Abstract
As a valuable and powerful platform technology, yeast surface display (YSD) has been widely used in various biotechnological fields, but little in agricultural applications. Xylanase can be used as an elicitor in the plant–pathogen interaction system and induce corn defence response. In this study, the YSD technology was used to express the xylanase protein family from Setosphaeria turcica, which is a novel strategy for plant protection. First, five genes encoding xylanase were selected from the S. turcica genome database. The conserved domains of these genes were analyzed, which revealed that these genes were highly conserved. Here, we selected two of the five genes and expressed them by a prokaryotic expression system, but the two xylanase genes were expressed in inclusion bodies and showed no activity. To make them available in the application, we successfully constructed them in a YSD system. The products were detected for enzyme activities and the activities of two xylanases were 16.72 and 19.12 U/mL, respectively. We finally proved that the yeast-displaying xylanase was an efficient strategy to induce corn defence response, as four defence genes in corn, PR-1, PR-4, SOD and CAT were induced after treatment with the yeast displaying xylanases.
Introduction
In nature, plants are constantly subject to a variety of pests and diseases, which can lead to great crop losses. To date, biopestcides derived from plants and including insect pathogens, botanicals, jasmonic acid (JA) and synthetic chemical pesticides are the main control methods used against pests and diseases [Citation1,Citation2]. However, potential damage of massively using biopestcides and synthetic chemical pesticides to the environment and human health has raised consumers concern. Therefore, there is a need to develop new control strategies such as using the plant immune response and plant elicitors to deal with this problem and at the same time ensure the approach is environmentally friendly, harmless to humans and reduces the risk of pest resistance.
In plant defence mechanisms, a group of hormones such as salicylic acid (SA), JA and ethylene (ET) induce plant defence responses [Citation3]. In addition, reactive oxygen species (ROS) also activate the plant defence responses, including the production of enzymatic and non-enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT) and glutathione reductase (GR) [Citation4,Citation5]. Pathogenesis-related (PR) proteins such as PR-1, PR-2 and PR-3 play vital roles in defence responses as well [Citation6]. Hence, these genes encoding enzymatic and non-enzymatic antioxidants are extensively used to study plant defence mechanisms.
It is reported that xylanase can be used as an elicitor in the plant–pathogen interaction system, but there is little application on it [Citation7,Citation8]. Xylanase, consisting of endo-1,4-β-xylanase, β-xylosidase, α-l-arabinase, α-d-glucosidase, acetylxylan esterase and phenolic esterase [Citation9], can degrade xylan hemicellulose, which is the second most common biopolymer in plant cell walls and an important component of hemicellulose [Citation10]. Xylanase is essential to plant–phytopathogen interaction. On the one hand, it can promote pathogen infection via decomposing plant cell walls; on the other hand, as an elicitor, it can also induce plant disease resistance and defence responses including plant cell wall strengthening, antimicrobial composition, ethylene biosynthesis and rapid local cell death [Citation11].
Currently, the main ways to obtain proteins of interest are direct extraction from cells or a prokaryotic expression system. The prokaryotic expression system, however, is the most widely used protein expression system both on a laboratory and industrial scale. Although large quantities of recombinant proteins can be obtained in a short time and the host cells can be cultured easily and inexpensively in prokaryotic expression systems, there are still some problems, e.g. the expressed proteins cannot be folded correctly sometimes and overexpressed proteins are often produced in the form of inclusion bodies, which are insoluble aggregates that have little or no activity.
Yeast surface display (YSD) is a useful technique, which is based on the fusion of target peptides or proteins with a cell wall anchoring protein. This fusion protein can be directed to the yeast cell wall and displayed on the yeast cell surface [Citation12,Citation13]. Saccharomyces cerevisiae Aga2p is the most commonly used system where anchor in C-terminal fusion displays is α-agglutinin [Citation14]. In this system, the Aga2p subunit is linked by two disulphide bonds to the Aga1p subunit, which anchors to the yeast cell wall while allowing the target protein to interact with substrates.
In this study, the members of the xylanase gene family from Setosphaeria turcica were selected by bioinformatics analysis. For the purpose of comparison, a prokaryotic expression system was used to express these genes as well, but the xylanase was expressed as inclusion bodies and showed no activity. We took advantage of the yeast-surface display strategy and demonstrated that yeast surface displayed xylanase could be an efficient strategy to induce corn defence response. Further studies are needed to improve and develop it for agricultural application.
Materials and methods
Strains, culture media and enzymes
Escherichia coli DH5α (TransGen Biotech), used as the recipient strain for recombinant plasmids, and BL21(DE3) (TransGen Biotech), used for expression of the xylanase, were grown in Luria-Bertani (LB) medium (1% tryptone, 0.5% yeast extract, 1% NaCl, pH 7.0) at 37 °C. Yeast were grown in complete medium (YPD: 1% yeast extract, 2% peptone, 2% glucose). Synthetic Dextrose Minimal Medium without Tryptophan (SD-Trp) (0.67% yeast nitrogen base, 2% glucose, 0.02% adenine, 0.02% uracil, 0.02% leucine and 0.02% histidine) was used to cultivate S. cerevisiae. Synthetic Galactose Minimal Medium without Tryptophan (SG-Trp) (0.67% yeast nitrogen base, 2% galactose, 0.02% adenine, 0.02% uracil, 0.02% leucine and 0.02% histidine) was used to display xylanase to yeast surface following the protocol described previously [Citation15]. The restriction enzymes BamH I, EcoR I and Sfi I were purchased from New England Biolabs (Beijing, China).
Multiple sequence alignment, phylogenetic analysis and domain analysis
To identify the S. turcica xylanase family GH11 coding genes, a blast search was carried out in the S. turcica genome database(JGI, http://genome.jgi.doe.gov/Settu1), using as queries amino acid sequences of well-characterized xylanases from other fungi. Multiple alignments of amino acid sequences were carried out with ClustalX. A phylogenetic tree was constructed by using MEGA6.0 software based on amino acid sequence similarity analysis.
The online software Pfam (http://pfam.xfam.org/) and SMART (http://smart. embl-heidelberg.de/) were used to analyze the conserved domain of the S. turcica xylanase GH11 family genes. And the software IBS (http://ibs.biocuckoo.org/) was used to draw the conserved domain following the procedures described previously [Citation16].
Cloning and prokaryotic expression of Stxyl1 and Stxyl2
The gene sequences of Stxyl1 and Stxyl2 gained from JGI were cloned from cDNA of S. turcica, which contained BamH I and EcoR I restriction enzyme sites and homologous arm of pGEX-6P-3, and was cloned into a pGEX-6P-3 vector containing a GST tag at the N-terminus by BamH I and EcoR I through One Step Cloning Kit (Beijing Biomed Co., Ltd). Recombinant plasmids pGEX-6P-3-Stxyl1 and pGEX-6P-3-Stxyl2 were transformed into DH5α. A colony of positive cells was selected on LB agar plate containing ampicillin (1‰) and screened by direct colony PCR with Stxyl1 and Stxyl2 primers. The extracted plasmids designated pGEX-6P-3-Stxyl1 and pGEX-6P-3-Stxyl2 were subjected to DNA sequencing by BGI, Beijing, China.
Then recombinant plasmids pGEX-6P-3-Stxyl1 and pGEX-6P-3-Stxyl2 were transformed into BL21 (DH3). And the transformed cells were cultured in LB medium containing ampicillin (1‰) at 37 °C with shaking until the optical density at 600 nm (OD600) reached 0.6. Isopropyl-β-d-thiogalactoside (IPTG) (Sigma, USA) was then added to a final concentration of 1 mmol/L to induce expression at 30 °C for 3 h. The empty pGEX-6P-3 vector transformed culture was used as control. E. coli cells were collected by centrifugation (10,300 g for 1 min at 4 °C) and then the pellets were resuspended in lysis buffer (50 mmol/L Tris–HCl, 1.5 mmol/L EDTA, 500 mmol/L NaCl, 1 mol/L DTT, 0.4 mol/L NaF, 1 mmol/L PMSF, 5 mmol/L pepstatin, 0.15 mmol/L aprotinin, 1 mmol/L leupeptin, 1% Triton X-100, pH 8.0). The pellets were lysed by supersonic treatment at 140 W for 10 min (each for 10 s with 10-s intervals) in an ice water bath and then centrifuged (10,300 g for 20 min at 4 °C). The supernatant was collected and the pellet was resuspended in loading buffer. The cell lysate, supernatant and pellet of cell lysate were subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).
Construction of the Stxyl1 and Stxyl2 surface expression vectors
The gene sequences of Stxyl1 and Stxyl2 were amplified from cDNA of S. turcica, containing an Sfi I restriction enzyme site and homologous arm of pCTcon2, which was cloned into a pCTcon2 vector through One Step Cloning Kit. The recombinant plasmids pCTcon2-Stxyl1 and pCTcon2-Stxyl2 were transformed into DH5α. A colony of positive cells was selected on LB agar plates containing ampicillin (1‰) and screened by direct colony PCR with Stxyl1 and Stxyl2 primers. The extracted plasmids designated pCTcon2-Stxyl1 and pCTcon2-Stxyl2 were subjected to DNA sequencing by BGI, Beijing, China.
Detection of the displayed xylanase by Western blotting
The proteins were obtained from the solution with and without induction and treated in loading buffer. Then the proteins were separated by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) blotting membrane (GE Healthcare). The membrane was blocked with 5% skim milk for 45 min at room temperature and then incubated with the anti-c-Myc (9E10, monoclonal mouse hybridoma supernatant; TransGen Biotech; HT01) for 2 h at room temperature with shaking. Next, the membranes were washed with 1 × TBST buffer (1% 10 × TBS buffer (0.3% Tris, 0.8% NaCl), 0.1% Tween 20) three times (5 min each time) and incubated with goat anti-mouse IgG (rabbit polyclonal; Dako, P0161) for 1 h at room temperature with shaking. After washing again, the proteins were visualized with MaxiLumin-WB (Biokits Technologies Inc., Beijing). A Coomassie blue-stained region of the same membrane used for immunoblotting is shown as a loading control.
Detection of the displayed xylanase activity
The xylanase activity was measured in the SG-Trp medium following the DNS method [Citation17]. Sodium dihydrogen phosphate-citrate buffer (pH 5.0) and SD-Trp were used as control. One unit of xylanase activity was defined as the amount of enzyme required to generate 1 µmol of xylose equivalent per minute at 55 °C and pH 5.0.
RNA extraction and quantitative RT-qPCR
The single colony from SD-Trp agar plate was inoculated into SD-Trp medium, cultured in the shaker and induced in the SG-Trp medium. The solution then was sprayed on the maize leaves. The maize inbred line B73, which was used as the susceptible host for S. turcica infection, was grown in artificial climate chambers under long-day conditions (16 h light/8 h dark). The temperature within the chambers during maize growth was maintained at 25 °C during the light period and 18 °C during the dark period. The temperature was kept at 25 °C when the maize leaves were inoculated with S. turcica. After 5d, 7d, 9d of incubation at 25 °C, the leaves were collected and ground with liquid nitrogen in a mortar, one leaf was selected from each group and each treatment was repeated three times. The total RNA was extracted by using Trizol Up (TransGen Biotech). The concentration of total RNA was measured by BioPhotometer plus (Eppendorf, Germany). And then first-strand cDNA was synthesized by using TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech). Quantitative real time-PCR was performed using CFX Connect Real-Time System (Bio-Rad). The S. turcica tub gene was used as an internal reference control. Levels of mRNA were calculated using the 2−ΔΔCt method.
Data analysis
The GrahPad prism 6.0 software was used for statistical analysis. The results are shown as the mean values with standard deviation (±SD) of three independent experiments and were analyzed by Student's t-test. Differences in all data were considered statistically significant at a level of p < 0.05.
Results and discussion
Phylogenetic analysis of the S. turcica xylanase GH11 family genes
The amino acid sequences of GH11 family genes from S. turcica and other species were downloaded from the JGI database and NCBI, respectively. In order to deeply associate the similarity among these genes, a phylogenetic tree was constructed based on the neighbour-joining method (). It showed that genes from the GH11 family have a high similarity. Stxyl1 and Stxyl3 presented a high similarity with xylanase genes from Stagonospora sp., where Stxyl3 is 100% identical to the xylanase gene from Stagonospora sp. And the similarity between Stxyl2 and the xylanase gene from Alternaria alternate was close to 99%. In addition, Stxyl4 and Stxyl5 also showed a high similarity with xylanase genes from Aureobasidium namibiae.
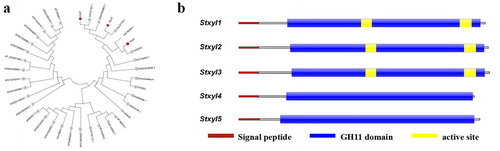
Figure 1. Phylogenetic and conserved domain analysis of the S. turcica xylanase GH11 family of genes. (a) Phylogenetic tree constructed based on the neighbour-joining method to show the relationship between the S. turcica xylanase GH11 family and other accessions. (b) Predicted conserved domain structure of the five genes. Catalytic GH11 domains (the blue part) and the signal peptide (the red part) were drawn to represent their relative positions along the chains of family GH11 xylanase genes. The active site (the yellow part) was analyzed for the prediction of conserved domain structures, which was measured in bits.
Conserved domain analysis of the S. turcica xylanase GH11 family genes
To further describe the domain structures of GH11 family genes, the conserved domains were analyzed using Pfam and SMART online tools (). It showed that a signal peptide composed of 19 amino acids was identified at N-terminus of four genes Stxyl1, Stxyl2, Stxyl3 and Stxyl4, whereas Stxyl5 had a signal peptide of 20 amino acids. These genes all had an extremely conserved GH11 domain, which indicates a high genetic similarity.
In addition, InterProScan online software was also used to predict GH11 genes functional domains. The result showed that five genes all contained a C-terminus domain which belongs to Concanavalin A-like lectin/glucanase domain superfamily. Five genes were predicted to have hydrolase activity and hydrolyze O-glycosyl compounds. Stxyl1 was predicted to have two active sites (aa 118-128 and aa 209-220), Stxyl2 and Stxyl3 were predicted to also have two active sites (aa 122-132 and aa 213-224), whereas Stxyl4 and Stxyl5 were predicted to have no active site. Here, we selected two genes Stxyl1 and Stxyl2 for the next steps in the study.
Prokaryotic expression of Stxyl1 and Stxyl2
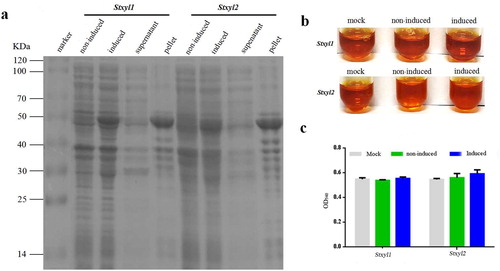
Recombinant plasmids pGEX-6P-3-Stxyl1 and pGEX-6P-3-Stxyl2 were constructed successfully, which were verified by restriction enzyme reaction. Then they were transformed into E.coli BL21 (DE3), subsequently induced by 1 mmol/L IPTG and lysised by ultrasonic disruption. Proteins were separated by SDS-PAGE, which showed that the two proteins were expressed in the form of inclusion bodies (). The pellet was used for analysis of xylanase activity. As shown in ,c), there was no difference in the colour of the enzymatic hydrolysate, which means that no xylanase activity was detected. The absorbance of the enzymatic hydrolysate was determined spectrophotometrically at 450 nm.
Figure 2. Prokaryotic expression of recombinant Stxyl1 and Stxyl2. (a) SDS–PAGE analysis of recombinant Stxyl1 and Stxyl2 at different conditions. (b) Quantitative activity of two xylanases obtained from prokaryotic expression system with the method of DNS. (c) Quantitative activity of two xylanases obtained from prokaryotic expression system by measurement of the optical density at 540 nm (OD540) of the reaction solution. Data are expressed as means with standard errors from three independent experiments.
Yeast surface display of Stxyl1 and Stxyl2
We then took advantage of the Aga2p system of S. cerevisiae to display Stxyl1 and Stxyl2 on the cell surface fusing firmly with the C-terminal of the Aga2p subunit (). First, the recombinant plasmids pCTcon2-Stxyl1 and pCTcon2-Stxyl2 for displaying indicated proteins on S. cerevisiae surface were constructed successfully, where the myc tag was used for immuno-blotting detection of the target proteins.
Figure 3. Yeast surface displaying of recombinant Stxyl1 and Stxyl2. (a) Pattern of yeast surface display system. The protein of interest (xylanase) can be displayed on the yeast cell surface by fusion of its N-terminal end to the C-terminus of Aga2p. (b) Western blot analysis of the expression levels of two xylanases, Stxyl1 and Stxyl2. (c) Qualitative detection of the activity of two xylanases obtained from yeast surface display of recombinant Stxyl1 and Stxyl2 with the method of DNS. (d) Quantitative measurement of the activity of two xylanases obtained from yeast surface display of Stxyl1 and Stxyl2 based on the optical density at 540 nm (OD540) of the reaction solution. Data are expressed as means with standard errors from three independent experiments. (***) P < 0.001.
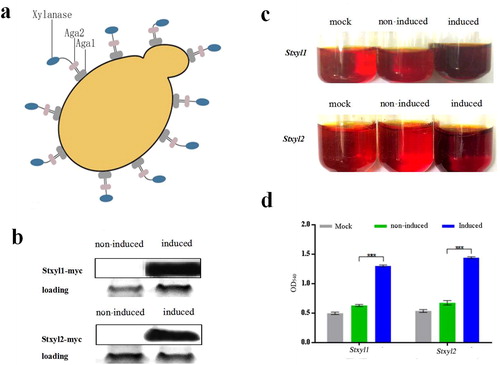
After induction as described in materials and methods, the proteins were extracted for Western Blotting analysis. The mouse anti-myc tag antibody was used as the primary antibody and the goat anti-rabbit IgG was used as the secondary antibody. The results proved that two proteins had been displayed on the surface of yeast cells as single bands of both about 50 kDa (). Then the protein activities were measured. The activities of the two proteins were 16.72 and 19.12 U/mL, respectively ().
Expression analysis of defence genes in corn treated with yeast surface displaying xylanase
To determine whether the yeast-displaying xylanases induce the expression of the defence genes, real-time quantitative PCR analysis was performed to monitor the amount of these selected marker genes. The expression levels of four defense genes (PR-1, PR-4, SOD and CAT) in corn leaves were analyzed by using RT-qPCR after treatment with yeast surface displayed xylanases Stxyl1 or Stxyl2 (). After induction with xylanase Stxyl1, the expression level of PR-1 was lower than that in the control group on the fifth day, but it increased to 2 folds that of the control group on the 7th day, and the expression level decreased on the 9th day. The PR-4 and SOD expression levels showed a trend of increasing and then decreasing after being induced via xylanase Stxyl1. They reached the highest expression level on the seventh day. Compared with the control group, the expression level of gene PR-4′ in the induced and non-induced groups were three-fold and 2-fold higher; the expression level of gene SOD’ in the induced and non-induced groups were 7-fold and 4.7-fold higher, respectively. In addition, the expression level of PR-4 on the ninth day was similar to that on the seventh day, whereas the level of SOD decreased significantly. By contrast, the expression of CAT was continuously up-regulated after induction.
Figure 4. Expression analysis of defence genes in corn treated with yeast surface displaying xylanase. RT-qPCR analysis of the genes PR-1, PR-4, SOD and CAT transcript levels during corn leaves being treated with yeast surface displaying xylanase for 5d, 7d and 9d, respectively. Expression levels were normalized to the expression of the reference gene tubulin. Results are shown as mean ± SD for three independent replicates. Statistical significance compared with control was assessed using one-way ANOVA followed by Student–Newman–Keuls tests.
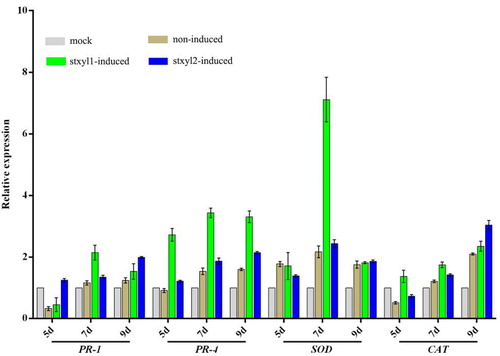
Similarly, the expression levels of PR-1, PR-4 and CAT were continuously up-regulated after induction by xylanase Stxyl2. The expression levels after treatment with the induced yeast were higher than in the other two groups, which were about two to three folds compared to the control group, respectively. However, the SOD expression level showed a descending trend after increasing and reached the maximum value on the seventh day, in which the expression level in the group induced by xylanase Stxyl2 was two-fold higher than that in the control group.
In short, these results indicated that xylanase could be used as an elicitor for YSD to induce corn defence response. As an elicitor, xylanase has been reported to trigger a plant defence response in the plant–pathogen interaction system. But few studies have attempted to demonstrate its application in practice. In this study, we found that almost all the xylanase expressed by prokaryotes existed in the form of inclusion bodies, and it had little activity. However, when xylanase was expressed by YSD, not only its activity increased, but also its yield increased with the increase of yeast content.
As a new eukaryotic expression technology, YSD technology is mainly used for the separation and screening of antibodies. In this study, to our knowledge, we tried to express xylanase by this new technology for the first time. Considerably, the products not only had extremely high enzyme activities but also could trigger expression of corn defence genes. Notably, corn defence genes were induced to a different degree following treatment with the two xylanases. It is possible that these two xylanases have different inducibility or different induction pathways, which need further study for better application.
Currently, eukaryotic and prokaryotic expression systems are still the efficient way to produce target proteins for various purposes. Prokaryotic expression systems, especially the E. coli expression system, are the most widely used protein expression system both on a lab scale and in industry because of the advantages of low cost, large yield of recombinant proteins in a short time, capacity for continuous fermentation, and the simplicity of genetic modifications and so on [Citation18,Citation19]. Nonetheless, the E. coli expression system lacks the posttranslational modifications such as glycosylation, proteolytic protein maturation or limited capacity for formation of disulphide bridges that can form the proper folding of the advanced structures of recombinant proteins [Citation20]. There are some problems arising from the inability of the expressed proteins to be folded correctly; and even overexpressed proteins are often produced in the form of inclusion bodies, from which insoluble aggregates have little or no activity. Although there are some strategies, including the use of high concentrations of denaturants and chaotropes like urea and guanidine hydrochloride (GdnHCl), and even mild solubilization processes using alkaline pH, high pressure, detergents and ganic solvents, for recovery of bioactive proteins from inclusion bodies [Citation21], the use of high concentrations of such agents can sometimes lead to complete denaturation of the existing secondary structures and often results in aggregation of protein molecules during the refolding process. Even regulation of the pH during expression can affect the quality of inclusion bodies [Citation22].
Molecular chaperones have been reported as proteins that can refold the proteins, degrade the misfolded proteins, and even participate in the expression of another protein and make it achieve its native and functional activity, without being present in the final structure [Citation23]. In our experiments, a molecular chaperone was introduced to assist the xylanase proteins to express correctly as soluble proteins; however the activities were still low (data not shown). As reported earlier, YSD is usually used for screening combinatorial protein libraries, isolating antibodies and antibody fragments, non-immunoglobulin protein scaffolds and enzymes [Citation24]. In this study, we take advantage of yeast display technology for the first time as a tool to express xylanase for agricultural application.
Conclusions
Here, we expressed two xylanase genes successfully on the surface of yeast cells. YSD technology was therefore used to express genes from S. turcica. Products were detected for enzyme activities, showing that the activities of two xylanases were high. We finally proved that the YSD xylanase was an efficient strategy to induce corn defense response, as the four defense genes, PR-1, PR-4, SOD and CAT, were induced after treatment with the YSD xylanases.
Acknowledgement
The authors are grateful to all laboratory members for helpful discussions and suggestions in this work.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Shrestha G, Reddy G. Field efficacy of insect pathogen, botanical, and jasmonic acid for the management of wheat midge Sitodiplosis mosellana and the impact on adult parasitoid Macroglenes penetrans populations in spring wheat. Insect Sci. 2019;26(3):523–535.
- Banumathi B, Vaseeharan B, Malaikozhundan B, et al. Green larvicides against blowflies, Lucilia sericata (Diptera, Calliphoridae): screening of seven plants used in Indian ethno-veterinary medicine and production of green-coated zinc oxide nanoparticles. Physiol Mol Plant Pathol. 2018;101:214–218.
- Bastias D, Martinez-Ghersa M, Ballare C, et al. Epichloë fungal endophytes and plant defenses: not just alkaloids. Trends Plant Sci. 2017;22(11):939–948.
- Hanaka A, Nowak A, Plak A, et al. Bacterial isolate inhabiting spitsbergen soil modifies the physiological response of Phaseolus coccineus in control conditions and under exogenous application of methyl jasmonate and copper excess. IJMS. 2019;20(8):1909–1909.
- Zhang P, Li S, Zhao P, et al. Comparative physiological analysis reveals the role of NR-derived nitric oxide in the cold tolerance of forage legumes. IJMS. 2019;20(6):1368–1368.
- Boccardo N, Segretin M, Hernandez I, et al. Expression of pathogenesis-related proteins in transplastomic tobacco plants confers resistance to filamentous pathogens under field trials. Sci Rep. 2019;9(1):1–13.
- Yang Y, Yang X, Dong Y, et al. The Botrytis cinerea xylanase BcXyl1 modulates plant immunity. Front Microbiol. 2018;9:2535–2535.
- Misas-Villamil J, van der Hoorn R. Enzyme-inhibitor interactions at the plant-pathogen interface. Curr Opin Plant Biol. 2008;11(4):380–388.
- Uday U, Choudhury P, Bandyopadhyay T, et al. Classification, mode of action and production strategy of xylanase and its application for biofuel production from water hyacinth. Int J Biol Macromol. 2016;82:1041–1054.
- Terrasan C, Guisan J, Carmona E. Xylanase and β-xylosidase from Penicillium janczewskii: purification, characterization and hydrolysis of substrates. Electron J Biotechnol. 2016;23:54–62.
- Chen H, Liu Y, Liu L, et al. Roles of endoxylanases and their inhibitors in plant-phytopathogen interaction. Plant Physiol Commun. 2009;45(2):176–182.
- Tanaka T, Yamada R, Ogino C, et al. Recent developments in yeast cell surface display toward extended applications in biotechnology. Appl Microbiol Biotechnol. 2012;95(3):577–591.
- Ren R, Jiang Z, Liu M, et al. Display of adenoregulin with a novel Pichia pastoris cell surface display system. Mol Biotechnol. 2007;35(2):103–108.
- Chao G, Lau W, Hackel B, et al. Isolating and engineering human antibodies using yeast surface display. Nat Protoc. 2006;1(2):755–768.
- Jia Y, Ren P, Duan S, et al. An optimized yeast display strategy for efficient scFv antibody selection using ribosomal skipping system and thermo resistant yeast. Biotechnol Lett. 2019;41(8–9):1067–1076.
- Zeng F, Meng Y, Hao Z, et al. Setosphaeria turcica ATR turns off appressorium-mediated maize infection and triggers melanin-involved self-protection in response to genotoxic stress. Mol Plant Pathol. 2020;21(3):401–414.
- McCleary B, McGeough P. A comparison of polysaccharide substrates and reducing sugar methods for the measurement of endo-1,4-β-xylanase. Appl Biochem Biotechnol. 2015;177(5):1152–1163.
- Porowińska D, Wujak M, Roszek K, et al. Prokaryotic expression systems. Postepy Hig Med Dosw (Online). 2013;67(863688):119–129.
- Kamionka M. Engineering of therapeutic proteins production in Escherichia coli. Curr Pharm Biotechnol. 2011;12(2):268–274.
- Yin J, Li G, Ren X, et al. Select what you need: a comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J Biotechnol. 2007;127(3):335–347.
- Singh A, Upadhyay V, Upadhyay AK, et al. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb Cell Fact. 2015;14(1):41.
- Castellanos-Mendoza A, Castro-Acosta RM, Olvera A, et al. Influence of pH control in the formation of inclusion bodies during production of recombinant sphingomyelinase-D in Escherichia coli. Microb Cell Fact. 2014;13(1):137.
- Smith HL, Li W, Cheetham ME. Molecular chaperones and neuronal proteostasis. Semin Cell Dev Biol. 2015;40:142–152.
- Cruz-Teran CA, Tiruthani K, Mischler A, et al. Inefficient ribosomal skipping enables simultaneous secretion and display of proteins in Saccharomyces cerevisiae. ACS Synth Biol. 2017;6(11):2096–2107. ].
