Abstract
SWEET family genes play an important role in different physiological processes, including sugar transport and adjustments, plant development and environmental adaptability. BLAST tools and Hidden Markov Model-directed sequence alignments in Eucalyptus grandis genome identified 52 Eucalyptus SWEET genes containing MtN3/saliva domain. Out of these, 40 genes encode predicted proteins that have 6 or more transmembrane regions with two MtN3/saliva domains. Six predicted proteins contained only one MtN3/saliva domain, similar to the structure of microbial SemiSWEETs. Of the 52 SWEET candidates, only 45 SWEET family genes were mapped on 8 chromosomes, and most of them showed clustered distribution. The expression profiles revealed that the SWEET genes have different expression in different tissues, showing that they might play different functions in Eucalyptus. Our results explored the evolutionary aspects, provided a systematic description of Eucalyptus SWEET genes and can help researchers to understand the functions of the SWEET family genes in Eucalyptus.
Introduction
Carbohydrates being an important source of food and energy are mainly secreted by plant nectaries and roots to attract pollinator and promote growth of beneficial microorganisms, respectively [Citation1]. Carbohydrates are mainly stored as sucrose, which later is moved to the phloem either via the apoplastic or symplastic pathways [Citation2–4]. Transport and partitioning of carbohydrates are vital for various development and growth processes as well as for plant response to biotic and abiotic stress [Citation5]. Sugar transport in plants is mediated by major facilitator superfamily (MFS family), which involves various proton-coupled sugar/H+ symporters, sugar/H+ antiporters and uniporters, mainly characterized by 12 transmembrane domains (TMs) [Citation6,Citation7]. SWEET, a novel class of sugar transporters, belongs to the MtN3-like clan and can be divided into three clades. The members of the first clade are associated with plants, those of the second one, with animals, whereas the third clade is associated with bacteria to archaea (Lactococcus) and nematodes. The protein family database (containing annotations and multiple sequence alignments (PFAM) (http://pfam.sanger.ac.uk/clan/MtN3-like)) search divides the MtN3-like clan into five families. These include MtN3/saliva (PF03083), PQ-loop (PF04193), UPF0041 (PF03650), ER Lumen Receptor (PF00810) and Lab-N (PF07578) [Citation8]. Growing evidence shows that eukaryotic SWEETs are more conserved than prokaryotic ones. Eukaryotic SWEET proteins mainly consist of seven transmembrane (TM1 to TM7) helices harboring two MtN3/Saliva domains, but prokaryotic SWEET homologs have only 3-TM. In the evolutionary process, prokaryotes have three transmembrane helices, while have eukaryotes evolved seven transmembrane helices with two MtN3/saliva domains [Citation9]. In addition, SWEET sugar transporter homologs from bacteria were identified and named SemiSWEETs [Citation8]. Although SWEET gene family is evolutionarily conserved, the existing data reveal that the SWEET genes participate in a variety of physiological processes, such as phloem loading, development, stress response reaction, ion transport [Citation8, Citation10]. According to the radio-tracer uptake or efflux assays, the sugar transporter SWEET proteins are low-affinity glucose transport proteins, bidirectional and independent of pH [Citation9]. Most SWEET family members are located in the plasma membrane [Citation11] but recently AtSWEET17 and AtWEET16 are reported in the vacuolar membrane [Citation12]. OsSWEET11 is characterized as a sucrose transporter [Citation11] located in the plasma membrane and preferentially expressed in rice phloem [Citation13]. These findings indicate that OsSWEET11 is important in preparation for phloem loading [Citation1]. AtSWEET1 was the first characterized plant SWEET transporter which acts as a glucose uniporter in various systems, while AtSWEET8 is reported as a bidirectional glucose transporter [Citation10]. AtSWEET9 is an essential efflux transporter involved in nectar production [Citation14]. AtSWEET11 and AtSWEET12 are involved in sucrose efflux from phloem parenchyma cells [Citation11]. Physiological function studies in mutant plants suggest the key role of AtSWEET11 and AtSWEET12 in phloem loading [Citation11]. Double mutant plants of Atsweet11 and Atsweet12 showed slower growth and excess accumulation of carbohydrate in Arabidopsis leaves [Citation11]. Overexpression of AtSWEET15(SAG29) in transgenic plants promotes senescence [Citation15], whereas recently identified AtSWEET16 and AtSWEET17 genes are major factors controlling the fructose content in the tonoplasts [Citation13, Citation16,Citation17]. AtSWEET17 may be crucial in carbohydrate allocation in plants because mutants of AtSWEET17 show stunted growth and lower seed yield [Citation17]. Genus Eucalyptus belonging to order Myrtales includes the world’s most widely planted hardwood trees [Citation18]. Their fantastic diversity, adaptability and growth have made them a global renewable resource of fiber, energy, pulp and timber [Citation19]. Eucalyptus grandis is mainly distributed in Australia, the Philippines and other islands of the Western Pacific as well as in China. In China, it is distributed from Wenzhou to the south coast area. E. grandis is an energy plant, with great industrial production, and important for investigating wood formation at the genomic level [Citation20]. All in all, the E. grandis genome sequence offers an opportunity for testing the impact of evolution in proteome modularity [Citation21].
The aim of this study was to lay a solid foundation for further investigations into the functional roles of SWEETs in the developmental processes and stress responses of E. grandis.
Materials and methods
Sequence retrieval and phylogenetic analysis
In order to identify SWEET genes in different species, MtN3/saliva domain (PF03083) genes were identified in 37 species with completely sequenced genomes, including plants from unicellular algae to multicellular plants. The proteins were retrieved from corresponding plant genome annotation resource by BLAST search performed in PLAZA (http://bioinformatics.psb.ugent.be/plaza/news/index) and Phytozome (http://www.phytozome.net/) [Citation22,Citation23] databases. To understand the phylogenetic relationships, a bootstrapped phylogenetic tree was constructed using the neighbor-joining (NJ) method [Citation24] and the genetic distance was calculated by MEGA v 5.1 (http://www.megasoftware.net/) [Citation25]. To extract all MtN3/saliva domain proteins from Arabidopsis and Eucalyptus, BLAST search was performed against Eucalyptus grandis (v1.1) [Citation20], Arabidopsis thaliana (TAIR 10) in Phytozome database.
Gene structure analysis
The exon and intron structures of 52 individual genes which have an MtN3/saliva domain in Eucalyptus were illustrated using Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/index.php) [Citation26] by aligning the cDNA sequences with the corresponding genomic DNA sequences from Phytozome.
Chromosomal location
The chromosomal locations of the SWEET genes were determined based on their physical position on Eucalyptus chromosomes. The Maplnspect software was used to generate the Eucalyptus SWEET genes chromosomal map [Citation20].
Plant material cultivation
About 20-cm tall, three-month-old E. grandis were used in hydroponics conditions. These trees were grown in the greenhouse at the Research Institute of Tropical Forestry, Chinese Academy of Forestry, Guangzhou, China. For the salt stress treatment, the E. grandis plants were transferred to 200 mmol/L NaCl solution and cultured for a total of 168 h; leaves were collected after 0, 1, 6, 24 and 168 h.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from five different tissues of Eucalyptus (root, young leaves, mature leaves, phloem and xylem) by using RNAprep Pure Plant Plus Kit (Polysaccharides & Polyphenolics-rich) (Tiangen biotech, Beijing, China). A total of 1 μg RNA per sample was reverse transcribed into cDNA with the PrimeScript™ II reverse transcription kit (TaKaRa Biotech. Co. Ltd., Dalian, China). The cDNA samples were diluted 1:20 with nuclease-free water prior to the qRT-PCR analyses. The qRT-PCR primers for 52 Eucalyptus SWEET family genes were designed by Primer premier 5.0 software (). qRT-PCR was performed in quadruplicate using the SYBR Premix Ex Taq™ II Kit (TaKaRa, Dalian, China) on a Roche Light Cycler® 480 (Roche Ltd. Mannheim, Germany). The PCR reaction was performed in a total volume of 20 μL, containing 10 μL of 2 × SYBR Premix, 2 μL of cDNA template and 0.4 μL of each specific primer to a final concentration of 100 mmol/L. The reactions were performed using the following conditions: pre-degenerated 95 °C for 30 s; followed by 40 cycles of 95 °C for 5 s, 58 °C for 50 s and 72 °C for 18 s, extension 72 °C for 2 min, melting curve 95 °C for 5 s, 58 °C for 30 s, 95 °C continuous; cooling 40 °C for 30 s. Eucons08 was used as the reference gene [Citation27]. The qPCR data were analysed by Livak 2-ΔΔCT.
Table 1. List of 40 SWEET genes which encode predicted proteins that have 6 or 7 TMs identified in E. grandis and their sequence characteristics.
Results and discussion
Identification of the SWEET genes family in Eucalyptus grandis and other plant species
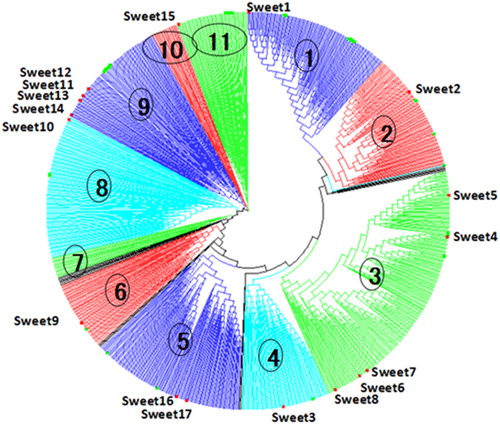
Gene duplication to meet environmental changes enriches SWEET family in various plants [Citation28–30]. From Phytozome database, the proteins containing the MtN3/saliva domain (http://pfam.xfam.org/clan/MtN3-like) were retrieved and 52 homologous sequences were observed in Eucalyptus. To study the evolution of genes with MtN3/saliva domains in different plants, a total of 947 genes were found from 37 different species. All the 947 proteins were divided into some outgroups (OG, these were a small number of proteins which were located as clades outliers) and 11 branches with phylogeny (). We retrieved E. grandis genes containing the MtN3/saliva domain. Comparing Arabidopsis 17 SWEET genes [Citation10,Citation11], rice 21 SWEET genes [Citation9, Citation22, Citation31] and rubber tree (36 genes) [Citation32], Eucalyptus has 52 SWEET genes sharing the highest homology with soybean [Citation33]. These results indicate that Eucalyptus SWEET genes are diversified like soybean and after monocot and dicot division [Citation33]. Clade 3 grouped as A1 sub-group containing EgSWEET7, EgSWEET5a, EgSWEET4b, EgSWEET5c, EgSWEET4a and EgSWEET5b genes. The protein encoded by Arabidopsis AtSWEET8 in this subgroup was characterized as a bidirectional glucose transporter [Citation10], indicating it might be important for glucose transport. Clade 5 contained EgSWEET17b, EgSWEET17c, EgSWEET17d, EgSWEET17a, EgSWEET16 and EgSWEET16a genes and was named A2. The proteins encoded by Arabidopsis AtSWEET16 and AtSWEET17 in the same subgroup were identified as major factors controlling fructose content in tonoplast [Citation13,Citation16,Citation17]. Clades 1, 2 and 4 contained EgSWEET1b, EgSWEET1a, EgSWEET1c, EgSWEET3a3, EgSWEET3a4, EgSWEET3a1, EgSWEET3a2, EgSWEET3a5, EgSWEET2a, EgSWEET2c and EgSWEET2b genes, and formed the A3 sub-group. Like the proteins encoded by Arabidopsis AtSWEET1 and AtSWEET3, they might serve as glucose uniporters in various systems [Citation10,Citation33]. Clades 6-11 containing 24 Eucalyptus members were grouped as B1. Similar to Arabidopsis AtSWEET11 and AtSWEET12, these genes might encode proteins that play a significant role in phloem loading [Citation11]. Compared with Arabidopsis, tree development needs more sugar from phloem, so wood plants have more SWEET gene members.
Figure 1. Phylogenetic relationship of MtN3/saliva motif proteins in 37 different species.
Note: A total of 947 SWEET proteins containing MtN3/saliva domain (PF03083) were identified by PFAM. The phylogenetic tree was constructed using MEGA5.1 software by using ClustalX2.1 alignment and neighbour joining (NJ) method with 1000 bootstrap replications. Eleven main clades were numbered with different colors.
Comparative analysis of the genetic properties of eucalyptus SWEET family genes
The structural characteristics and conserved regions of 52 Eucalyptus SWEET genes were compared with online GSDS 1.0 software (http://gsds.cbi.pku.edu.cn/index.php) [Citation26]. To obtain the transmembrane domain of E. grandis SWEET family, the amino acid sequences were submitted to TMHMM Server v.2.0 (http://www.cbs.dtu.dk/services/TMHMM) in FASTA format with default settings [Citation34]. Out of 52 Eucalyptus homologous sequences, E. grandis had 40 SWEET genes containing two MtN3/saliva domains and 6 or more transmembrane regions. Moreover, the transmembrane domains of half of the genes for the other 12 sequences were less than 6; the other half had only one MtN3/saliva domain, similar to the structure of microbial SemiSWEETs, which were not considered as SWEET family members. The results revealed that the SWEET family of genes in E. grandis has also undergone expansion and new functions may have differentiated during the species’s evolution.
Most of these 40 Eucalyptus SWEET genes had 5 or 6 extrons in their nucleotide sequences (). The similar structure with tomato [Citation24], soybean [Citation23] and Medicago truncatula [Citation35] reveals that SWEET genes may be highly conserved during evolution. It is still unclear whether the genes with less than six exons, EgSWEET1a/b, EgSWEET2c, EgSWEET4a/b, EgSWEET7, EgSWEET9a/b, EgSWEET15a/b/e and EgSWEET16a, have acquired new functions. There were 40 genes having 6 or 7 TMs. And their protein molecular weight (MW) and isoelectric point (PI) were analyzed by DNAMAN. The amino acid length of the predicted proteins encoded by these 40 genes ranged from 215 to 290 aa with an average length of 254 units (). Among them, EgSWEET3c was the longest gene coding for 290 amino acids. The 40 genes coded for six or seven transmembrane helices bearing two MtN3/saliva domains except for EgSWEET9c, which encoded only three MtN3/sailva domains. There were approximately 90 amino acids in these two MtN3/saliva domains. The open reading frame (ORF) ranged from 1208 to 7084 ().
Figure 2. Exon-intron structures of 40 SWEET genes identified in Eucalyptus.
Note: Shown is a graphic representation of the gene models of 40 SWEETs which have 6 or 7 TMD. Exons and introns are represented by green boxes and black lines, respectively. The number indicates the splicing phases of the SWEET genes. 0, phase 0; 1, phase 1; 2, phase 2.
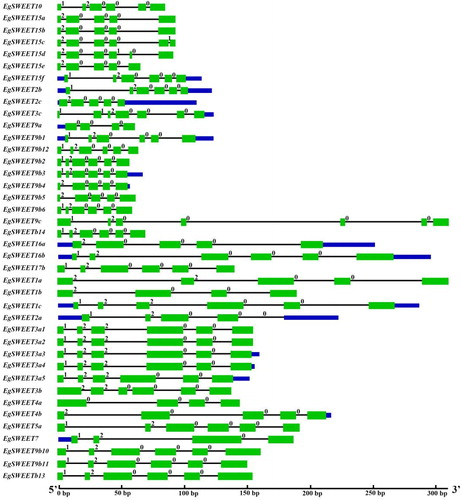
We also analyzed the other 12 genes for which the TMs were less than six (). The lengths of the proteins encoded by these 12 genes ranged from 97 to 284 amino acids. EgSWEET2d was the smallest gene, while EgSWEET17e was the longest one. The open reading frame (ORF) ranged from 529 to 4615, whereas the exons ranged from 3 to 6. One half of the genes encoded 2 MtN3/saliva domains, whereas the other half encoded 1 MtN3/saliva domain, these including EgSWEET5c, EgSWEET5b, EgSWEET17a, EgSWEET17e, EgSWEET2d and EgSWEET12 genes ().
Table 2. List of 12 SWEET genes which encode predicted proteins that have less than 6 TMs identified in E. grandis and their sequence characteristics.
We further compared the MtN3/saliva domain genes in Poplar and found that only 25 genes contained an MtN3/saliva domain (). Out of these 25 genes, 21 genes encoded 6 or more transmembrane regions and two MtN3/saliva domains, regarded as SWEET family members. The other four genes coded for two MtN3/saliva domains as well, but less than six transmembrane domains (). There were 2 genes (PtSWEET3c and PtSWEET12a) that encoded only one MtN3/saliva domain and were similar in structure to microbial SemiSWEETs. Two MtN3/saliva domains are typical of eukaryots, whereas one MtN3/saliva domain is found in prokaryotes. The genes containing sequences corresponding to one MtN3/saliva domain may have retained some of the characteristics of prokaryotes in the process of biological evolution and lay a vital foundation to the evolutionary relationship in the study of prokaryotes and eukaryotes.
Chromosomal locations and gene duplications
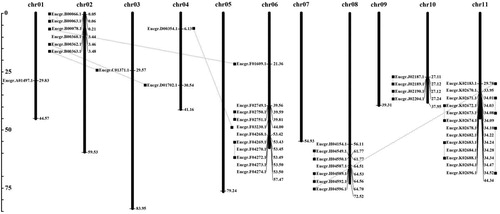
We obtained the chromosomal locations of the 52 Eucalyptus genes which encode an MtN3/saliva domain from Phytozome (http://www.phytozome.net/). Among them, 45 genes are located on 8 chromosomes. Most of the SWEET genes were identified to be distributed on chromosomes 2, 6, 8 and 11, and no genes were found on chromosomes 5, 7 and 9. Two genes were present on chromosome 4 and only one gene was located on chromosome 1 and 3 each. At the same time, we found that the genes on chromosomes 2, 6, 8, 10 and 11 were clustered. This indicates that they belong to the same ancestral gene amplification product. According to the phylogenetic tree (Supplementary material, ), we linked 16 pairs of the paralogous SWEET family genes.
From the distribution of 45 Eucalyptus SWEET genes on chromosomes (), most of these genes located in Chr11, including EgSWEET9b11/b12, EgSWEET9b5/6, EgSWEET9b7/b8, EgSWEET9b4/b9 and EgSWEET15f. EgSWEET7 was located on Chr01; EgSWEET9a/d, EgSWEET10 and EgSWEET17c/d were the tandem distribution in Chr02; EgSWEET3b/EgSWEET17a and EgSWEET1a/b were the tandem distribution in Chr06; EgSWEET15b/c/d and EgSWEET16b/EgSWEET16a were distributed on Chr08; and EgSWEET4b/EgSWEET5c and EgSWEET4a/EgSWEET5b were tandemly distributed on Chr10. The bootstrap values of the EgSWEET4b/EgSWEET5c, EgSWEET3b/EgSWEET17a, EgSWEET1a/b, EgSWEET15b/c were more than 70%, which showed that each pair has closer genetic relationship.
Expression analysis of 52 genes which have MtN3/saliva domain in Eucalyptus
As previous studies have shown, SWEET genes specifically mediate different development stages. Further detailed analyses are expected to determine the spatial expression profiles and potential biological functions. In order to provide meaningful clues to the functions of Eucalyptus SWEET family genes, we measured their expression level in different tissues (root, young leaves, mature leaves, phloem, xylem) by quantitative RT-PCR. In general, the expression of EgSWEET2a was highest among the Eucalyptus genes followed by EgSWEET16b. All other genes were expressed at comparatively lower levels. Of the 52 SWEET genes of Eucalyptus, 36 genes showed higher transcript accumulations in the root. There were less than 30 genes expressed in young leaves (22 genes), mature leaves (20 genes), xylem (29 genes) and phloem (27 genes). The data revealed that Eucalyptus SWEET family genes had higher expression in the root than in other tissues and more than half of the Eucalyptus SWEET family genes were expressed in the xylem and phloem. We can speculate that Eucalyptus SWEET genes were closely related with phloem loading () [Citation2].
Figure 4. Expression of 38 genes which have an MtN3/saliva domain in Eucalyptus.
Note: These were quantified by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) in vegetative tissues (root, young leaves, mature leaves, phloem, xylem).
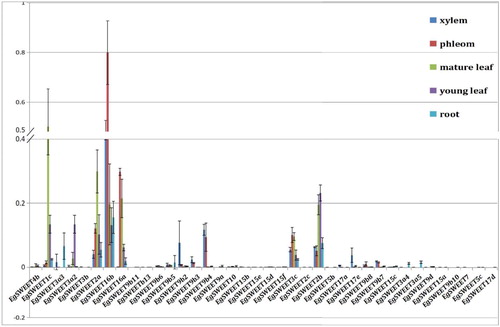
Eucalyptus is one of the fastest growing woody plants. Phloem loading acts primarily in the long-distance transport of carbohydrates from the leaves to various sinks to support plant growth. The expression of EgSWEET16a/b in the phloem was significantly greater than that of the other 38 EgSWEET genes. AtSWEET16 has been revealed to mediate fructose transport [Citation12,Citation16,Citation17]. EgSWEET16a/b might be associated with the transport of fructose; yet the particular mechanism is not understood. In leaves, EgSWEET1c, EgSWEET2a/b had EgSWEET3a2/3c had higher expression levels (). AtSWEET2 determines the glucose contents in tonoplasts of Arabidopsis leaves and roots [Citation36]. CsSWEET1, 2, 3 are predicted to encode monosaccharide efflux transporters in cucumber [Citation37] and may not directly participate in phloem loading but rather in regulating the monosaccharide contents in cucumber leaves [Citation38]. All these functions deserve further exploration. The observation that different Eucalyptus SWEET genes had their highest levels in different tissues also points toward their role in different processes.
SWEET family genes were induced under salinity stress
When three-month-old Eucalyptus seedlings were grown in 200 mmol/L NaCl, the expression levels of different genes showed different response patterns. Salinity stress inhibits plant growth by reducing water uptake and may also disrupt the metabolic balance between Na+ and K+ [Citation39], which are closely related with sugar transport. The expression patterns of 23 SWEET genes in three-month-old Eucalyptus seedlings responded to 200 mmol/L NaCl treatment (). After treatment, we found that the expression of 11 SWEET genes was up-regulated and the expression of 9 other genes was down-regulated. The expression level of EgSWEET1c, EgSWEET2a, EgSWEET4b, EgSWEET9b6, EgSWEET9b7, EgSWEET9b8, EgSWEET9d7, EgSWEET10, EgSWEETb13, EgSWEET15d and EgSWEET15c increased with salt treatment in a time-dependent manner ().
Figure 5. Expression analysis of 20 EgSWEET genes following NaCl treatment as determined by qRT-PCR. Up-regulated (A) and down-regulated (B) genes under 20 mmol/L NaCl treatment.
Note: The relative expression levels were calculated using the 2-△△Ct method.
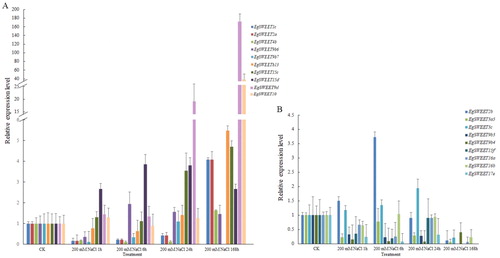
The expression levels of EgSWEET9d and EgSWEET10 had a greater rise than others. As with AtSWEET15 [Citation40], EgSWEET15c/d/f may also be involved in salinity regulation. The expression level of EgSWEET9d, which is essential for nectar secretion [Citation41], showed a marked increase. In contrast, EgSWEET2b, EgSWEET3a5, EgSWEET3c, EgSWEET9b3, EgSWEET9b4, EgSWEET15f, EgSWEET16a, EgSWEET16b and EgSWEET17e had lower expression under NaCl treatment (). Most of the EgSWEET genes like EgSWEET9, EgSWEET17 were down-regulated with the salt supplementation, indicating that the distribution of carbohydrates, such as sucrose, fructose, glucose etc, was seriously interfered. EgSWEET16a and EgSWEET16b were similar to AtSWEET16, primarily as a fructose transporter. Similarly, SWEET17, expressed highly in roots and positioned in vacuolar membranes, might be closely related to SWEET16. We speculate that these genes may have analogous functions, yet further function has not been reported. All of these results showed that some salt-stress responsive genes may be involved in sugar transport, suggesting that the expression levels of EgSWEET family genes were affected by salt stress and may be primarily associated with the sucrose transport.
SWEET family is associated with the growth and development of all plant organs in terms of expression patterns. Specifically, there exists a set of SWEET genes closely involved in the vegetative growth, flower, and seed development in each plant species. EgSWEET family might also play a key role in wood biomass. This gene family is of particular interest for further research, since Eucalyptus species, fast-growing woody plants, have the potential to be planted both in sub-tropical and in tropical zones. The Asia Pacific Region is already the most important of the tropical zones for Eucalyptus plantation and utilization.
Conclusions
In the present study, we performed a comprehensive genome-wide analysis of 52 EgSWEET genes with regard to their characteristics, exon-intron structures, genome distribution, phylogeny and expression analysis under salt stress. The results from this study provide a basic understanding of the EgSWEET genes and may also facilitate future research that may elucidate the detailed roles of SWEET genes in Eucalyptus and other woody plants.
Supplemental Material
Download PDF (157 KB)Acknowledgments
The authors would like to thank Chen Chen (South China Agricultural University) and Xianhai Zhao (South China Agricultural University) for their comments on this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ayre BG. Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol Plant. 2011; 4(3):377–394.
- Voitsekhovskaja OV, Rudashevskaya EL, Demchenko KN, et al. Evidence for functional heterogeneity of sieve element-companion cell complexes in minor vein phloem of Alonsoa meridionalis. J Exp Bot. 2009; 60(6):1873–1883.
- Giaquinta RT. Phloem loading of sucrose: involvement of membrane ATPase and proton transport. Plant Physiol. 1979; 63(4):744–748.
- Lemoine R, La Camera S, Atanassova R, et al. Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci. 2013; 4:272–272.
- Chen L. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 2014; 201(4):1150–1155.
- Reuscher S, Akiyama M, Yasuda T, et al. The sugar transporter inventory of tomato: genome-wide identification and expression analysis. Plant Cell Physiol. 2014; 55(6):1123–1141.
- Doidy J, Grace E, Kühn C, et al. Sugar transporters in plants and in their interactions with fungi. Trends Plant Sci. 2012; 17(7):413–422.
- Xuan YH, Hu YB, Chen L, et al. Functional role of oligomerization for bacterial and plant SWEET sugar transporter family. Proc Natl Acad Sci USA. 2013; 110(39):E3685–E3694.
- Yuan M, Wang S. Rice MtN3/Saliva/SWEET family genes and their homologs in cellular organisms. Mol Plant. 2013; 6(3):665–674.
- Chen L, Hou B, Lalonde S, et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature. 2010; 468(7323):527–532.
- Chen L, Qu X, Hou B, et al. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science. 2012; 335(6065):207–211.
- Klemens PAW, Patzke K, Deitmer J, et al. Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis. Plant Physiol. 2013; 163(3):1338–1352.
- Chu Z, Yuan M, Yao J, et al. Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 2006; 20(10):1250–1255.
- Lin IW, Sosso D, Chen L, et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature. 2014;508(7497):546–549.
- Seo PJ, Park J, Kang SK, et al. An Arabidopsis senescence-associated protein SAG29 regulates cell viability under high salinity. Planta. 2011; 233(1):189–200.
- Guo W, Nagy R, Chen H, et al. SWEET17, a facilitative transporter, mediates fructose transport across the tonoplast of Arabidopsis roots and leaves. Plant Physiol. 2014; 164(2):777–789.
- Chardon F, Bedu M, Calenge F, et al. Leaf fructose content is controlled by the vacuolar transporter SWEET17 in Arabidopsis. Curr Biol. 2013; 23(8):697–702.
- Paiva JA, Prat E, Vautrin S, et al. Advancing Eucalyptus genomics: identification and sequencing of lignin biosynthesis genes from deep-coverage BAC libraries. BMC Genomics. 2011;12(1):137 [2019 Mar 04].
- Zhu X, Chase MW, Qiu Y, et al. Mitochondrial matR sequences help to resolve deep phylogenetic relationships in rosids. BMC Evol Biol. 2007; 7:217–217.
- Myburg AA, Grattapaglia D, Tuskan GA, et al. The genome of Eucalyptus grandis. Nature. 2014;510(7505):356–362.
- Kersting AR, Mizrachi E, Bornberg-Bauer E, et al. Protein domain evolution is associated with reproductive diversification and adaptive radiation in the genus Eucalyptus. New Phytol. 2015; 206(4):1328–1336.
- Yuan M, Zhao J, Huang R, et al. Rice MtN3/saliva/SWEET gene family: Evolution, expression profiling, and sugar transport. J Integr Plant Biol. 2014; 56(6):559–570.
- Patil G, Valliyodan B, Deshmukh R, et al. Soybean (Glycine max) SWEET gene family: insights through comparative genomics, transcriptome profiling and whole genome re-sequence analysis. BMC Genomics. 2015;16(1):520 [2019 Jul 11];
- Feng C, Han J, Han X, et al. Genome-wide identification, phylogeny, and expression analysis of the SWEET gene family in tomato. Gene. 2015;573(2):261–272.
- Guo AY, Zhu QH, Chen X, et al. GSDS: a gene structure display server. Yi Chuan. 2007; 29(8):1023–1026.
- Eom J, Chen L, Sosso D, et al. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr Opin Plant Biol. 2015; 25:53–62.
- Van Bel M, Proost S, Wischnitzki E, et al. Dissecting plant genomes with the PLAZA comparative genomics platform. Plant Physiol. 2012; 158(2):590–600.
- Ganko E, Meyers B, Vision T. Divergence in expression between duplicated genes in Arabidopsis. Mol Biol Evol. 2007;24(10):2298–2309.
- Cannon SB, Mitra A, Baumgarten A, et al. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4:10.
- Li X, Si W, Qin Q, et al. Deciphering evolutionary dynamics of SWEET genes in diverse plant lineages. Sci Rep. 2018;8(1):13440.[2019 Sep 17].
- Goodstein DM, Shu S, Howson R, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012; 40(Database issue):D1178–D1186.
- Sui J, Xiao X, Qi J, et al. The SWEET gene family in Hevea brasiliensis - its evolution and expression compared with four other plant species. Febs Open Bio. 2017;7(12):1943–1959.
- Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci Usa. 2004; 101(30):11030–11035.
- Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28(10):2731–2739.
- Hu B, Wu H, Huang W, et al. SWEET gene family in Medicago truncatula: genome- wide identification, expression and substrate specificity analysis. Plants. 2019;8(9):338.
- Chen HY, Huh JH, Yu YC, et al. The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection. Plant J. 2015;83(6):1046–1058.
- Chen L, Cheung L, Feng L, et al. Transport of sugars. Annu Rev Biochem. 2015;84:865–894.
- Hu L, Zhang F, Song S, et al. Genome-wide identification, characterization, and expression analysis of the SWEET gene family in cucumber. J Integr Agr. 2017;16(7):1486–1501.
- Munns R. Genes and salt tolerance: bringing them together. New Phytol. 2005;167(3):645–663.
- Slewinski TL. Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: a physiological perspective. Mol Plant. 2011;4(4):641–662.
- Oliveira LA, Breton MC, Bastolla FM, et al. Reference genes for the normalization of gene expression in Eucalyptus species. Plant Cell Physiol. 2012;53(2):405–422.