Abstract
The stingless bee Heterotrigona itama honey is generating interest among consumers due to unique antibacterial properties. Despite the substantial health benefits of this honey, its action mode of inhibiting and killing pathogenic bacteria remains unresolved. The present study aimed to unravel the effect of raw H. itama honey against four clinically important bacteria, Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis and Staphylococcus aureus. Total phenolic and total flavonoid contents, alongside antioxidant and antibacterial activities of honey samples were established to identify the ‘best’ raw honey. Honey Sy-1 showing the highest amount of phenolics (80.71 mg GAE/100 g) and antioxidant capacity (82.67%) and, the lowest MIC (1.56% (w/v)) and MBC (3.125% (w/v)), was tested against four bacterial cells, wherein any alterations in cellular morphology were observed under scanning electron microscope. The micrographs revealed significant elongation/filamentation in B. subtilis cells, de-flagellation in P. aeruginosa, membrane perturbation and leakage of intracellular contents in E. coli alongside non-dividing septated cells in S. aureus. H. itama honey inhibited bacterial growth by disrupting the cell membrane in conjunction to interacting with a cytosolic target or possibly interrupted septum formation and blocking DNA and protein syntheses. The evidence strongly supported the natural antibacterial properties of the H. itama honey.
Introduction
One of the factors that primarily distinguishes the Malaysian stingless bee Heterotrigona itama from other honey varieties is the combination of its sour and sweet tastes, with a hint of fruitiness, along with its slightly more viscous texture compared to water. This unique superfood often exists in an array of coloured tones, has lower contents of sugars, glucose and fructose but a higher protein content than honey from sting bees, Apis mellifera [Citation1]. The Malaysian H. itama bee, or commonly known as Kelulut by the locals, is easily distinguishable from the typical yellow black Apis mellifera honeybee. The former has an all-black, relatively smaller and slimmer body, together with a notably longer set of wings. Likewise, the foraging pattern of H. itama bees differs from that of honeybees, with the former producing lesser yields of honey [Citation2].
Farming of stingless bees has become increasingly popular in Malaysia as the species can tolerate seasonal changes and can endure extreme environmental conditions [Citation3]. Aside from the premium honey it produces, as well as being an excellent pollinating agent, beekeepers believed that farming of stingless bees can aid their conservation, in view of their dwindling original habitat due to human activities [Citation4]. According to the Malaysian Agricultural Research Development Institute (MARDI), at least 32 species of stingless bees show high potential for domestication in Malaysia, of which four species, including H. itama, are receiving wider attention for their highly valuable honey. As a matter of fact, honey from H. itama species occupies a considerable portion of the stingless bee honey market compared to other varieties [Citation1,Citation5].
The growing incidence of antimicrobial resistance (AMR) due to various factors has brought about recent massive outbreaks of infectious epidemics, topping as the most complex global health crisis [Citation6]. The World Health Organization (WHO) has since called for urgent corrective actions including expansion of effective antibacterial therapies [Citation7]. Combined with the higher demand for better quality food with consistent qualities, the search for foods naturally rich in bioactive compounds showing broad antibacterial activities has since been stepped up [Citation8]. Studies have shown that raw H. itama honey is a possible candidate for the complementary treatment to combat AMR, following numerous claims of its excellent antibacterial activity against many superbugs [Citation9,Citation10]. It is worth mentioning here that honey-resistant bacterial phenotypes have yet to be reported in the literature. So far, two recent studies showed that raw H. itama honey yielded exceptional antioxidant activity compared to other honey varieties including the popular Manuka honey, a widely used ‘gold standard’ for comparison [Citation9,Citation11]. H. itama honey was previously reported to be effective in reducing obesity in rats [Citation12], probably via the synergistic interplay between the major constituents viz. sugar, phenolics and flavonoids, along with other minor constituents, for example, enzymes, minerals organic acids and amino acids, present in honey.
The bioactivity seen in H. itama honey is also due to the existence of beneficial live probiotic microbiota such as Lactobacillus sp. and Fructobacillus sp., inhabiting the gastrointestinal tract of the bees. These microorganisms are then transferred into honey during the enzymatic transformation of nectar [Citation13]. The bacterial species work in concert with oligosaccharides in honey to provide nourishment, by facilitating the absorption of fatty acids, ions and vitamins through the gut while simultaneously protecting the host from pathogenic bacterial invasion. The ability of H. itama honey to hamper and/or kill pathogenic bacteria has been demonstrated by numerous studies, with the general acceptance that gram-positive bacteria are more susceptible to this honey than gram-negative ones [Citation9,Citation10]. Despite the promising antibacterial properties reported for H. itama honey, to the best of our knowledge, so far there are no studies elucidating the effects of this honey on bacterial cellular morphology. The underlying mechanism of bacterial inhibition at the cellular level by honey remains unexplained in different pathogens.
One approach to address this matter is by the use of instrumental microscopic techniques to view the ultrastructure and morphological changes of bacteria [Citation14]. This is to allow a deeper understanding of the disruptive physical events that occur, as a result of exposure to raw H. itama honey. Therefore, in the present study, we carried out scanning electron microscopy (SEM) to observe cell architecture disruption in four different bacterial species (gram-positive: Bacillus subtilis ATCC 21332, Staphylococcus aureus ATCC 25923 and gram-negative: Escherichia coli ATCC 11775 and Pseudomonas aeruginosa ATCC 27853), after direct exposure to raw H. itama honey. P. aeruginosa, E. coli and S. aureus were used as test organisms following reports of their critical pathogenicity for which new antibiotics are constantly being developed [Citation7]. Meanwhile, the pathogenicity of B. subtilis merits investigation as it is a prevalent spore-forming bacterial species that causes food poisoning [Citation15]. In this study, the obtained micrographs were compared to untreated and streptomycin-treated bacteria.
Since the H. itama honey contains a plethora of antibacterial compounds that work synergistically to impart antibacterial action, it is hypothesised that the multi-compound regimen may target more than one type of cellular disruption, and ultimately inhibition of the test bacteria. Also, each tested microorganism may exhibit a unique response profile to this honey. As far as we know, this is the first study to assess the effect of raw H. itama honey on the tested organisms at the cell structural level. Therefore, we tested four H. itama honey samples from different known geographical origins in Malaysia. Only H. itama honey showing the highest quantity of phenolic compounds, in addition to good antibacterial and antioxidant activity was used in the bacterial morphology study. The four test bacteria exposed to raw H. itama honey were then subjected to SEM analysis.
Materials and methods
H. itama honey collections
To verify the diversity of samples, the present study used four samples of H. itama honey collected from four different established farms, located across different cardinal areas of Peninsular Malaysia. The collection sites spanned the north, south, east and west coast of the country. Each honey sample was withdrawn aseptically from sealed colonies of H. itama hives using a sterilize 25 mL syringe, and then samples were stored in a sterile dark glass recipient at room temperature for subsequent analysis. lists the assigned unique code and information for each sample.
Table 1. Physicochemical profile of four samples H. itama honey.
Quality and antioxidant capacity assessments
pH of each raw honey sample was read using a pH meter (Mettler Toledo S220, Ohio, USA) The phenolic and flavonoid contents of the samples were quantified using a modified Folin-Ciocalteu method [Citation16] and an assay proposed by Isla et al. [Citation17], respectively. The free-radical scavenging activity of raw H. itama honey was assayed using 2,2-diphenyl-1-picryhydrazyl (DPPH), whereas the antioxidant power was assessed by ferric reducing (FRAP) assay, as previously reported [Citation18].
Bacterial strains and growth conditions
Standard strains from American Type Culture Collection (ATCC) comprising of B. subtilis ATCC 21332, S. aureus ATCC 25923, P. aeruginosa ATCC 27853 and E. coli ATCC 11775 were grown on nutrient agar (Oxoid, Hants, United Kingdom) and incubated overnight at 37 °C. The bacterial inoculum was prepared by transferring a selected colony into a 5 mL test tube of sterile distilled water, followed by vortexing for 15 s before the turbidity was adjusted to 108 colony forming units, CFU/mL.
Agar well diffusion assay
Susceptibility of the pathogenic bacterial strains to raw H. itama honey was examined using the agar well diffusion assay. An aliquot of 100 μL of the bacterial suspension was uniformly spread on a nutrient agar using the tip of sterile cotton swab. Then, 8 mm diameter wells were punched aseptically in the nutrient agar using the end of a sterile P1000 tip. Raw H. itama honey was diluted in sterile distilled water to concentrations of 75% and 50% (w/v), while the honey sample diluted in catalase solution at 50% (w/v) was used to observe for non-peroxide activity. Freshly prepared filter-sterilized (0.2 µm) honey mixture was delivered into individual test wells (100 μL), whereas wells containing sterilized distilled water and catalase solution were used as negative controls. Antibiotics consisting of streptomycin, ampicillin and kanamycin prepared at 100 µg/mL were incorporated as positive controls to determine the reproducibility and reliability of the assay, as well as to illustrate the bacterial resistant profiles. All plates were incubated overnight at 37 °C and the produced inhibitory zones were measured in millimetre using a vernier calliper. For the susceptibility test, the results were interpreted as: <6, insignificant activity; 6–10, low activity; 11–15, significant activity; >16, high activity [Citation19].
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The resazurin assay used in this study was modified from a previous protocol by Drummond and Waigh [Citation20], for better accuracy in determining the minimum inhibitory concentration (MIC). The test was performed using aseptically prepared fresh-daily two-fold serial dilutions of honey samples [from 50% to 0.39% (w/v)] in nutrient broth. Bacterial inoculum (50 µL of 108 CFU/mL) was added into each test tube and was incubated for 24 h at 37 °C. Antibiotics, ampicillin, streptomycin and kanamycin, were prepared in the same manner, at a starting concentration of 100 − 0.78 mg/mL. Mueller Hinton (MH) broth containing bacteria was used as the positive control, whereas an uninoculated MH broth was the negative control. After 24 h, all test tubes were centrifuged (16,000 g, 5 min), the supernatant was discarded, and the bacterial pellet collected. The cell pellet was rinsed with sterile saline (0.97% w/v) twice, in order to remove any honey from the tube. This was followed by resuspension of the cell pellet in a 500 µL of nutrient broth. Then, 300-µL aliquots of the mixture were dispensed into a flat-bottom 96-well plate, followed by the addition of 20 µL of resazurin solution (2 mg/mL) and incubation for 1 h at 37 °C. All assays were triplicated, and changes in colour were recorded. Once the incubation was completed, columns with no colour change (blue resazurin) were scored as above the MIC value. The minimum biocidal concentration (MBC) was determined by plating directly the content of wells with concentrations higher than the MIC value onto a MH agar followed by incubation for 2 h at 37 °C. The concentrations causing >99% reduction in bacterial growth were determined. In this study, the experimental data were expressed as mean values with standard deviation (±SD).
Scanning electron microscopy (SEM)
Initially, a glass slide cleaned using acidified alcohol (1% HCl in 70% ethanol) and a drop of poly-l-lysine solution (100 µL) (Sigma Aldrich, Steinhem, Germany) was left to stand for 24 h. Overnight cultures of the bacterial strains were incubated at MIC and MBC of selected H. itama honey for 4 h at 37 °C. An aliquot of 500 µL H. itama honey treated bacterial cells was allowed to adhere to the poly-l-lysine coated glass slide, air dried for 1 h and fixed using 2.5% glutaraldehyde at 4 °C overnight. Next, the glass slide was dehydrated with graded ethanol (70%, 90% and 100%), followed by drying for 2 h using hexamethyldisilazane (HMDS) (Fluka, Castle Hill, Australia). The glass slide was mounted on a carbon adhesive tab and platinum paint was applied under vacuum to assist in sample conductivity. Microscopic analyses of the raw honey treated bacterial cultures were performed on a JEOL variable pressure scanning electron microscope (JSM-IT500, US). Untreated bacteria were used as the control to substantiate any morphological alterations.
Data analysis
For comparison of means, data were subjected to analysis of variance (ANOVA) followed by Tukey's Honest Significant Difference test. All statistical analyses used the SPSS version 24 and Origin software, where differences were considered statistically significant at a p value of less than 0.05. One sample t-test with two-tailed distribution was used to assess the significant difference between i) susceptibility of pathogenic strain at different dilution levels and ii) total activity and non-peroxide activity.
Results and discussion
Physicochemical analysis of H. itama honey
pH value
summarizes the physicochemical features of the four tested H. itama honey samples. All samples were acidic, with values ranging between pH 2.59 and 2.74, lower than those reported by Bakar et al. [Citation11] for H. itama honey samples (pH 3.24 − 3.42) collected from the south of Peninsular Malaysia. The honey samples collected in this study were considerably more acidic than honey produced by Apis mellifera (pH 3.3 to 6.56) from countries such as Italy, Spain, India and Algeria [Citation21–24].
This is consistent with reports showing the pH variability in honey samples being strongly correlated with differences in biomass, geographical foraging area and mineral content of the nectar collected by the bees [Citation25]. Moreover, the microbial flora of the Malaysian H. itama honey tends to harbour different kinds of Lactobacillus (LAB) bacterial species [Citation13] that produce various organic acids that increase the acidity of honey, thus giving the unique sour taste. Most importantly, the low pH of H. itama honey can inhibit the growth of most pathogenic bacteria that grow optimally at pH 4.0 − 4.5. The findings of this study, hence, corresponded well with the better shelf life and antibacterial activity of raw H. itama honey [Citation26].
Phenolic and flavonoid content
The Malaysian H. itama honey samples assessed in this study exhibited a broad ranging phenolic content, largely between 52.71 − 80.71 mg GAE/100 g, and the results were similar to that reported for stingless bee honey from Northern Brazil (1.30 − 66.0 mg GAE/100 g) [Citation27]. The ohenolic content in the four H. itama honey samples observed in this study was considerably higher than that in acacia honey from Romania (2 − 39 mg GAE/100 g) and Slovenia (4.48 mg GAE/100 g), but lower than the Malaysian Tualang (110.394 mg GAE/100 g honey), Gelam (159.743 mg GAE/100 g honey) and acacia honeys (196.5 mg GAE/100 g honey) [Citation28]. This was expected, as the high variation in phenolic content correlated well with the different geographical and botanical origins of the H. itama honey samples. Likewise, the selective floral behaviour of the bees, alongside their longer and earlier foraging time were also contributing factors. The small body size of the H. itama bee is another contributing factor that influenced their foraging capability for nectar [Citation29]. Literature has shown that ellagic (28.3%) and benzoic (25.0%) acids are the dominant phenolic compounds in raw H. itama honey, followed by hesperetin (12.3%) and rutin (5.7%) [Citation29].
Flavonoids that impart aroma and antioxidant properties in raw H. itama honey are typically found as glycosides in nectar. The compounds are then are hydrolysed in the bee stomach as aglycones before transformation into honey [Citation22]. Iurlina et al. [Citation30] reported that flavonols (45% quercetin) were the predominant flavonoids in raw honey samples. The flavonoid content, measured as quercetin equivalents (QE), of the four raw H. itama honey samples broadly ranged from 29.17 to 91.23 mg QE/100 g of honey, averaging at 54.48 ± 26.98 mg QE/100 g. Remarkably, the highest flavonoid content was observed in the Sy-4 honey followed by Sy-1, Sy-3 and Sy-2.
Antioxidant activity
The four Malaysian H. itama honey samples showed increasing radical-scavenging ability with increasing honey concentration, whereby Sy-1 exhibited the highest inhibition at 82.67% for 60 mg/mL, corresponding to an IC50 of 21.63 ± 0.66 mg/mL, followed by Sy-4 (26.25 ± 0.50 mg/mL), Sy-3 (31.17 ± 2.83 mg/mL) and Sy-2 (47.92 ± 1.44 mg/mL). Consistently, the percentage inhibition values recorded in the four H. itama honey samples were higher than those in the Algerian honey (44.57% inhibition by 120 mg/mL of honey) [Citation22], thus indicating a stronger antioxidant potential of the Malaysian H. itama honeys. This outcome could be attributed to the multiflora nature of H. itama honey, which has highly blended nectars from different flowers, consequently yielding greater antioxidant properties [Citation31]. Likewise, the corresponding FRAP value of the four honey samples ranged from 385.97 to 624.72 µmol/L Fe(II), with the highest seen in the Sy-1 honey followed by Sy-4, Sy-3 and Sy-2.
Zones of inhibition (ZOI), minimum inhibitory concentration (MIC) and minimum Bactericidal concentration (MBC)
The results of the agar-well diffusion assay on the four Malaysian H. itama honey samples displayed broad-spectrum antibacterial activity (). On average, the inhibition zones of undiluted honey against gram-positive bacteria were 22.8 ± 2.6 mm, whereas the inhibition zones of gram-negative bacteria were considerably smaller at 15.9 ± 5.6 mm. The study found that the H. itama honey samples were significantly more effective in inhibiting gram-positive bacteria than gram-negative ones (p < 0.05). The gram-positive B. subtilis was the most susceptible (23.3 ± 2.9 mm) followed by S. aureus (22.3 ± 2.9 mm), whereas gram-negative bacteria, for instance, E. coli (20.5 ± 1.7 mm) and P. aeruginosa (10.25 ± 1.9 mm), were less affected. This can be correlated to the outer membrane of E. coli and P. aeruginosa, which provides greater resistance to morphological changes caused by raw H. itama honey. The unique structural components in gram-negative bacteria essentially hinder access and prevent the bioactive compounds from disrupting the cell wall. The lipopolysaccharide outer membrane of gram-negative bacteria consisting of lipid A as the core polysaccharide, and the O-side chain, is likely responsible for their higher resistance to inhibition by raw H. itama honey. Conversely, the 90–95% peptidoglycan-based cell wall of gram-positive bacteria permits easier entry of the hydrophobic molecules found in raw H. itama honey into the bacteria [Citation32]. However, the differences in the size of inhibition zones between the gram-positive S. aureus and gram-negative E. coli were not significantly different (p > 0.05). Since H. itama honey inhibited both gram-staining groups of bacteria, it was indicative of a broad-spectrum antibacterial activity. Likewise, the bioactivity of some constituents in the raw honey may be organism specific. However, more bacterial strains from both gram groups must be tested to validate this assumption.
Table 2. Mean zone of inhibition (mm) determined by agar well diffusion method.
In general, the largest inhibition zones were observed when raw H. itama honey samples were applied undiluted to the test bacteria. However, the difference in the sizes of inhibition zones between undiluted and diluted honey at 75% (w/v) were found non-significant (p < 0.05). It is presumed that the glucose oxidase enzyme in the H. itama honey samples was instrumental in liberating hydrogen peroxide from a two-phase colloidal structure that is made up of multiplexes of micron-sized proteins, polyphenols and oligosaccharides [Citation33]. Unlike raw H. itama honey diluted at 50% (w/v), the presence of additional water permits the unpacking and dissociation of the large, micro-size, superstructures into smaller size particles, specifically at the threshold concentration of the molecular crowding [Citation33]. This in turn, produced a less active glucose oxidase which contributed to the significant difference (p < 0.05) in antibacterial activity in the 50% (w/v) diluted H. itama honey over the undiluted one. Based on the findings, consumption of H. itama honey at 75% (w/v) will offer the same therapeutic benefits as that consuming the undiluted honey.
Notably, all four H2O2-neutralized H. itama honey samples remained active after treatment with catalase (p > 0.05), suggesting the possible involvement of bioactive compounds other than H2O2. According to Allen et al. [Citation34], non-peroxide antimicrobial activity in Manuka honeys were either of floral origin, largely derived from Leptospermum species or due to the existence of methylglyoxal. Perceptively, this study believed that the non-peroxide antibacterial activity seen in the Malaysian H. itama honey samples came from cerumen pots that were used to store the honey. The pots are constructed from wax and propolis, both of which are rich in plant-derived antimicrobial compounds, unlike honey from the Apis bee species, which is stored in wax-constructed brood combs [Citation35]. Likewise, the uniquely high content of benzoic acids in H. itama honey can produce peroxyacids upon reaction with hydrogen peroxide [Citation29]. The more stable peroxyacids are not destroyed after catalase treatment prior to antibacterial analysis [Citation36,Citation37]. This explained the retention in bioactivity of the tested H. itama samples. Moreover, peroxyacids are highly active in a low pH environment [Citation36,Citation37], a typically observed feature of the H. itama honey (pH: 2.59 − 2.74) tested in this study.
It was apparent that the Sy-1 honey exerted the strongest antibacterial activity, with an overall mean diameter of inhibition zones of 22 ± 6.2 mm, followed by Sy-3 (19 ± 6.2 mm), Sy-4 (17.8 ± 6.1 mm) and Sy-2 (17.5 ± 6.0 mm). The differences were statistically significant (p < 0.05), affirming the high radical scavenging activity that originated from the high concentration of phenolics in the H. itama honey samples. Presumably, the observed inhibition was due to production of hydroxyl radicals from H2O2 that acted as the substrate in a polyphenol mediated Fenton-like reaction.
In this study, the MIC value was established by visual inspection method according to NCCLS M27-A2. Results for honey susceptibility testing are usually subjective and less reproducible, as the strong colour of honey tends to obscure judgement of turbidity levels. Thus, the present study resorted to a modified colorimetric resazurin assay, which demonstrated good reproducibility and yielded a more clear-cut MIC endpoint. The resazurin assay is popular for toxicity and biofilm screening, as well as for determining MIC of variety of plant compound [Citation38]. As far as we know, this is the first study reporting on MIC of H. itama honey.
enlists the MICs of the four H. itama honey samples tested against four bacteria. The average MICs calculated for gram-positive bacteria were 3.91% ± 2.05 (w/v), whereas a 5.47% ± 1.45 (w/v) average was recorded for gram-negative bacteria. The statistically significant strong inhibitory effect of Malaysian H. itama honey seen here on gram-positive bacteria (p < 0.05) concurred with our earlier findings using the agar well diffusion test. The Sy-1 honey was the most effective against B. subtilis and S. aureus, as seen from the lowest MIC of 1.56% (w/v), compared to 6.25% (w/v) for Sy-2, and 3.125% (w/v) for Sy-3 and Sy-4, respectively. The MICs and MBCs established for all samples in this study were either comparable or only varied by one dilution factor, thus verifying the potent bactericidal effect of the four Malaysian H. itama honey samples.
Table 3. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of H. itama honey and antibiotics determined by resazurin assay.
For comparison, the antibiotic tests revealed that streptomycin showed stable antibacterial activity against all tested bacteria. Ampicillin was most inhibiting on P. aeruginosa, but weakly inhibited E. coli, whereas kanamycin imparted the weakest inhibition against all bacterial strains except for E. coli. Therefore, it was deduced that bacterial inhibition caused by raw H. itama samples seen in this study varied in terms of antibiotic resistance and the response was not associated to the Gram-group.
Morphological changes of bacterial cells
To uncover the cellular targets of H. itama honey, scanning electron microscopy (SEM) was used. SEM micrographs can provide clear visuals on the effect of raw H. itama honey on target cell structures [Citation39]. In this study, exponentially growing bacterial cells were tested as they are more vulnerable to antibacterial compounds [Citation40].
Characteristically, B. subtilis growing in broths without H. itama honey appeared as well-separated rods with lengths of 1.88 ± 0.12 µm (). The same was seen for the growth of E. coli () and P. aeruginosa (), evident from their noticeably smooth cell surfaces with average lengths of 2.83 ± 0.12 µm and 1.95 ± 0.11 µm, respectively. The structure of untreated S. aureus cocci-shaped cells captured in seemed intact and healthy, characterized by an evenly distributed cellular contents alongside a closely integrated membrane.
Figure 1. Effect of H. itama honey on the appearance of mid-exponential phase B. subtilis as seen by SEM under 8000× magnification. (A) Control culture of B. subtilis, (B) B. subtilis treated with inhibitory concentration (MIC), (C) B. subtilis treated with bactericidal concentration (MBC), (D) B. subtilis treated with streptomycin. Bar = 2 µm.
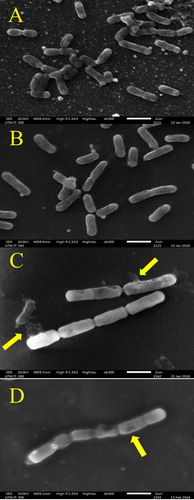
Figure 2. Effect of H. itama honey on the appearance of mid-exponential phase S. aureus as seen by SEM under 8000× magnification. (A) Control culture of S. aureus, (B) S. aureus treated with inhibitory concentration (MIC), (C) S. aureus treated with bactericidal concentration (MBC), (D) S. aureus treated with streptomycin. Bar = 2 µm.
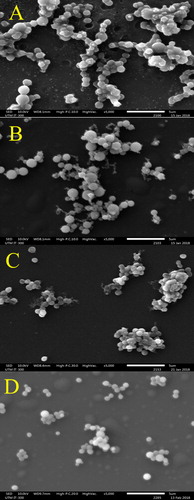
Figure 3. Effect of H. itama honey on the appearance of mid-exponential phase E. coli as seen by SEM under 8000× magnifications. (A) Control culture of E. coli, (B) E. coli treated with inhibitory concentration (MIC), (C) E. coli treated with bactericidal concentration (MBC), (D) E. coli treated with streptomycin. Bar = 2 µm.
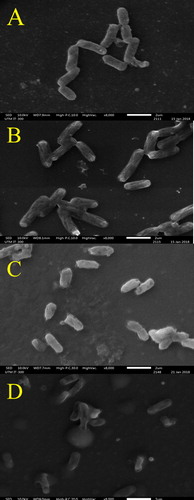
Figure 4. Effect of H. itama honey on the appearance of mid-exponential phase P. aeruginosa as seen by SEM under 8000× magnifications. (A) Control culture of P. aeruginosa, (B). P. aeruginosa treated with inhibitory concentration (MIC), (C). P. aeruginosa treated with bactericidal concentration (MBC), (D) P. aeruginosa treated with streptomycin. Bar = 2 µm.

However, the morphological structures of the bacterial cells were markedly altered after a 4 h incubation in raw H. itama honey at MIC. Although the cells of B. subtilis (), E. coli () and P. aeruginosa () seemingly retained their rod-shape, the cell lengths were reduced, hence implying cell shrinkage. The micrographs (8000× magnification) of each bacterial culture revealed a coarser surface of the bacterial cell membrane with pronounced collapse of cell components in the cytoplasm. The increased distance between the cytoplasmic membrane and outer membrane in the honey treated P. aeruginosa cells (314.78 ± 29.50 nm) when compared to untreated ones (170.76 ± 6.46 nm) was distinctive of a detach cell envelope (). The cellular changes were likely due to the higher external sugary concentration (in the broth) in the presence of raw H. itama honey that imparted a high osmotic effect. This caused the osmotic pressure to drop across the cell envelope that consequently led to retraction of the cytoplasmic membrane with a concomitant outflow of water molecules from the interior of the bacterial cell. Shrinkage in honey-treated E. coli cells was similarly reported by Oliveira et al. [Citation41]. Detachment of bacterial cell envelope was probably caused by an extended periplasmic space [Citation42], which often occurs if an antibacterial agent triggers the release of endotoxin or β-lactamase. As a matter of fact, Brudzynski and Sjaarda [Citation38] successfully demonstrated that Canadian honey induced cell wall damage in bacteria as a result of liberated endotoxin.
It is worth mentioning here that cell shrinkage was more pronounced in gram-negative bacteria, E. coli () and P. aeruginosa (), as compared to gram-positive, B. subtilis (). This observation correlated well with the higher MICs of E. coli and P. aeruginosa (6.25%) than B. subtilis (3.125%). A higher MIC value meant that the bacteria were exposed to higher quantities of antibacterial compounds from raw H. itama honey, thus intensifying cellular disruptions. Moreover, negatively charged phenolic compounds in H. itama honey (due to presence of hydroxyl groups, -OH) tend to bind to the divalent cations, i.e. magnesium (Mg2+) stabilized outer membrane of gram-negative bacteria by electrostatic interactions and, consequently chelate the ions. This would have disrupted the lipid–protein interaction and destabilized the membrane structural integrity. Membrane permeability was increased, enabling entry of more antibacterial compounds [Citation43] from H. itama honey, in addition to accelerating leakage of intracellular constituents into the surrounding broth.
Exposure to MBC of H. itama honey led to more serious alterations in the tested bacterial cells, characterised by the presence of a higher amount of debris in the SEM micrographs. B. subtilis cells exposed to MBC of H. itama honey for 4 h were abnormally long filamentous in shape, similar to cells that had been treated with streptomycin. The lengths of honey-treated and streptomycin-treated B. subtilis cells were approximately 12.23 µm and 12.34 µm, respectively, almost four times longer than healthy ones. The exception was that the honey-treated bacterial cells remained intact and connected to each other.
Survey of the literature described that filamentation is normally observed when rod-shape bacteria produce peptidoglycan for their lateral wall but failed to do so for septal wall during growth [Citation44]. This failure could be attributed to the inhibition of penicillin binding protein 3 (PBP3) essential for cross-linking peptidoglycan at the septal wall but not the lateral wall [Citation45,Citation46]. As a result, the cells elongate without division, as seen in the raw H. itama honey treated bacterial cells. Noguchi et al. [Citation46] observed a similar outcome on β-lactam treated cells of E. coli and P. aeruginosa. Filamentation of bacterial cells has been reported when DNA synthesis mediated by a process known as SOS response, is interrupted or damaged. Typically, this process halts further septum formation until the DNA is repaired, thereby the damaged DNA is not transferred to daughter cells [Citation47]. Hence, it was not surprising to find septa-lacking cells in B. subtilis treated at MBC of the raw H. itama honey (). Similar morphological changes are observed when formations of EzrA and GpsB membrane proteins are interrupted, as both proteins are intimately involved in co-ordinating the formation of peptidoglycan in the lateral septal cell wall during cell cycle progression [Citation48]. Brudzynski et al. [Citation49] successfully showed that polyphenols from honey were responsible for bacterial DNA degradation. However, the class of polyphenols responsible for such disruption remains to be elucidated.
For the gram-negative E. coli, H. itama honey at MBC reduced the viability of the initial bacterial count (±130 bacterial colonies per plate) by ≥99.9% and produced bacilli with less-defined capsules or spheroidal cells. The cells were considerably shorter, in the range of 1.81 ± 0.14 µm, which is almost half of the original length of healthy E. coli cells (2.83 ± 0.12 µm). In this study, the term ‘spheroidal cells’ refers to cells that have lost their peptidoglycan layer but retained their outer membrane. In this case, the membrane permeability in E. coli seen here was likely triggered at MBC of raw H. itama honey. Lacking the rigid, shape-determining structure and membrane tension caused the bacteria to acquire a spherical shape. Taking these considerations into account, we propose that a higher concentration of the tested H. itama honeys can better inhibit bacterial cell wall synthesis in the four tested bacteria, perhaps by direct binding to the PBP1A protein responsible for cell shape and deactivation of transpeptidase activity. A few types of organic acids with such deactivating capabilities have been identified in raw H. itama honey, with gluconic acid as the major common component [Citation50]. The acid is produced by oxidase activity from glucose in the presence of water and oxygen. It is possible that the acids can intercalate into the phospholipid membrane of pathogenic bacteria, decrease the intracellular pH and interact with the bacterial cellular constituents, thereby causing cell death. This was evident from the remaining very few intact bacterial cells and strikingly large quantities of debris seen in this study ().
The presence of numerous septated coccoid cells of S. aureus at MIC () were observed. Septa were also seen at MBC but with larger and more noticeable clumping cells (). This was consistent with a report by Jenkins et al. [Citation51] describing the ability of raw honey to inhibit the normal cell division of S. aureus. The mechanism of inhibition begins with the disruption of hydrolytic activity of murein, causing a build-up of septated non-dividing cells, as observed in the H. itama treated bacterial cultures (). Also, the highly acidic nature of the H. itama honey itself can denature and alter protein and fatty acids involved in cell wall synthesis, as described by a previous study [Citation52]. De-flagellation of P. aeruginosa cells seen at MBC () proved that high concentrations of H. itama honey can suppress flagellin-associated genes, thus reducing bacterial pathogenicity, as similarly reported for P. aeruginosa cells exposed to Manuka honey [Citation53]. It is important to note that bacterial flagella are needed for adhesion, the initiation of infection and biofilm establishment. Roberts et al. [Citation53] discovered that the production of major structural flagellin was coincidently suppressed after treatment with Manuka honey. Nevertheless, these are hypotheses that warrant further proteomic and genomic studies to fully comprehend the action of the antibacterial compounds in the Malaysian H. itama honey.
Conclusions
This study details some important insights into the effects of raw Malaysian H. itama honey on bacterial morphology based on SEM analysis. Similar to previous findings, raw H. itama honey was less effective against gram-negative bacteria particularly P. aeruginosa, which in part, was due to poorer permeability of the gram-negative bacterial outer membrane for the bioactive compounds. The observed altered membrane and cellular protein morphology, membrane perturbation, leakage of bacterial cell contents and cell lysis evidenced from SEM micrographs seen in this study, were indicative of raw H. itama honey mode of action which targeted those sites on bacteria. In future, additional experiments involving in vivo models may prove helpful in validating cellular alteration actions of H. itama honey of gram-positive and gram-negative bacteria.
Ethical standards
This article does not contain any studies with human or animal subjects.
Informed consent
There is no patient care involved in this article.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Se KW, Ibrahim RKR, Wahab RA, et al. Accurate evaluation of sugar contents in stingless bee (Heterotrigona itama) honey using a swift scheme. J Food Compos Anal. 2018;66:46–54.
- Basari N, Ramli SN, Khairi N. Food reward and distance influence the foraging pattern of stingless bee. Heterotrigona Itama Insects 2018; 9(4):138.
- Ismail MM, Ismail W. Development of stingless beekeeping projects in Malaysia. In: E3S Web of Conferences. EDP Sciences; 2018. p. 28.
- Kelly N, Farisya MSN, Kumara TK, et al. Species diversity and external nest characteristics of stingless bees in meliponiculture. Pertanika J Trop Agric Sci. 2014;37(3):293–299.
- Zulkhairi AFA, Sabri S, Ismail M, et al. Probiotic properties of Bacillus strains isolated from stingless bee (Heterotrigona itama) honey collected across Malaysia. IJERPH. 2019;17(1):278.
- Varaldo PE, Facinelli B, Bagnarelli P, et al. Antimicrobial resistance: a challenge for the future. In: The first outstanding 50 years of “Università Politecnica delle Marche. Springer; 2020. p. 13–29.
- Organization WH. Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. Genewa: World Health Organization; 2017.
- Mokaya HO, Bargul JL, Irungu JW, et al. Bioactive constituents, in vitro radical scavenging and antibacterial activities of selected Apis mellifera honey from Kenya. Int J Food Sci Technol. 2020;55(3):1246–1254.
- Tuksitha L, Chen Y-L, Chen Y-L, et al. Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak). J Asia Pac Entomol. 2018;21(2):563–570.
- Zainol MI, Yusoff KM, Yasim M. Antibacterial activity of selected Malaysian honey. BMC Complement Altern Med. 2013;13(1):129–121.
- Bakar MFA, Sanusi SB, Bakar FIA, et al. Physicochemical and antioxidant potential of raw unprocessed honey from Malaysian stingless bees. Pakistan J Nutr. 2017;16(11):888–894.
- Rafie M, Zulkifli A, Syahir A, et al. Supplementation of stingless bee honey from Heterotrigona itama improves antiobesity parameters in high-fat diet induced obese rat model. Evid Based Complement Alternat Med. 2018;2018:6371582. https://doi.org/10.1155/2018/6371582.
- Syed Yaacob SN, Huyop F, Kamarulzaman Raja Ibrahim R, et al. Identification of Lactobacillus spp. and Fructobacillus spp. isolated from fresh Heterotrigona itama honey and their antagonistic activities against clinical pathogenic bacteria. J Apic Res. 2018; 57(3):395–405.
- Szöke-Nagy T, Porav AS, Coman C, et al. Characterization of the action of antibiotics and essential oils against bacteria by surface-enhanced Raman spectroscopy and scanning electron microscopy. Anal Lett. 2019;52(1):190–200.
- Setlow P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. 2006;101(3):514–525.
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999; 299:152–178.
- Isla MI, Craig A, Ordoñez R, et al. Physico chemical and bioactive properties of honeys from Northwestern Argentina. LWT Food Sci Technol. 2011;44(9):1922–1930.
- Turkut GM, Degirmenci A, Yildiz O, et al. Investigating 5-hydroxymethylfurfural formation kinetic and antioxidant activity in heat treated honey from different floral sources. Food Measure 2018;12(4):2358–2365.
- Hussain MB, Hannan A, Akhtar N, et al. Evaluation of the antibacterial activity of selected Pakistani honeys against multi-drug resistant Salmonella typhi. BMC Complement Altern Med. 2015;15(1):32.
- Drummond AJ, Waigh RD. The development of microbiological methods for phytochemical screening. Recent Res Dev Phytochem. 2000;4:143–152.
- Esti M, Panfili G, Marconi E, et al. Valorization of the honeys from the Molise region through physico-chemical, organoleptic and nutritional assessment. Food Chem. 1997;58(1–2):125–128.
- Khalil MI, Moniruzzaman M, Boukraâ L, et al. Physicochemical and antioxidant properties of Algerian honey. Molecules 2012;17(9):11199–11215.
- Saxena S, Gautam S, Sharma A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010;118(2):391–397.
- Terrab A, Recamales AF, Hernanz D, et al. Characterisation of Spanish thyme honeys by their physicochemical characteristics and mineral contents. Food Chem. 2004;88(4):537–542.
- Lemos MS, Venturieri GC, Dantas Filho HA, et al. Evaluation of the physicochemical parameters and inorganic constituents of honeys from the Amazon region. J Apic Res. 2017;57(1):1–10.
- Manyi-Loh CE, Clarke AM, Ndip RN. An overview of honey: therapeutic properties and contribution in nutrition and human health. African J Microbiol Res. 2011;5(8):844–852.
- da Silva IAA, da Silva TMS, Camara CA, et al. Phenolic profile, antioxidant activity and palynological analysis of stingless bee honey from Amazonas, Northern Brazil. Food Chem. 2013;141(4):3552–3558.
- Chua LS, Lee JY, Chan GF. Honey protein extraction and determination by mass spectrometry. Anal Bioanal Chem. 2013;405(10):3063–3074.
- Ismail NI, Abdul Kadir MR, Mahmood NH, et al. Apini and Meliponini foraging activities influence the phenolic content of different types of Malaysian honey. J Apic Res. 2016;55(2):137–150.
- Iurlina MO, Saiz AI, Fritz R, et al. Major flavonoids of Argentinean honeys. Optimisation of the extraction method and analysis of their content in relationship to the geographical source of honeys. Food Chem. 2009;115(3):1141–1149.
- Islam A, Khalil I, Islam N, et al. Physicochemical and antioxidant properties of Bangladeshi honeys stored for more than one year. BMC Complement Altern Med. 2012;12(1):177.
- Mai-Prochnow A, Clauson M, Hong J, et al. Gram positive and gram negative bacteria differ in their sensitivity to cold plasma. Sci Rep. 2016;6:38610..
- Brudzynski K, Miotto D, Kim L, et al. Active macromolecules of honey form colloidal particles essential for honey antibacterial activity and hydrogen peroxide production. Sci Rep. 2017;7(1):7637.
- Allen KL, Hutchinson G, Molan PC. The potential for using honey to treat wounds infected with MRSA and VRE. In: FirstWorld Wound Healing Congress. 2000. p. 10–13.
- Oddo LP, Heard TA, Rodríguez-Malaver A, et al. Composition and antioxidant activity of Trigona carbonaria honey from Australia. J Med Food. 2008;11(4):789–794.
- Molan PC. The antibacterial activity of honey 1. The nature of antibacterial activity. Bee World. 1992;73. http://dx.doi.org/10.1080/0005772X.1992.11099109
- Weston RJ. The contribution of catalase and other natural products to the antibacterial activity of honey: a review. Food Chem. 2000;71(2):235–239.
- Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007;42(4):321–324.
- Golding CG, Lamboo LL, Beniac DR, et al. The scanning electron microscope in microbiology and diagnosis of infectious disease. Sci Rep. 2016;6:26516..
- Henriques AF, Jenkins RE, Burton NF, et al. The effect of manuka honey on the structure of Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2011;30(2):167–171.
- Oliveira A, Ribeiro HG, Silva AC, et al. Synergistic antimicrobial interaction between honey and phage against Escherichia coli biofilms. Front Microbiol. 2017;8:2407..
- Chen K, Sun GW, Chua KL, et al. Modified virulence of antibiotic-induced Burkholderia pseudomallei filaments. Antimicrob Agents Chemother. 2005;49(3):1002–1009.
- Chen CZ, Cooper SL. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002;23(16):3359–3368.
- Cushnie TPT, O'Driscoll NH, Lamb AJ. Morphological and ultrastructural changes in bacterial cells as an indicator of antibacterial mechanism of action. Cell Mol Life Sci. 2016;73(23):4471–4492.
- Kong K, Schneper L, Mathee K. Beta-lactam antibiotics: from antibiosis to resistance and bacteriology. APMIS. 2010;118(1):1–36.
- Noguchi H, Matsuhashi M, Takaoka M, et al. New antipseudomonal penicillin, PC-904: affinity to penicillin-binding proteins and inhibition of the enzyme cross-linking peptidoglycan. Antimicrob Agents Chemother. 1978;14(4):617–624.
- El-Hajj ZW, Newman EB. How much territory can a single E. coli cell control? Front Microbiol. 2015;6:309..
- Claessen D, Emmins R, Hamoen LW, et al. Control of the cell elongation-division cycle by shuttling of PBP1 protein in Bacillus subtilis. Mol Microbiol. 2008;68(4):1029–1046.
- Brudzynski K, Abubaker K, Miotto D. Unraveling a mechanism of honey antibacterial action: polyphenol/H2O2-induced oxidative effect on bacterial cell growth and on DNA degradation. Food Chem. 2012;133(2):329–336.
- Shamsudin S, Selamat J, Sanny M, et al. Influence of origins and bee species on physicochemical, antioxidant properties and botanical discrimination of stingless bee honey. Int J Food Prop. 2019;22(1):239–264.
- Jenkins R, Burton N, Cooper R. Manuka honey inhibits cell division in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2011;66(11):2536–2542.
- Ahmer B. Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Mol Microbiol. 2004;52(4):933–945.
- Roberts AEL, Maddocks SE, Cooper RA. Manuka honey reduces the motility of Pseudomonas aeruginosa by suppression of flagella-associated genes. J Antimicrob Chemother. 2015;70(3):716–725.
