Abstract
The increasing antibiotic application in agriculture has raised a potential risk to human health worldwide. Although high concentrations of antibiotics are lethal for plants without a resistant gene, it is not clear what detoxifying processes act in plants under low antibiotic concentration. Arabidopsis thaliana was selected to characterize the effects of kanamycin antibiotic toxicity on seedling growth in MS plates and pots, respectively. We investigated only control plants treated with kanamycin; but without comparison with transgenic ones, since we had no positive control transgenic plants. Gene expression profiles from plant material in pots were evaluated by customized oligonucleotide microarray. Morphological investigation indicated that kanamycin concentration is negatively correlated with A. thaliana seedling growth. Gene expression profile analysis detected that 542 and 116 genes were up- and down-regulated over five-fold in leaf tissue under the influence of kanamycin. The glutathione S-transferase and peroxidase gene families were involved in antioxidant defense mechanisms by preventing the accumulation of toxic products of reactive oxygen species (ROS). An exceeding amount of ROS after antibiotic-induced oxidative burst stimulated several over-presented processes for the balance between ROS and antioxidants. The significant increase in detoxification enzymes may indicate that efficient antioxidant machinery is the main method to ensure survival.
Introduction
Kanamycin is the most widely used selection antibiotic in plant genetic transformation [Citation1]. Due to its significant screening effect and the biosafety of resistance genes, it has received widespread attention [Citation1,Citation2]. The screening function for transgenic plants is determined by the kanamycin resistance gene, nptII [Citation3]. Kanamycin interferes with protein synthesis in the chloroplasts and mitochondria of plant cells and causes yellowing of the green organs of plants. This process eventually leads to the death of plant cells without the detoxifying ability of nptII gene [Citation4]. Because of the wide use of kanamycin antibiotic, there has been growing public concern about the safety of marker genes that remain in transgenic plants. Although the biosafety of kanamycin-resistant transgenic plants proves to be acceptable [Citation2], there is interest in tolerance of non-transformed plants to antibiotic contaminated soil [Citation5,Citation6]. Therefore, the levels of kanamycin tolerance need to be evaluated, in order to provide informative preliminary data about the detoxifying pathways that act during kanamycin-induced stress.
The toxic effect depends on the sensitivity of the organisms, the duration of exposure and the concentration of toxic substances [Citation7]. The kanamycin effects in plants are species-specific for selection of transgenic plants within a specific growing environment [Citation8]. For example, in cotton (Gossypium hirsutum L.), the optimal kanamycin concentration is >50 mg/L in order to inhibit callus formation [Citation9] and seed germination [Citation10]. In comparison, the optimal concentration for selection in genetically engineered shoots is 20 mg/L in annual wormwood (Artemisia annua) [Citation11]. However, toxicity can already occur at low concentrations. In Arabidopsis thaliana, leaf etiolation and root reduction were worsened as the concentration of kanamycin increased (>10 mg/L) [Citation12]. These studies demonstrated that low kanamycin concentration influences plant growth. Although it is clear that the selective kanamycin concentration inhibits the development of non-transformed plants, we need to throw light on the changes associated with the detoxifying process in harmed plants under low kanamycin concentration.
A wide range of gene expression responses are responsible for the tolerance to antibiotics, such as kanamycin. High-density gene microarray has provided us with an opportunity to study the relative levels of gene transcripts for thousands of genes simultaneously in cells under normal and treatment conditions. Using an Arabidopsis full-length cDNA microarray containing 1300 full-length cDNAs, Seki et al. [Citation13] analyzed the expression of A. thaliana in drought and cold conditions [Citation13]. They found that 30 genes have not previously been reported among 44 drought-induced genes and 10 genes have not been reported among 19 cold-induced genes. Using the same method, Oztur et al. [Citation14] studied the changes in gene expression under drought and high salt stress, and found that 38% of new genes and their functions are unknown [Citation14]. Microarray technology also allows for the study of kanamycin toxicity effects in comparison of transcript abundance in plants. Determining the changes in the gene expression profile is important to explain clearly the detoxifying process and adverse effects on plants under kanamycin treatment.
In the present study, A. thaliana was selected to examine the toxicity of kanamycin antibiotics. The objective was to study the duration and concentration level of kanamycin toxicity and evaluate the gene expression changes to understand the effects of kanamycin on the detoxifying metabolism and physiological functions in non-transgenic plants, compared with kanamycin-resistant transgenic plants.
Materials and methods
Seed germination and kanamycin treatments in experiment
Seeds of A. thaliana L. cv. Columbia obtained from Shanghai Agrobiological Gene Center were surface-sterilized and rinsed with distilled water. We did not investigate the performance of nptII transgenes since we had no positive control transgenic plants. We used 60 seeds in each experiment, and they were sown in solid Murashige and Skoog (MS) medium supplemented with 3% sucrose. The medium was treated with kanamycin (Sigma, St. Louis, MO, USA) at different concentrations: 0, 2.5, 10, 30, 40 and 50 mg/L, respectively. Seeds in the MS plates without kanamycin treatment were used as control. Each treatment had three replicates of the culture vessel. The seeds were subjected to 3 days of cold stratification at 4 °C and then were incubated in greenhouse at constant long-day conditions (16/8 h light/dark, 23 ± 1 °C). About 10 days after growing with the plates oriented vertically, the kanamycin phytotoxicity and growth responses were evaluated by measuring the primary root length and leaf color.
Seedling growth and kanamycin tolerance
The seeds were planted in plastic pots with a mixture soil of vermiculite, peat moss and perlite (18:6:1 v/v), and were grown at 23 °C and 60% humidity. Pot preliminary experiments were conducted by using over ten times the kanamycin concentration applied to MS plates, to determine the kanamycin concentrations. These preliminary results showed that the inhibitor concentration was high in 1400 mg/L kanamycin. Thus, we applied a continuous double concentration of kanamycin (350 mg/L). When A. thaliana seedlings had grown for about 3 weeks, the dry pots were selected for exposure to 0, 350, 700 and 1400 mg/L kanamycin. To avoid kanamycin volatilization, we poured the same volume (100 mL) of different kanamycin concentrations into plastic bags, put each pot with seedlings into a plastic bag, and then used elastic strings to bundle the bag and pot together. This method enabled the soil to absorb kanamycin as quickly as possible. During the period of kanamycin treatments, we added the same volume of water into the bag when the liquid was completely absorbed. After 10 days of treatment, plant growth status was observed.
RNA extraction
The 4-week-old seedlings in pots were selected to study gene expression profiles using microarray. After 10 days of kanamycin treatments, the seedlings were harvested, immediately frozen in liquid nitrogen and stored at −70 °C for RNA isolation. Total RNAs were extracted from pooled leaf tissue by a mixture of three plants in a pot using Trizol reagent (Invitrogen, Carlsbad, CA, USA). By observing the morphological responses of Arabidopsis seedling growth to kanamycin tolerance, we selected the restrained growth of plants under 1400 mg/L kanamycin. Equivalent amounts of RNA from three duplicate plants with 0 (the control) and 1400 mg/L kanamycin treatments were combined into one sample pool for the microarray experiment, respectively.
Microarray analysis
According to the Affymetrix standard protocol, microarray hybridization was conducted by using Arabidopsis (V4) gene expression microarray (Agilent, Palo Alto, CA, USA) in the CapitalBio Corporation (Beijing, China). The Agilent Arabidopsis GeneChip arrays G2519F-021169, which contained 45,220 probes, were used for subsequent hybridization. Two micrograms of total RNA were reverse transcribed into double-strand cDNA. The labeled RNA samples were used for microarray hybridization and scanned in an Agilent GeneArray Scanner (Agilent). Signal intensities were extracted from scanned images using Agilent Feature Extraction Software 10.5.1.1 (protocol: GE1_105_Dec08 and Grid: 21,169_D_F_20100217). Extracted data were filtered with signal values >25, and detected the P flag in all samples. The normalization and fold-change calculations of signal intensities were performed using GeneSpring 11.1 (Agilent). Differentially expressed genes (DEGs) were identified using the signal log ratio (Fold change >3). Gene ontology (GO) enrichment analysis was conducted using AgriGO database (http://systemsbiology.cau.edu.cn/agriGOv2/) [Citation13]. The overrepresented GO terms related to antioxidant machinery are listed in . The dataset from signal intensities was deposited at Gene Expression Omnibus (accession GSE125396).
Table 1. DEGs of kanamycin antibiotic-induced oxidative burst in etiolation of seedling leaves.
Results and discussion
Morphological responses of Arabidopsis seedling growth to kanamycin
The ability to prevent antibiotic-induced oxidative damage is an important feature of plant growth [Citation15]. To observe the morphological responses and transcriptional changes in Arabidopsis seedlings grown in the presence of kanamycin, we sowed seeds of A. thaliana in MS plates and pots with mixture soil in a greenhouse.
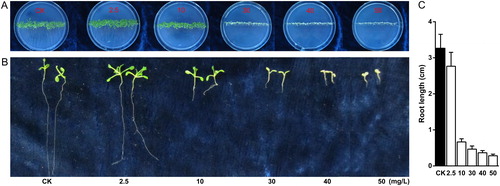
Different kanamycin concentrations, namely, 0, 2.5, 10, 30, 40 and 50 mg/L, were administered in the MS plates and were used to exert stress on seed germination and growth. Following 10 days of culture, we compared the seedling morphology to observe the responses to different kanamycin concentration. Varied performances between seedling color or root length with kanamycin and control (0 mg/L) were highly evident (). The low kanamycin concentrations (<10 mg/L) did not affect the seedling growth. Interestingly, Arabidopsis leaf area increased rapidly with a low kanamycin concentration of 2.5 mg/L in MS plates in comparison to control. When the kanamycin concentration was 10 mg/L, the leaves were yellower () and the main roots were shorter () compared with those of the control. When the kanamycin concentration in MS was 30 mg/L, kanamycin rapidly damaged the growth of A. thaliana seedlings (). Furthermore, increased kanamycin concentration exacerbated the extent of seedling injury, yellowness of seedling color and reduction of root growth, as well as inhibition of seed germination. Therefore, the kanamycin concentration is negatively correlated with Arabidopsis seedling growth, especially over 10 mg/L.
Figure 1. Effect of kanamycin treatment on Arabidopsis seedling (A) and root (B and C) growth.
Note: Seedling growth (A): Seeds were sown on 0 (CK), 2.5, 10, 30, 40 and 50 mg/L kanamycin plates. Root growth (B and C) on kanamycin-containing plates: Root length was measured after 10 d growth. Data points show mean standard deviation, n = 3 for every concentration.
Furthermore, the Arabidopsis seedlings grown in pots were treated with kanamycin solution to observe their tolerance to kanamycin. Using potted plant experiments, A. thaliana seedlings that had grown for 21 days were treated with kanamycin stock solutions in three different concentrations (350, 700 and 1400 mg/L) (). The Arabidopsis seedlings showed poor growth in pots with 1400 mg/L kanamycin stock solution after 10 days of treatment. The seedlings were yellower than the controls, and this color indicated that increased kanamycin concentration decreased the photosynthetic efficiency.
Figure 2. Kanamycin suppresses plant growth.
Note: Arabidopsis seedlings were grown on soil for 21 days then irrigated with 0, 350, 700, and 1400 mg/L kanamycin solution. The picture was taken after 30 d growth.

The seedling morphology in response to kanamycin treatment was similar to that reported by Duan et al. [Citation12]. We found that kanamycin, to a certain extent, disrupted the normal growth of Arabidopsis, but in high concentration caused seedling etiolation and plant death. The leaf is the most important tissue for the products of photosynthesis [15]. The etiolation of seedling leaves in our study indicated decreased chlorophyll content and photosynthetic activity. Furthermore, kanamycin restrained the formation and growth of roots [Citation12]. Roots are essential organs involved in the efficient uptake of water and nutrients [16]. Kanamycin caused shorter roots, without formation of lateral roots. We hypothesize that the observed changes in root formation influenced the supply of nutriments and hormone synthesis. The phenotypic effects on leaves and roots suggested that high kanamycin concentrations decreased photosynthesis and nutrient uptake for the growth and development of Arabidopsis seedlings.
Transcriptional response of Arabidopsis seedlings to kanamycin treatments in pots
To determine the cellular processes responsible for kanamycin tolerance, we conducted gene expression profiling only from plant material in pots to observe the transcriptional response to kanamycin in Arabidopsis seedlings. After 10 days of treatment with 1400 mg/L kanamycin, we found 2320 DEGs induced, with above three-fold changes in expression levels. In this study, we focused our analysis on the genes that showed more than five-fold changes in their expression levels. As a result, we identified 542 up-regulated genes and 116 down-regulated ones. The DEGs indicated that the high concentration of kanamycin induced mainly the up-regulated genes to protect seedling growth. These functional features of DEGs in kanamycin treatments can be easily expected because antibiotics are known to induce cell dysfunction [17].
Functional features of DEGs in kanamycin treatments
An important goal of this study was to dissect cellular processes induced by kanamycin in Arabidopsis seedlings. To characterize the effects of antibiotics on cells in Arabidopsis, we examined the over-represented categories of the DEGs with above three- or five-fold changes. The GO-enrichment analysis of 2320-induced DEGs with above three-fold changes indicated that the response to this stimulus was more frequent among several categories, including cellular response to stimulus, nitrogen compound metabolic process, secondary metabolic process, developmental process, and so on. Furthermore, the developmental process was highly associated with structural developments, including anatomy, phloem, shoots, fruit and seeds. In particularly, the genes related to metabolic processes, such as toxin metabolic process, were highly associated with seedling growth. Several genes that were enriched in the category of transcription and transport further highlighted the differential regulations taking place in kanamycin tolerance. The enriched GO terms were similar when 658 DEGs with above five-fold changes in expression levels were analyzed using GO analysis. DEGs were mainly divided into four groups based on their molecular functions: molecule catalytic function, binding function, transporter function and transcription regulator function. These DEGs were involved in various functional areas, thus indicating that the response to kanamycin stress in Arabidopsis is a complex network that includes multiple physiological and metabolic pathways. Therefore, these functional features of DEGs likely reflected the adaptation of metabolic processes upon exposure to kanamycin.
Functional mechanism of antibiotic-induced DEGs in seedling leaves
To further investigate cell dysfunction induced by kanamycin, we focused on the following GO terms: response to extracellular stimulus (GO:0009991), innate immune response (GO:0045087), toxin catabolic process (GO:0009407), shoot development (GO:0048367) and antioxidant activity (GO:0016209) (). The functional categories of DEGs enabled us to correlate the phenotypic observations with the biological processes [18]. Aminoglycoside antibiotics cause accumulation of reactive oxygen species (ROS) in bacteria; thus, we inferred that oxidative tissue damage was induced. In promoting tolerance to kanamycin antibiotics, plants initially enhance their defense response and innate immune responses [19]. First, DEGs, in response to extracellular stimulus, were up-regulated and induced ROS formation. Second, an excessive amount of ROS acts as a signal for the activation of innate immune responses in plants. The activation of immune response genes significantly enhanced and improved the cell defenses against pathogens and toxins. Additionally, DEGs in the toxin catabolic process were significantly up-regulated. Third, oxidative damage after antibiotic-induced oxidative burst stimulates the over-expression of antioxidants. It was reported that ROS balance has been associated with resistance to pathogens in cotton [Citation22,Citation23]. A total of 15 DEGs as key genes of oxidative stress response were involved in antioxidant defense mechanisms (). These DEGs associated with peroxidase activity (GO:0004601) and the production and scavenging of H2O2 and O2−, which are responsible for the regulation of dynamic changes in ROS levels. Over-accumulation of ROS leads to oxidative stress, and impairs the integrity of cell structure, reduces cell metabolism and even results in cell death [22]. Studies have reported that AT5G06720 is down-regulated in response to dynamic changes in ROS levels. Finally, antibiotic-induced oxidative stress suppresses shoot development by regulating gene expression. These DEGs were mainly composed of transcription factors, which are responsible for defense responses.
We observed that several gene families were involved into the fundamental cellular stress defense mechanism. The glutathione S-transferase (GST) gene family involved in toxin catabolic process acts as detoxification enzymes to allow plants to survive in the presence of environmental toxins and pollutants. GST transcriptome-level responses of AtGSTU1, AtGSTU3, AtGSTU4, AtGSTU6, AtGSTU12, AtGSTU14 and AtGSTU24 in Arabidopsis exposed to kanamycin were significantly induced. Among these, the AtGSTU12 was the most up-regulated gene in non-transgenic Arabidopsis. On the other hand, 15 peroxidase genes were enriched in the category of antioxidant activity. Peroxidases can catalyze the oxidation of a number of peroxides and prevent the accumulation of toxic products, e.g. ROS [Citation25]. The increasing ROS levels can cause significant damage to plant cells exposed to stress environment [Citation26]. PeroxiBase database collected peroxidase sequences from a great variety of plant species, which show that antioxidant defenses are widely present in plants [Citation27]. Sparfloxacin antibiotic induces antioxidative responses, including ascorbate peroxidase, a key enzyme in ROS detoxification [Citation28]. Therefore, this significant increase in expression levels of genes encoding detoxification enzymes may indicate that efficient antioxidant machinery is the main method to ensure survival from kanamycin-induced oxidative tissue damage.
Conclusions
High kanamycin concentration decreased nutrient uptake for the growth and development of Arabidopsis seedling due to a shorter root and fewer lateral roots. The identified DEGs were associated with cellular processes mainly involved in antioxidant defense mechanisms in response to cellular oxidative damage by ROS in seedling etiolation. However, the special functions of the genes involved in kanamycin response should be concretely and further studied by using other methods, such as transgenic genes and mutation analysis.
Acknowledgements
The authors acknowledge the Shanghai Platform of Crop Germplasm Resources (18DZ2293700) and Platform of National Crop Germplasm Resources (NICGR2019-021) for providing them with Arabidopsis thaliana seeds.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Gay PB, Gillespie SH. Antibiotic resistance markers in genetically modified plants: a risk to human health? Lancet Infect Dis. 2005;5(10):637–646.
- Nap JP, Bijvoet J, Stiekema WJ. Biosafety of kanamycin-resistant transgenic plants. Transgenic Res. 1992;1(6):239–249.
- Reiss B, Sprengel R, Will H, et al. A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene. 1984;30(1–3):211–217.
- Wang X, Ryu D, Houtkooper R H, et al. Antibiotic use and abuse: A threat to mitochondria and chloroplasts with impact on research, health, and environment. BioEssays. 2015;37(10):1045–1053. doi:10.1002/bies.201500071.
- McManus PS, Stockwell VO. Antibiotic use for plant disease management in the United States. Plant Health Prog. 2001;2(1):14.
- McManus PS, Stockwell VO, Sundin GW, et al. Antibiotic use in plant agriculture. Annu Rev Phytopathol. 2002;40:443–465.
- Berry M, Gurung A, Easty DL. Toxicity of antibiotics and antifungals on cultured human corneal cells: effect of mixing, exposure and concentration. Eye. 1995;9(1):110–115.
- Joersbo M, Okkels FT. A novel principle for selection of transgenic plant cells: positive selection. Plant Cell Rep. 1996;16(3–4):219–221.
- Zhang B, Liu F, Liu Z, et al. Effects of kanamycin on tissue culture and somatic embryogenesis in cotton. Plant Growth Regul. 2001;33(2):137–149.
- Umbeck P, Swain W, Yang N. Inheritance and expression of genes for kanamycin and chloramphenicol resistance in transgenic cotton plants. Crop Sci. 1989;29(1):196–201.
- Chen D, Ye H, Li G. Expression of a chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci. 2000;155(2):179–185.
- Duan H, Ding X, Song J, et al. Effects of kanamycin on growth and development of Arabidopsis thaliana seedling, cotyledon and leaf. Pak J Bot. 2009;41:1611–1618.
- Seki M, Narusaka M, Abe H, et al. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell. 2001;13(1):61–72.
- Oztur ZN, Talame V, Deyholos M, et al. Monitoring large-scale changes in transcript abundance in drought- and salt-stressed barley. Plant Mol Biol. 2002;48(5–6):551–573.
- Jones J D G, Vance R E, Dangl J L. Intracellular innate immune surveillance devices in plants and animals. Science. 2016;354(6316):aaf6395–aaf6395. doi:10.1126/science.aaf6395.
- Tian T, Liu Y, Yan H, et al. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017;45(W1):W122–W129.
- Luo R, Wei H, Ye L, et al. Photosynthetic metabolism of C3 plants shows highly cooperative regulation under changing environments: a systems biological analysis. Proc Natl Acad Sci U S A. 2009;106(3):847–852.
- Rogers ED, Benfey PN. Regulation of plant root system architecture: implications for crop advancement. Curr Opin Biotechnol. 2015;32:93–98.
- Darqui FS, Radonic LM, Lopez N, et al. Simplified methodology for large scale isolation of homozygous transgenic lines of lettuce. Electron J Biotechnol. 2018;31:1–9.
- Su Z, Ma X, Guo H, et al. Flower development under drought stress: morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis. Plant Cell. 2013;25(10):3785–3807.
- Bao Z, Hua J. Linking the cell cycle with innate immunity in Arabidopsis. Mol Plant. 2015;8(7):980–982.
- Lehmann S, Serrano M, L'Haridon F, et al. Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry. 2015;112:54–62.
- Yang J, Zhang Y, Wang X, et al. HyPRP1 performs a role in negatively regulating cotton resistance to V. dahliae via the thickening of cell walls and ROS accumulation. BMC Plant Biol. 2018;18(1):339.
- Cui HP, Zhou W, Guo CH. The role of plant peroxisomes in ROS signalling network. Chin J Biochem Mol Biol. 2017;33:220–226.
- Karthikeyan M, Jayakumar V, Radhika K, et al. Induction of resistance in host against the infection of leaf blight pathogen (Alternaria palandui) in onion (Allium cepa var aggregatum). Indian J Biochem Biophys. 2005;42(6):371–377.
- Caverzan A, Passaia G, Rosa SB, et al. Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol. 2012;35(4(suppl)):1011–1019.
- Koua D, Cerutti L, Falquet L, et al. PeroxiBase: a database with new tools for peroxidase family classification. Nucleic Acids Res. 2009;37:D261–D266.
- Margas M, Piotrowicz-Cieslak AI, Michalczyk DJ, et al. A strong impact of soil tetracycline on physiology and biochemistry of pea seedlings. Scientifica. 2019;2019:3164706.
