals normally accumulate in the root, stem and leaves of the plant, and they are harmful for plant growth and human health [Citation3–5]. Many crops are polluted by heavy metal from soil, such as rice [Citation5,Citation6], vegetables [Citation6,Citation7] and tobacco [Citation8]. The Agriculture Organization and World Health Organization [Citation9,Citation10] established a provisional tolerable Cd intake for humans and maximum Cd levels in food crops [Citation9]. In the Hunan province of China, Cd contamination is one of the most serious problems of heavy metal pollution. The Cd content from the rice and vegetables from some farmers in some districts of Hunan province, China is not consistent with China’s and FAO’s standards [Citation6,Citation11]. Cd contaminant is a serious threat for human health and influences the income of the farmers. Reducing the Cd++content in crops is important for the safety of the foods made from the crops.
Some genes confer resistance to heavy metals. Overexpressing the AtPDR12 gene significantly reduces the Pb content in Arabidopsis plants [Citation12]. Plants overexpressing the AtPDR8 gene had lower Cd or Pb contents than the wild-type plants [Citation13]. The OsHMA3 gene limits the translocation of Cd from the roots to the above-ground tissues by selectively sequestrating Cd in the root vacuoles [Citation14]. The mutant 1 and mutant 2 of OsNramp5 from paddy greatly reduce the Cd content of straw and rice grain [Citation5]. The resistance genes of plants are safe for human. Some plant resistance genes are also safe for plants. For example, the mutant 1 and mutant 2 alleles of OsNramp5 from paddy have no influence on the growth of plants [Citation5]. The regulation of OsLCT1 enables the generation of ‘low-Cd rice’ without negatively affecting other agronomical traits [Citation15]. The PvSR2 gene was isolated from bean plant (Phaseolus ulgaris L.) [Citation16,Citation17]. The PvSR2 gene can enhance the resistance of the bacterial strain DH5a to Cd [Citation16] and reduce theCd content in PvSR2 transgenic tobacco seedlings exposed to Cd solution [Citation16,Citation18] but without total reversion of injury from Cd [Citation18].
Plant viruses can continuously exist in the living plant. The generation of plants overexpressing resistance genes using attenuated virus-based vectors can enhance the resistance of plant. The attenuated virus ZYMV AGII-Bar can facilitate weed control in cucurbits and can be applied during field crop production [Citation19]. However, the use of an attenuated virus-based vector to express a resistance gene to reduce the Cd content in tobacco plants was not reported. The CMV△2b vector has been developed [Citation20,Citation21]. CMV△2b is an attenuated virus and is used to express medicinal proteins in plants.
In this paper, the PvSR2 gene was expressed in the Nicotiana benthamiana plant using the attenuated CMV△2b virus, and the effects of PvSR2 overexpression on the resistance of the N. benthamiana plant to Cd were examined.
Material and methods
Plasmid construct
The infectious clone plasmids pCB301-CMV Fny109, pCB301-CMV Fny 209 and pCB301-CMV Fny 309 were from our laboratory, where these plasmids were constructed according to YAO’s methods [Citation21]. The pCB301-CMV Fny 209△2b-MCS vector was constructed according to Matsuo’s and YAO’s methods [Citation19,Citation20]. Individually, the sequence of the 2b gene of CMV Fny 209 was deleted and replaced by the sequence ‘agaaggcctgacgcgtgactagtgggcccgggatcc’, which introduces both a termination codon in the 2a gene and a multi-cloning site (Stu I, Mlu I, Spe I, Apa I, BamH I).
The sequence of the PvSR2 gene is from the Genebank (ID: U54704). The ORF of the PvSR2 was synthesized by Thermor Fisher Scientific (China) Co., Ltd. and amplified with a pair of primers (MluPvSR2F: 5′CGACGCGTATGCGTTGCGCCATCCTCT-3′; BamPvSR2R: 5′CGGGATCCTCAATTTCCACTGAAGACTTTG-3′) and pyrobest Taq (Takara company, Dalian, China). The PCR products and the plasmid pCB301-CMV Fny 209△2b-MCS were digested with Mlu I and BamH I. The two digestion products were ligated with DNA ligase. The PvSR2 gene was inserted into the cloning site using the Mlu I and BamH I sites in pCB301-CMV Fny 209△2b-MCS. The construct pCB301-CMV Fny209△2b-PvSR2 was transformed into Escherichia coli DH5α, and it was then extracted and confirmed by PCR and sequencing identification.
Plant culture, inoculation and RT-PCR analysis
Three germinant seeds of N. benthamiana were sowed in separate earthen bowls, and the seedlings were grown in a plant growth chamber under 16 h of light at 25 °C. The infectious clone plasmids were transformed into Agrobacterium tumefaciens GV3101. A few of plants were inoculated at the 4-6 leaf stage by agroinfiltration with plasmid-Agrobacterium corresponding to each treatment in the experiment. After the virus had been detected in the non-inoculated leaves of the plant inoculated with plasmid-Agrobacterium, the plants of each treatment in the experiment were inoculated with sap of plant leaves containing virus corresponding to each treatment, except for the control. The treatments in the experiment were as follows:
Treatment A: pCB301-CMVFny109+ pCB301-CMVFny309+ pCB301-CMVFny209△2b-PvSR2 (tentatively termed CMV△2b-PvSR2),
Treatment B: pCB301-CMVFny109 + pCB301-CMVFny309 + pCB301-CMVFny209△2b-Bar (tentatively termed CMV△2b-Bar),
Treatment C: pCB301-CMVFny109+ pCB301-CMVFny309+ pCB301-CMVFny209△2b-MCS (tentatively termed CMV△2b),
Treatment D: Control, the leaves of the plant were inoculated with sterile distilled water.
The RT-PCR analyses of the samples were conducted according to the operating instructions of the Reverse Transcriptase M-MLV reagent (Takara Company, Dalian, China). Individually, a pair of primers was designed from the sequence of the 2a gene from CMV Fny 209△2b. The forward primer started with a NcoI digestion site and was named NcoF (primer sequence: 5′ ccatggctgagtttgcctg 3′), and the reverse primer started with a 2bAvrII digestion site and was named 2bAvrR (primer sequence: 5′ ccgaagaaacctaggag 3′). Both primers were from the CMV Fny209△2b sequence. In the first step, the synthesis of single-stranded DNA was conducted. The components of the synthesis reaction consisted of the following: 2 μL of RNA sample, 1 μL of reverse primer (2bAvrR) and 2.5 μL of DEPC treated water. The reaction condition was as follows: denaturation for 10 min at 65 °C. Afterwards, the reaction tube was placed on ice for 5 min, and 0.25 μL of Recombinant RNase Inhibitor, 2 μL of dNTP mixture, 2 μL of 5 × Reverse Transcriptase M-MLV Buffer and 0.25 μl of Reverse Transcriptase M-MLV were added. The reaction continues for 60 min at 42 °C and then for 15 min at 70 °C. In the second step, PCR was conducted. The PCR components consisted of 2 μL of 10x buffer, 13.8 μL of ddH2O, 2 μL of dNTPS, 0.5 μL of forward primer, 0.5 μL of reverse primer, 1 μL of the RT production and 0.2 μL of rTag. The PCR reaction conditions included 3 min at 94 °C, then 35 cycles of 30 s at 94 °C, 30 s at 56 °C and 80 s at 72 °C, with a final 8 min incubation at 72 °C. The third step, electrophoresis of the PCR products, was used to check if there is the proper band.
Cd treatment, analysis of the Cd content from plant straw and leaves and data analysis
Cd treatment was conducted after the plants had been inoculated with the infectious clone plasmids for 8 days. A total of 10 mg/L of Cd++ solution was prepared from CdCl2. Then, 50 mL of Cd++ solution was evenly poured on the surface of the soil in each earth bowl in the experiment. The Cd++ concentration in the soil was required to change to 2.5 mg/kg soil.
Cd content was assessed in the straw and leaves of the plants. Samples of plant straw and leaves were collected after Cd treatment had been conducted for 15 days; the samples were dried to a constant weight at 60 °C in a baking box with an air-blower. The samples were analyzed using inductively coupled plasma atomic emission spectrometry (ICP-OES) on a spectrophotometer Agilent 720(Agilent Technologies, Inc.) at the Institute of Subtropical Agriculture, Chinese Academy of Science. The data were analyzed using the DPS Data Procession System (DPS) [Citation22].
Results and discussion
Construction of the pCB301-CMVFny209△2b-PvSR2 plasmid
To express the PvSR2 gene in a tobacco plant using the attenuated CMV△2b virus, the plasmid pCB301-CMV Fny 209△2b-MCS was constructed by deleting the 2b gene sequence in pCB301-CMV Fny 209. Next, the pCB301-CMVFny209△2b-PvSR2 plasmid was constructed using pCB301-CMV Fny 209△2b-MCS and the PvSR2 gene sequence. These plasmid constructs were confirmed to be successful via PCR and sequencing identification. The scheme of the construction is shown in .
The attenuated CMV△2b virus and PvSR2 gene expression in the tested plants
To confirm that the attenuated CMV△2b virus and the PvSR2 gene were expressed in the tested plants, RT-PCR analysis of the tested plants was used. A pair of primers was used for the RT-PCR in all of the treated samples (CMV△2b, CMV△2b-bar, CMV△2b-PvSR2 and control) from the tested N. benthamiana plants. The results of the analysis showed that bands were produced by the PCRs for every sample that were consistent with the predicted band sizes (CMV△2b treatment, 800 bp; CMV△2b-bar treatment, 1350 bp; CMV△2b-PvSR2 treatment, 1300 bp; control, 0 bp) (). The results indicated that the CMV△2b virus, the bar gene and the PvSR2 gene were expressed in the treated plants.
Figure 2. The results of the RT-PCR analysis of the attenuated CMV△2b virus and PvSR2 gene expression in the tested plants. Line 1, H2O; Line 2, Control; Line 3, CMV△2b; Line 4, Marker; Line 5, CMV△2b-PvSR2; Line 6, CMV△2b-bar.
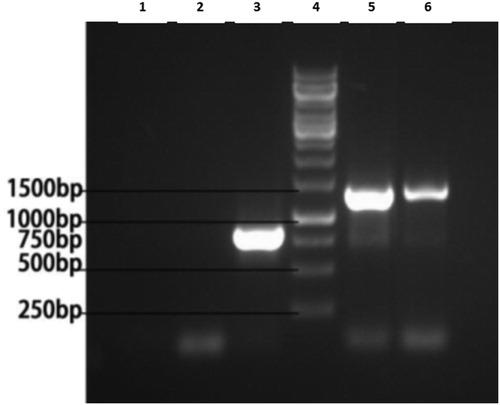
In this study, a pair of primers from the sequence of CMV F209 was used for the RT-PCR in all of the treated samples. The RT-PCR analyses are simple, effective and sufficient to identify if the attenuated CMV△2b virus and the PvSR2 gene were expressed in the tested plants because the technique avoids the false positive results gives comparable results.
The PvSR2 gene reduced the Cd content in the plants
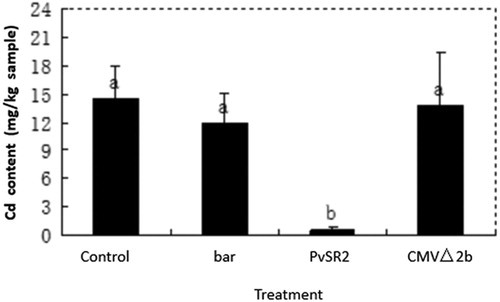
To determine if the PvSR2 gene can reduce the Cd content in the plant, we designed an experiment in which N. benthamiana plants were inoculated with CMV△2b-PvSR2 by agroinfiltration. The experiment included 4 treatments: CMV△2b, CMV△2b-bar, CMV△2b-PvSR2 and control. After the N. benthamiana plants were inoculated with CMV△2b, CMV△2b-bar, CMV△2b-PvSR2 and H2O for 10 days, the plants were uniformly treated with Cd++ (2.5 mg Cd++/kg soil). Samples from the plants were analyzed via ICP-OES. The results showed that the Cd content in the plants treated with CMV△2b-PvSR2 were significantly lower than those of the other treatments (such as CMV△2b, CMV△2b-bar and control) (). There were great differences between the Cd levels in N. benthamiana plants treated with CMV△2b-PvSR2 compared to those receiving the other treatments. The average Cd content of the N. benthamiana plants treated with CMV△2b-PvSR2 was 0.643 mg Cd++/kg sample. The average Cd contents of the N. benthamiana plants receiving the other treatments ranged from 12.24 to 14.57 mg Cd++/kg sample. The average Cd content of the N. benthamiana plants treated with CMV△2b-PvSR2 was less than 5.25% of those of the N. benthamiana plants receiving the other treatments.
Figure 3. The Cd content in the treated plants. Treatment: Bar, CMV△2b-bar; PvSR2, CMV△2b-PvSR2; Control; CMV△2b. The Duncan’s method was used to test for significance (p < 0.05), indicated by different lowercase letters.
In this study, the growth of tobacco plants expressing the PvSR2 gene was studied under a high concentration of Cd++. It has previously been reported that plants of the Nicotiana genus have high tolerance to heavy metal compounds in soil [Citation23]. In our experiment, when the Cd concentration in the soil was 10 mg/kg soil, the growth of all of the N. benthamiana plants was normal (data not shown). When the Cd concentration in the soil was 1250 mg/kg soil, the growth of the N. benthamiana plants treated with CMV△2b-PvSR2 was normal, but the growth of the plants treated with CMV△2b or CMV△2b-bar and the control were greatly inhibited, and most of the plants were dead ().
Figure 4. The growth of plant treated with high concentration of CdCl2. Treatment: Bar, CMV△2b-bar; PvSR2, CMV△2b-PvSR2; Control; CMV△2b.

It has previously been reported that PvSR2 gene can enhance the resistance of transgenic tobacco seedlings exposed to Cd solution; however, at a Cd concerntration of 0.1 mmol/L, the transgenic tobacco seedlings still lose their green colour and grow much slower than the controls that were not exposed to Cd solution [Citation18], so is not possible to be used to produce tobacco plants. It has not been reported whether the PvSR2 gene can reduce the Cd content of older seedlings or adult plants and protect plant’s growth following Cd contamination. In our study, it was confirmed that using recombinant virus CMV△2b containing the PvSR2 gene can greatly reduce the Cd content of older seedlings and adult N. benthamiana plants and that plants expressing the PvSR2 gene grow normally in soil with between 2.5 to 1250 mg Cd/kg soil. These results indicated that using recombinant virus CMV△2b containing the PvSR2 gene may be a possible approach to protect tobacco plants from Cd contamination.
Most virus vectors are used to express pharmaceutical proteins or RNAi [Citation24,Citation25], including the CMV△2b vector [Citation20]. CMV is a plant virus with a broad host spectrum host. The CMV△2b is an attenuated plant virus, and it can infect plants from the Nicotiana genus without symptoms and lastingly exist in the living plant. The high expression can be obtained when plants are inoculated with the virus vector to express exogenous genes [Citation20,Citation24,Citation25]. It is convenient, effective, rapid and low cost to have seedlings of plant being inoculated with the sap of plant leaves containing virus vector in the field crop production [Citation26].
Some remediation approaches of heavy metal contaminants have been reported [Citation27,Citation28]. One of these approaches is the physical and chemical remediation approach. For example, Li et al. [Citation29] reported that using soil amendments reduced cadmium accumulation in rice by changing Cd distribution in soil. Xie et al. [Citation30] reported that applying the lime amendment reduced the lead accumulation in rice grains. Zhang et al. [Citation31] indicated that three amendments effectively reduced the content of Cd and Ni in different organs of wheat plants. Huang et al. [Citation32] suggested that silicon (Si) fertilizer can effectively alleviate heavy metal toxicity in plants. However, the physical and chemical remediation approaches are mainly applied to wastewater and contaminated soils [Citation27]. The approaches are generally expensive and possibly produce secondary noxious end-products [Citation28]. Compared to physical and chemical remediation approaches, our approach is directly applied to the plant by simple inoculation, and it is low cost and have no secondary noxious end-products.
Another remediation approach is to utilize the microorganisms with ability to remediate and tolerate heavy toxicity [Citation28]. Wang et al. [Citation33] reported that two urease-producing bacteria strains can produce the effects of heavy metal immobilization and can guarantee vegetable safety in situ in heavy metal-polluted farmland. Bacillus megaterium N3 and Serratia liquefaciens H12 significantly reduced the Cd and Pb content in wheat [Citation34]. However, in most cases, microorganism’s proliferation requires some amount of time to reach the necessary population level to remedy the heavy metal contaminated soil, only then can the leaf-edible crops be planted in the remedied field, such as vegetables and tobacco crops. Compared to the microorganisms approach, the leaf-edible crop can normally be planted in the contaminated field when applying the approach in our study; the resistance genes against heavy metals can be delivered into the plants within a short period [Citation26]. The leaf-edible crop is protected from heavy metal contamination.
The third approach is phytoremediation [Citation27,Citation28]. Plants utilized in phytoremediation are the hyperaccumulators with a very high heavy metal accumulation potential [Citation28]. The transgenic plants possessing improved heavy metal accumulation or remediation capacity have been developed [Citation27]. Nanotechnology has also been used in soil remediation. Phytoremediation coupled with nanomaterials can modifyand stabilize a broad category of soil contaminants [Citation28,Citation35]. However, phytoremediation can take up at least one year, and the edible crop cannot be planted in the contaminant soil during remediation. Compared to phytoremediation, transgenic phytoremediation and phytoremediation coupled with nanomaterials, our approach also has the benefit of not requiring waiting for the remediation to be finished to plant the edible crop.
The fourth approach is utilizing the transgenic plant expressing resistance gene against heavy metal [Citation12–18]. The resistance genes against heavy metals were genetically transformed into the plant, then the transgenic plant gained the resistance to heavy metals and is safe in contaminated soil because it significantly reduced the content of heavy metals in the transgenic plant. However, it is necessary to take up to a few years to culture the transgenic plant with resistance gene to heavy metals; if the transgenic plant was planted in the entire contaminated field, the varieties diversity of crop would be lost. In comparison, the approach in our study is convenient and does not require genetic transformation; the resistance genes against heavy metals can be expressed in the plant within a short period by simple inoculation [Citation26]. Regarding the expression level of resistance genes in plants, virus vector systems are superior to the transgenic approach [Citation26]. Each variety of crop can be planted in the contaminated field according to peasant’s expectations, and the varieties diversity of crop will not be affected.
Conclusions
In summary, use of the virus CMV△2b containing the PvSR2 gene is a new method to reduce the heavy metal content of plants, and it is a possible approach to protect tobacco plants from Cd contamination.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Mohite BV, Koli SH, Patil SV. Heavy metal stress and its consequences on exopolysaccharide (EPS)-producing Pantoea agglomerans. Appl Biochem Biotechnol. 2018;186(1):199–216.
- Daghan H, Arslan M, Uygur V, et al. The cadmium phytoextraction efficiency of ScMTII gene bearing transgenic tobacco plant. Biotechnol Biotecnol Equip. 2010;24(3):1974–1978.
- Tsuchiya K. Epidemiological studies on cadmium in the environment in Japan: etiology of itai-itai disease. Fed Proc. 1976;35(12):2412–2418.
- Van HL, Kuraishi S, Sakurai N. Aluminum-induced rapid root inhibition and changes in cell-wall components of squash seedlings. Plant Physiol. 1994;106(3):971–976.
- Ishikawa S, Ishimaru Y, Igura M, et al. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc Natl Acad Sci USA. 2012;109(47):19166–19171.
- Chen H, Yang X, Wang P, et al. Dietary cadmium intake from rice and vegetables and potential health risk: a case study in Xiangtan, southern China. Sci Total Environ. 2018;639:271–277.
- Li X, Li Z, Lin CJ, et al. Health risks of heavy metal exposure through vegetable consumption near a large-scale Pb/Zn smelter in central China. Ecotoxicol Environ Saf. 2018;161:99–110.
- Zhao F, Gao X, Wang Z, et al. Research progress on the distribution, prevention and control method of tobacco heavy metals (in Chinese). Chin Agric Sci Bul. 2013;29(25):69–73.
- Food and Agriculture Organization/World Health Organization. 2010. Joint FAO/WHO Expert Committee on Food Additives, Seventy-Third Meeting, Geneva, 8–17 June 2010. Summary and Conclusions. Available from: www.who.int/foodsafety/publications/chem/summary73.pdf
- Codex Alimentarius Commission. 1995. Codex general standard for contaminants and toxins in food and feed. Available from: http://www.codexalimentarius.net/download/standards/17/CXS_193e.pdf
- Liu X, Tian G, Jiang D, et al. Cadmium (Cd) distribution and contamination in Chinese paddy soils on national scale. Environ Sci Pollut Res Int. 2016;23(18):17941–17952.
- Lee M, Lee K, Lee J, et al. AtPDR12 contributes to lead resistance in Arabidopsis. Plant Physiol. 2005;138(2):827–836.
- Kim DY, Bovet L, Maeshima M, et al. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007;50(2):207–218.
- Ueno D, Yamaji N, Kono I, et al. Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci USA. 2010;107(38):16500–16505.
- Uraguchi S, Kamiya T, Sakamoto T, et al. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc Natl Acad Sci USA. 2011;108(52):20959–20964.
- Zhang Y, Chai T, Dong J, et al. Cloning and expression analysis of the heavy-metal responsive gene PvSR2 from bean. Plant Sci. 2001;161(4):783–790.
- Chai T, Zhang Y, Burkard G. Heavy metal-responsive genes in kidney bean: cloning of cDNAs and gene expression analysis (in Chinese). Acta Photophysiologica Sinica. 1998;24(4):399–404.
- Chai T, Chen Q, Zhang Y, et al. Cadmium resistance in transgenic tobacco plants enhanced by expressing bean heavy metal-responsive gene PvSR2. Sci China C Life Sci. 2003;46(6):623–630.
- Shiboleth YM, Arazi T, Wang Y, et al. A new approach for weed control in a cucurbit field employing an attenuated potyvirus-vector for herbicide resistance. J Biotechnol. 2001;92(1):37–46.
- Matsuo K, Hong JS, Tabayashi N, et al. Development of Cucumber mosaic virus as a vector modifiable for different host species to produce therapeutic proteins. Planta. 2007;225(2):277–286.
- Yao M, Zhang T, Tian Z. Construction of Agrobacterium-mediated cucumber mosaic virus infectious cDNA clones and 2b deletion viral vector (in Chinese). Scientia Agricultura Sinica. 2011;44(14):3060–3068.
- Tang QY, Zhang CX. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013;20(2):254–260.
- Vera-Estrella R, Gómez-Méndez MF, Amezcua-Romero JC, et al. Cadmium and zinc activate adaptive mechanisms in Nicotiana tabacum similar to those observed in metal tolerant plants. Planta. 2017;246(3):433–451.
- Hefferon KL. Plant virus expression vectors set the stage as production platforms for biopharmaceutical proteins. Virology. 2012;433(1):1–6.
- Liu Y, Schiff M, Dinesh-Kumar SP. Virus-induced gene silencing in tomato. Plant J. 2002;31(6):777–786.
- Choi B, Kwon SJ, Kim MH, Choe S, et al. A plant virus-based vector system for gene function studies in pepper. Plant Physiol. 2019;181(3):867–880.
- Kumar S, Prasad S, Yadav KK, et al. Hazardous heavy metals contamination of vegetables and food chain: role of sustainable remediation approaches - a review. Environ Res. 2019;179(Pt A):108792.
- Ojuederie OB, Babalola OO. Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health. 2017;14(12):1504.
- Li S, Wang M, Zhao Z, et al. Use of soil amendments to reduce cadmium accumulation in rice by changing Cd distribution in soil aggregates. Environ Sci Pollut Res Int. 2019;26(20):20929–20938.
- Xie T, Li Y, Dong H, et al. Effects and mechanisms on the reduction of lead accumulation in rice grains through lime amendment. Ecotoxicol Environ Saf. 2019;173:266–272.
- Zhang JJ, Zhu SG, Zhu LN, et al. [Effects of different amendments on fractions and uptake by winter wheat in slightly alkaline soil contaminated by cadmium and nickel]. Huan Jing Ke Xue. 2020;41(1):460–468.
- Huang H, Rizwan M, Li M, et al. Comparative efficacy of organic and inorganic silicon fertilizers on antioxidant response, Cd/Pb accumulation and health risk assessment in wheat (Triticum aestivum L.). Environ Pollut. 2019;255(Pt 1):113146.
- Wang T, Wang S, Tang X, et al. Isolation of urease-producing bacteria and their effects on reducing Cd and Pb accumulation in lettuce (Lactuca sativa L.). Environ Sci Pollut Res Int. 2020;27(8):8707–8718.
- Han H, Wang XY, Cai H, et al. [Isolation of heavy metal immobilizing and plant growth-promoting bacteria and its effects on reducing heavy metal accumulation in wheat] . Huan Jing Ke Xue. 2019;40(7):3339–3346.
- Yadav KK, Gupta N, Kumar A, et al. Mechanistic understanding and holistic approach of phytoremediation: a review on application and future prospects. Ecol. Eng. 2018;120:276–298.

