Abstract
The SQUAMOSA promoter binding protein (SBP)-box consists of a family of transcription factors unique to plants and mediates important roles in plant growth and development. We identified 57 SBP genes in the Brassica juncea (swollen-stem mustard) genome, and analyzed the structural characteristics, evolutionary relationships, as well as their involvement in regulation and response to environmental stress. By the help of phylogenetic analysis, the SBP genes were divided into seven subgroups (G1-G7). Interestingly, most members of the same group shared conserved gene structure and motifs as well as gene duplications. This suggests that their functions are redundant highlighting evolutionary relatedness. Identification of the cis-acting elements of the promoter revealed multiple regulatory elements that responded to phytohormone or abiotic stresses. In addition, data from GO annotation indicated that BjuSBPs play a role in biological processes. Further interrogation of the expression of BjuSBPs in different stages of swollen-stem mustard showed spatiotemporal expression patterns. On the other hand, quantitative real-time polymerase chain reaction (qRT-PCR) showed that the expression levels of SBP genes at the development stage correlated with their transcriptome data but treatment with phytohormones affected their expression levels. Our study showed that the SBP-box responds to phytohormone signals and the genes might confer different functions in the development of the mustard plant. Our data lay the foundation for further studies evaluating the regulatory mechanisms of SBP-box family of genes.
Introduction
Gene expression is central to efficient plant growth and development. Regulation of the gene expression by transcription factors ensures faithful and timely gene transcription and expression that enhances specificity in many important biological processes [Citation1]. At least 58 transcription factor families have been identified in the plant genome [Citation2]. More than 5% of genes in the Arabidopsis genome code for more than 1500 transcription factors [Citation3]. In rice, 4% of genes code for transcription factors [Citation4].
SQUAMOSA promoter binding protein (SBP)-box is a family of plant-specific transcription factors. SBP-box proteins contain a highly conserved SBP domain of approximately 76 amino acid residues. This domain is involved in DNA-binding and nuclear localization with two zinc-binding sites (Cys-Cys-His-Cys and Cys-Cys-Cys-His) [Citation5, Citation6]. The initially identified SBP-box genes, AmSBP1 and AmSBP2, have been shown to be involved in the control of early flower development in Antirrhinum majus [Citation7]. Since then, the SBP-box family of genes have been isolated and identified in the main flora, such as Physcomitrella patens [Citation8], Oryza sativa (rice) [Citation9], Malus pumila (apple) [Citation10], Petunia [Citation11] or Gossypium (cotton) [Citation12].
Previous research has demonstrated that the SBP-box transcription factors are involved in the regulation of plant growth and development as well as in stress response [Citation13]. In Arabidopsis, SPL3, SPL4 or SPL5 can regulate the development and photoperiod flowering through interaction with bZIP protein mediated-signals [Citation14]. During fruit development, ZjSBPs were specifically expressed in the mature leaves and fruits of Ziziphus jujuba [Citation15], and papaya CpSBP1 was also involved in the transcriptional regulation mechanism of fruit ripening [Citation16]. BplSPL2 obtained from Betula platyphylla was specifically expressed during inflorescence development as well [Citation17]. On the other hand, AtSPL1 and AtSPL12 made Arabidopsis thermo-tolerant during the reproductive phase, whereas CaSBPs were differentially expressed under Phytophthora capsici stress [Citation18, Citation19]. In addition, the SBP gene in Chlamydomonas carried a copper response element that maintained copper homeostasis in the body [Citation20]. Other studies have shown that the SBP-box is involved in regulating hormone signal transduction pathways which affect plant growth and development. For example, AtSPL8 in Arabidopsis affected gibberellin-mediated development [Citation21], whereas some CaSBP genes in pepper may be involved in hormone regulation pathways such as salicylic acid or methyl jasmonate [Citation19]. With the identification and characterization of microRNAs (miRNAs), important gene regulatory functions in plants have been discovered. SBP-box plays a key role in these miR156-mediated regulatory functions that aid in plant development [Citation22]. In tomatoes, miR156 regulates the SBP-box gene in ovarian and fruit developmental functions [Citation23].
Mustard plant (Brassica juncea) is rich in nutrients and glucosinolate. After a long period of evolution, its vegetative organs have differentiated, forming 16 varieties of 4 major categories of roots, stems, leaves and calyxes [Citation24]. Tuber mustard (Brassica juncea var. tumida Tsen et Lee) is a stalk variety of mustard whose swollen fleshy stems are mainly used for mustard processing [Citation25]. Stem mustard can swell to 20 cm, which reflects quality and yield. The process of stem enlargement has been explored through cell activity studies, linkage maps and whole-genome analysis. These studies have laid the theoretical foundation for the molecular mechanism for the tumor stem development and its molecular breeding [Citation26, Citation27].
However, data on the roles of the SBP-box family of genes on growth and development of the mustard plant are scarce. This study reports the whole genome identification of the exon-intron structure and homology analysis. Differential gene expression levels at different stages of stem expansion as well as research on response to hormones were also determined. Our results provide theoretical basis for the study of BjuSBPs in mustard, and highlight the role of SBP-box family of genes in the development of the mustard plant.
Materials and methods
Identification and screening of SBP-box genes in B. juncea
To build a local database, the gene and protein sequences were downloaded from the B. juncea genome (V1.5) (http://brassicadb.org/brad/). The HMMER3.0 profile of the SBP-box domain (PF03110) was used to search the database using BLASTP program [Citation28], which uncovered 57 B. juncea transcript IDs from the SBP-box gene family. All the transcripts with E > 1e−10 were removed. The number of amino acids, molecular weight, predicted theoretical isoelectric point (pI), exon number and subcellular localization were calculated using the ProtParam tool (http://www.expasy.org/tools/protparam.html). Subcellular localization analysis was performed using WOLF PSORT (https://www.genscript.com/wolf-psort.html). The ExPASy tool (https://www.expasy.org/tools/ProtParam.html) was used to predict the physicochemical properties of the protein [Citation29].
Phylogenetic and syntenic relationships among species
The SBP sequences of Arabidopsis thaliana and rice were downloaded from TAIR (https://www.arabidopsis.org/) and PlantTFDB (http://planttfdb.cbi.pku.edu.cn/). The amino acid sequences of all SBPs were aligned using ClustalX (V2.1) with default settings, and the neighbor-joining method with 1000 bootstrap replicates was used to construct a phylogenetic tree by MEGA7.0 [Citation30]. The genome sequence and annotations of Brassica vegetables were obtained from the Brassica Database (http://brassicadb.org/brad/). The symbiotic relationship between species was analyzed by MCScanX software, and visualized by TBtools software [Citation31].
Analysis of gene structure, conserved motif and promoter region
The structures of the SBP-box genes were generated using GSDS, whereas MEME was used for protein sequence motif analysis [Citation32]. The motif structure was visualized using TBtools software. The SBP conserved domain analysis of BjuSBP protein was performed on the ClustalX alignment and the results were viewed in GenDOC.
Gene location, gene family replication and promoter region
The chromosomal distribution of the genes was drawn by MapChart (Version 2.1), whereas MCScanX was used to find synteny blocks in the genome [Citation33]. A promoter sequence of 1500 bp upstream of the initiation codon of each BjuSBP gene was obtained from the mustard genome database, and the cis-acting element was identified using the PlantCARE program [Citation34].
Analysis of functional annotation and interaction network
Based on sequence similarities, we used Blast2GO to annotate the BjuSBP protein sequence with GO (Gene Ontology). Both the GO terms and annotations were assigned to each BLAST hit [Citation35]. In addition, the STRING program was used to analyze the specific protein interactions between SBP-box transcription factors in mustard, whereas the interaction network and expression synergistic interaction between gene pairs was visualized by Cytoscape v3.7.
Plant materials, phytohormone treatments and RNA extraction
The experimental samples used for the analysis of the expression pattern at different stages of development were obtained from the four stages of the swelling stem of tuber mustard variety ‘Fuza No. 2’. All samples were grown in pots in a controlled-environment growth chamber under a photoperiod of 16 h with 300 μmol/(m2 s) light intensity at 25 °C and 8 h/16 h dark/light regime at 16 °C, and 55% humidity. For hormone treatment, plants at four weeks of age with 5-6 true leaves were used in the experiments. The seedlings were sprayed separately with auxin (IAA) (10 μmol/L), abscisic acid (ABA) (75 μmol/L), salicylic acid (SA) (0.5 mmol/L) and gibberellin (GA) (50 μmol/L) and placed for 24 h, all treatments were set up three biological replicates. The samples were frozen in liquid nitrogen immediately after collection and stored in a −80 °C refrigerator. Total RNA was extracted from four stem swelling and hormone-treated seedling samples using a total RNA Kit (Tiangen, Beijing, China), and then using the PrimeScript RT Kit (TaKaRa, Dalian, China) with oligonucleotides (dT) and random primers for reverse transcription synthesis of cDNA.
Quantitative transcript analysis and qRT-PCR validation
The unique expression pattern of all genes in mustard during the stem swelling was obtained from the transcriptome sequencing that had been completed by our lab. Sequencing data was obtained through the NCBI website under the accession number SRP151320. The Pearson correlation test was used to analyze the differential gene expression during the development stages. The expression abundance of BjuSBP gene in different developmental stages of the mustard was based on transcriptome data as reflected by FPKM value. The heat map was constructed by MeV program available at (http://www.tm4.org/).
A Bio-Rad CFX96TM real-time PCR system (Bio-Rad, California, USA) was used for qRT-PCR. All of the experiments were performed in triplicate, and UBC was used as the internal control to normalize the gene expression [Citation36]. The relative expression levels of BjuSBPs were calculated using the 2−ΔΔCT method [Citation37]. Primer pairs ware designed using Primer Premier 5.0 software and shown in Supplemental Table S1.
Results and discussion
Identification of BjuSBP transcription factor genes in B. juncea
A total of 57 SBP-box genes, referred to as BjuSBP01 to BjuSBP57, were identified (Supplemental Table S2). Most of the genes were localized in the nucleus. The BjuSBP polypeptides had between 106 (BjuSBP52) and 9800 (BjuSBP04) amino acids, and had a molecular weight ranging from 12.16 (BjuSBP52) to 114.80 (BjuSBP06) KDa. The polypeptides had an isoelectric point of 5.06 to 10.26. Aliphatic or aromatic amino acids accounted for 14% or 8% respectively of the polypeptide. All BjuSBPs had negative GRAVY values, indicating that they were all hydrophilic. The hydrophobicity of G2 was generally low, with an average of −1.189, suggesting that the subgroup proteins were highly hydrophilic. At the same time, most of the BjuSBPs polypeptides were unstable except BjuSBP45.
Phylogenetic analysis of SBP-box genes
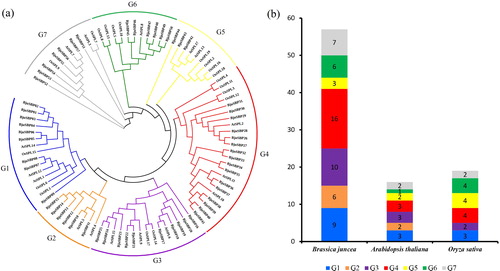
To dissect the evolutionary relatedness of the SBP domain-containing proteins in mustard, two model plants, Arabidopsis thaliana and Oryza sativa were selected for phylogenetic analysis. Phylogenetic analysis data showed that 92 plant sequences contained 57 BjuSBPs, 16 AtSPLs and 19 OsSPLs. The sequences were classified into seven distinct groups (). Group G4 had the highest number of BjuSBP genes (16 BjuSBP genes), whereas G2 formed the smallest group with only 5 members (). In contrast, there was high SBP gene homology between mustard and Arabidopsis. Group G2 contained only SBPs from Arabidopsis and mustard, revealing the diversity of SBP genes between monocot and dicot plants.
Figure 1. Phylogenetic analysis of SBP-box proteins among B. juncea, Arabidopsis and Oryza sativa by MEGA7.0. (a) The full-length SBP domain protein sequences from B. juncea (BjuSBP), Arabidopsis (AtSPL) and O. sativa (OsSPL), each of group were aligned by ClustalX, and the branched lines of the subtrees with different colors indicated different SBP-box subgroups. (b) SBP-box family members of B. juncea, Arabidopsis and O. sativa.
Analysis of BjuSBP genes structure
Except for BjuSBP52, all other genes contained from one to nine introns, with BjuSBP40 having the longest intron sequence (). The BjuSBPs with closer homology were more similar in structure. For instance, BjuSBP01-08 belonging to G1 and BjuSBP32-39 belonging to G4 had the same number of exons and introns, and were more conserved.
Figure 2. Phylogenetic relationship, exon–intron structure and conserved motifs analysis of SBP-box genes in B. juncea. (a) The phylogenetic tree created by MEGA 7.0 and exon–intron structures from online software GSDS. Also boxes and lines represent exons and introns, respectively. (b) Conserved motifs predicted in BjuSBP protein. The fifteen motifs were identified by MEME program, with each number of colored box representing a different motif.
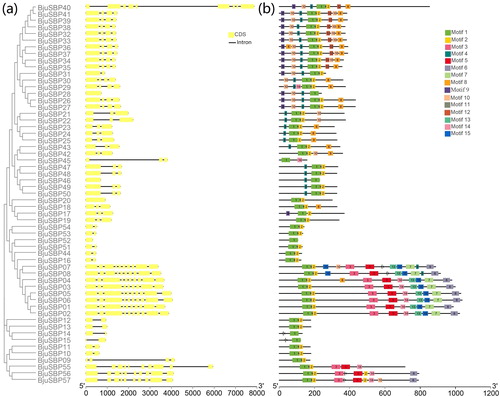
In order to better understand the composition and diversity of motifs in the mustard protein sequences, we used MEME Suite to search for protein motifs. We identified 15 distinct motifs with a length ranging between 6 and 50 amino acids as shown in and Supplemental Table S3. Analysis of the mustard SBP domain showed that motif 1 and 11 were Zn finger motifs, whereas motif 2 had a nuclear localization signal (Supplemental Figure S1). Besides, all the BjuSBPs contained the Zn finger motif 1, suggesting high level of amino acid conservation. Motifs 11 and 2 were absent in BjuSBP14, 15, 28, 45, 46, or 52, whereas there was a missing sequence in the BjuSBP25 of the SBP domain. The BjuSBP01-08 of group G1 had motifs 7, 13 and 15. However, there were also significant differences in the arrangement and combination of motifs between each subgroup, hence the sequence diversity in the mustard.
The BjuSBP gene structure displayed its own characteristics in different subgroups, ranging from different intron numbers to identification of motifs specific to G1 and G7 subgroups. Even though some BjuSBPs in the subgroup had similar structures and motif distributions, there were still differences, indicating that the structural diversity was proportional to the functional diversity of the SBP-box family of genes [Citation38]. In addition, the SBP domain in BjuSBPs was pivotal in the construction of phylogenetic relationships [Citation5].
Chromosomal distribution and duplication of BjuSBPs
A total of 52 genes were found to be unevenly distributed on 17 chromosomes except ChrB05, while the remaining five genes were located on unanchored contigs (). Whereas there were more genes in the A sub-genome, ChrB03 in the B sub-genome contained the most BjuSBPs (8 genes). Mapping and sequence alignment of the BjuSBP genes showed that there were at least 22 gene pairs with more than 90% sequence similarity. The data show that the genes are homologous, of which 15 pairs are between the sub-genome A and B (Supplemental Table S4). In addition, it was observed that the pairs BjuSBP28 and BjuSBP31, BjuSBP32 and BjuSBP34, BjuSBP36 and BjuSBP38, BjuSBP37 and BjuSBP39, or BjuSBP46 and BjuSBP50 clustered in ChrB02, ChrA09, ChrA07 or ChrB03, respectively, which may be attributed to chromosomal tandem duplication. Gene fragment and tandem replication are important ways that define gene family expansion and protein evolution. Our study found that a large number of SBP gene pairs had a cross-collinearity relationship, and more fragment repeats occurred on each chromosome ().
Figure 3. Duplications of BjuSBP genes in the B. juncea chromosomes. The homologous region is represented with different colors. Chromosome numbers are indicated at the top of each bar. The scale bar on the left indicates the size of chromosomes.
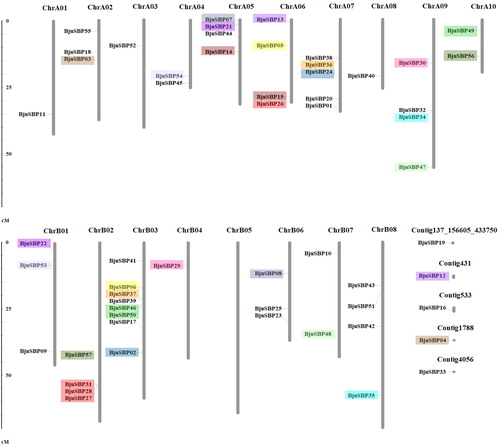
Figure 4. Analysis of the successive duplications of the SBP-box genes in the mustard genome. Among them, 18 chromosomes and four contigs were arranged in a circle with a single color. The gray line connected a pair of duplicate genes generated by doubling the genome. The red line connecting the chromosomes represented the syntenic region of the BjuSBP genes.
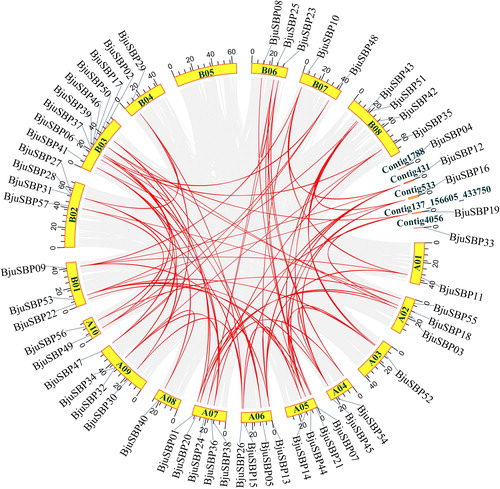
In summary, tandem repeats and fragment replication may be the main reasons that account for the diversity of the BjuSBP family of genes. Both A and B sub-genomes of the mustard showed gene tandem and fragment duplication. Besides the segment duplication defining expansion of the SBP-box family of genes in the evolution of the mustard [Citation39], it accounted for the large number of homologous gene pairs in the mustard genome. In addition, duplication also compensated for the chromosomal segment loss that occurred during the evolution [Citation40].
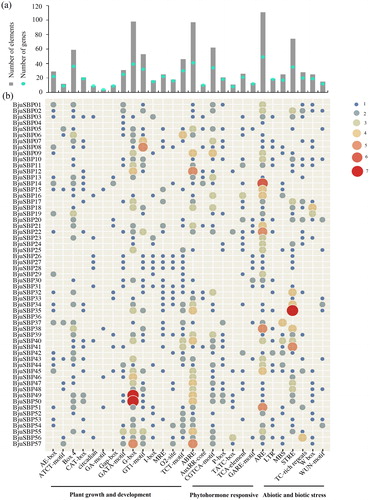
Analysis of the cis-regulatory elements in BjuSBPs promoter region
A large number of cis-acting elements related to plant growth and development, phytohormone and stress response were identified in all the BjuSBP genes, as shown in . Ninety-eight, 59 or 53 elements involved in light responsiveness were identified in G-Box, Box4 or GT1-motif, respectively, in BjuSBPs, whereas the most widely distributed element in growth and development was concentrated in G-Box. Twenty-one elements related to meristem expression during plant growth and development (CAT-box) and nine circadian control elements (circadian) were also identified. Among hormone-related elements, a total of 97 ABA-responsive elements (ABRE), 62 MeJA-responsive elements (CGTCA-motif), 45 gibberellin-responsive elements (P-box, GARE-motif and TATC-box), 26 salicylic acid (TCA-element) and 11 auxin-responsive elements (AuxRR-core) were found. ABRE elements were more abundant in BjuSBPs. The identification of a large number of hormone-responsive elements in the promoter region indicated that hormones play an important role in plant growth and development. On the other hand, 111 anaerobic induction elements (ARE), 74 stress response elements (STRE), 25 drought induction elements (MBS) and 19 low-temperature responsive elements (LTR) were detected in the BjuSBP gene promoters. Interestingly, ARE was the most widely distributed element in the BjuSBP family of genes. In addition, G-box and ABRE, two elements related to development and hormonal-responsiveness, were concentrated in two subgroups; G6 and G7. These findings suggest that BjuSBPs may be involved in the transcriptional regulation of various processes including development, phytohormone and stress response.
Figure 5. Cis-regulatory elements analysis in the promoter region of BjuSBP genes. (a) The total number of BjuSBPs for each homeopathic element (gray bars), and the total number of BjuSBPs including the corresponding cis-acting element (blue dots). (b) Cis-acting elements are divided into three categories based on functional annotation: plant growth and development, phytohormone responsive, or abiotic and biotic stresses-related cis-acting elements.
Functional annotation for the BjuSBP genes
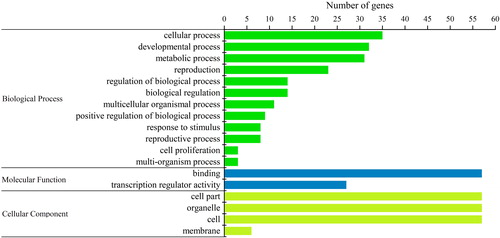
Functional annotations analysis for the 57 BjuSBP genes was performed by Blast2GO in the GO database. A total of 365 annotations were obtained and classified as biological process (BP), molecular function (MF) and cellular component (CC) or 18 terms ( and Supplemental Figure S2). Most of the BjuSBPs were categorized in the biological processes, especially cellular, developmental, metabolic or reproduction processes. Fifty-seven genes classified in the molecular function category had a binding role, while others showed transcriptional regulator roles.
Interaction network analysis of the BjuSBPs
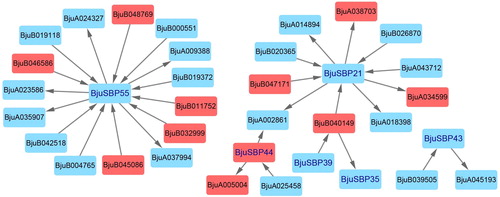
To further understand the regulatory network of the SBP-box transcription factor, a protein–protein interaction network between BjuSBPs and other proteins in B. juncea genome was built (). The results showed that a total of six BjuSBP proteins showed interactions with 29 proteins. The BjuSBP21 or BjuSBP55 showed interactions with 15 or 10 proteins, respectively, indicating that they had an effect on the regulation of transcription and biological processes of mustard. These genes were closely related to AtSPL7 and AtSPL9 as depicted by the phylogenetic analysis. Previous data have shown that BjuSBP55 may be involved in regulating the homeostasis of metal ions in plants [Citation41]. On the other hand, BjuSBP21 plays an important role in plant leaf formation and development [Citation42]. Like the functional annotation of StSBPs in potatoes, the GO terms showed that most of the BjuSBP genes were involved in the development process [Citation43]. This finding supports the role of the SBP gene in regulating mustard stem development. In addition, three BjuSBP proteins, BjuSBP21, BjuSBP35 and BjuSBP39, interacted with BjuB040149. On the other hand, most of the other mustard genes that interacted with the six BjuSBP genes were down-regulated, including BjuSBPs but not BjuSBP44.
Collinearity analysis of three cruciferous crops
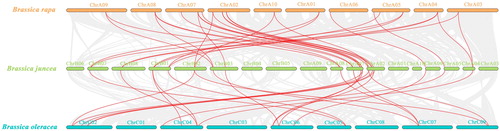
There were 2675 synteny blocks between the Brassica rapa and B. juncea genomes, and 72.07% of them were collinear. The largest linear region had 1401 pairs of genes shared between the B. rapa ChrA02 and B. juncea ChrA02. On the other hand, of the 3310 synteny blocks between the B. juncea and Brassica oleracea genomes, the longest was between the B. juncea ChrA09 and B. oleracea ChrC08, with 853 pairs of genes. Gene pairs that were collinear with 17 BjuSBP genes were detected in all three species (). Our data showed that there were more homologous genes between B. rapa and B. juncea, suggesting that their genomes had a high evolutionary relatedness.
Figure 8. Analysis of collinearity among B. rapa, B. juncea and B. oleracea genomes. Colored circular rectangles denote the chromosomes of three cruciferous plants. All bezier curves connect protein-coding genes in synteny blocks, and some synteny blocks of B. juncea and B. rapa, B. juncea and B. oleracea are removed, which are less than 80 and 60 pairs of homologous genes, respectively. The red curves represent gene pairs that are collinear with some BjuSBP genes.
In the ‘U-triangle’ model, B. juncea (AABB, 2n = 36) derive from the natural hybridization between B. rapa (AA, 2n = 20) and B. nigra (BB, 2n = 16), and B. napus (AACC, 2n = 38) is a natural hybridization between B. rapa and B. oleracea (CC, 2n = 18). Both of them belong to the heterotetraploid, and the number of SBP-box genes should be roughly equal [Citation44]. Fifty-eight SBP-box genes have been identified in B. napus genome, similar to the number of SBP-box genes in B. juncea, which is in line with the above theory. A collinearity analysis of B. rapa, B. juncea and B. oleracea genomes, showed a higher number of synteny blocks and homologous gene pairs between B. rapa and B. juncea genomes as compared to that between B. juncea and B. oleracea. Further, it was shown that B. rapa was part of the ancestry string for B. juncea [Citation45].
Gene expression analysis
Transcript abundance analysis of BjuSBPs across the stem developmental stages
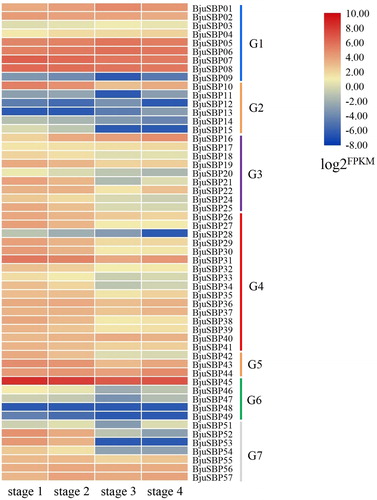
To explore potential functions of the SBPs in the development of mustard, transcriptome data from different periods of mustard stem enlargement were analyzed. With the exception of BjuSBP23 and BjuSBP50, the expression levels of the remaining 55 genes were detected and plotted as a heat map (). Interestingly, the expression patterns of different genes in the same group were different, suggesting different evolutionary fate even for homologous genes. However, the expression pattern for SBP gene in mustard was similar across the different groups. The expression levels of BjuSBP05, 06, 07 and 08 belonging to group G1 were high and similar in all the four stages of stem development. Similarly, the transcriptome data showed that most genes belonging to the G3 and G4 in the four stages were highly expressed in early stages of development but down-regulated in maturation, similar to peanut seed formation [Citation46]. The expression of some genes belonging to G2 and G6 was very low at all the stages, whereas some SlySBP genes in tomatoes were hardly expressed during fruit development [Citation12]. This finding shows that the SBP-box genes were involved in the entire process of growth and development of the mustard.
Figure 9. Expression profiles of BjuSBP genes by the analysis of transcriptome data of different developmental stages of mustard. The color scale bar on the right side of the heat map corresponds to the value converted by log2FPKM, where red indicates higher transcription abundance and blue indicates lower.
Quantitative expression profile of the BjuSBP genes at different stages of stem swelling
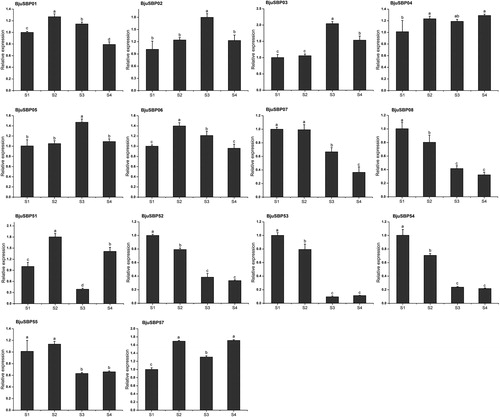
To find out the roles of BjuSBPs in the development of the mustard, we selected genes from G1 and G7 with higher transcript abundance and more cis-regulatory elements. The expression levels of BjuSBPs in the four stages of stem enlargement (S1 to S4) were detected by qRT-PCR (). As shown in the heat map, the relative expression at each stage had no obvious fluctuations except for BjuSBP53 and BjuSBP54, indicating that they were significantly down-regulated in the later stages of development. In G1, BjuSBP02, 03, and 05 exhibited significant relative expression levels at S3, whereas BjuSBP01 and BjuSBP06 showed relative expression at S2, which implied that these genes primarily regulate the middle stage of mustard development. The results of the qRT-PCR showed that the expression of BjuSBP02-06 in G1 was significantly up-regulated in the swelling stem. Similarly, VvSBPs belonging to G1 were highly expressed during late stages of fruit maturation in grape development [Citation47]; thus the five genes might play an important role in the mustard stem swelling process. However, compared with G1, the genes in G7 except BjuSBP57 had higher expression levels at S1 and S2. In other words, the expression of some genes in G7 in the early stage of stem swelling was much higher than that in the later stage. The phylogenetic analysis showed that the expression levels of SmSPL4 and SmSPL7, which were close to AtSPL7, decreased at maturation of Salvia miltiorrhiza [Citation48]. Furthermore, it was observed that BjuSBP53 and BjuSBP54 or BjuSBP56 and BjuSBP57 were homologous, thus they might have similarities in function.
Expression of BjuSBPs in response to the treatment with phytohormone
Since we identified phytohormone responsive elements in the promoter region, we explored the expression pattern of some genes belonging to G1 and G7 in response to gibberellin (GA), abscisic acid (ABA), salicylic acid (SA) or auxin (IAA) (). After SA treatment, the relative expression levels of eight genes in G1, except BjuSBP06, were higher compared to the untreated control. In addition, GA or ABA treatment increased the expression of BjuSBP02 or BjuSBP08 respectively. On the other hand, the expression levels of BjuSBP01 and BjuSBP06 were down-regulated after IAA treatment. Besides, unlike BjuSBP51, the expression of BjuSBP52, 55, 56 or 57 in group seven were significantly up-regulated under treatment with the four phytohormones.
Figure 11. Expression profiles of BjuSBPs in response to treatment with gibberellin (GA), abscisic acid (ABA), salicylic acid (SA) and auxin (IAA).
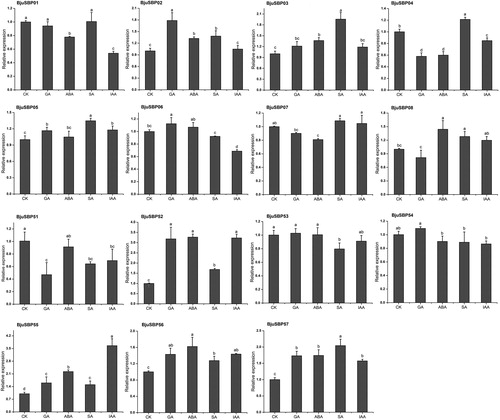
Certain phytohormones can mediate various defense-related signal transduction pathways and activate the transcription of related effector genes [Citation49]. For example, GA regulates the development of floral organs [Citation50], whereas SA is involved in the transmission of pathogen infection signals [Citation51]. Based on the promoter region analysis, we selected some BjuSBP genes to determine the expression levels of exogenous phytohormones. Most genes in mustard G1 were significantly up-regulated under SA treatment. After 12 h of SA treatment, the expression levels of BrSBP5 and BrSBP13 in Chinese cabbage G1 were significantly increased [Citation52]. Besides, PeSPLs were also found to play an important role in regulating stress response in Moso bamboo [Citation53]. AtSPL8 in Arabidopsis was involved in regulating the signal transduction pathway GA [Citation54]. In addition, it was observed that some genes in the mustard G7 could be up-regulated or down-regulated under the four phytohormones pressure. However, it can be speculated that BjuSBPs were involved in the response of various phytohormone signals.
Conclusions
In this study, 57 members of the SBP-box genes were identified in the B. juncea genome. Phylogenetic and collinearity analysis showed the homology relationship between mustard and other species. The corresponding functions were inferred following the functional annotations. The interaction network of BjuSBPs and other proteins was explored and maps developed. The expression profile of the selected BjuSBP genes showed that their expression was stage-specific, responded to and regulated signals from phytohormones. The identification and profiling of the BjuSBP genes show that they influence the development of mustard. These data give insights for further research on the SBP-box family genes.
Supplemental Material
Download PDF (85.4 KB)Supplemental Material
Download PDF (84.3 KB)Supplemental Material
Download PDF (149.5 KB)Supplemental Material
Download PDF (116.2 KB)Supplemental Material
Download PDF (223.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Gong Z, Xiong L, Shi H, et al. Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci. 2020;63(5):635–674.
- Jin J, Tian F, Yang D, et al. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017;45(D1):D1040–D1045.
- Riechmann JL, Heard J, Martin G, et al. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science. 2000;290(5499):2105–2110.
- Goff SA, Ricke D, Lan TH, et al. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science. 2002;296(5565):92–100.
- Birkenbihl RP, Jach G, Saedler H, et al. Functional dissection of the plant-specific SBP-domain: overlap of the DNA-binding and nuclear localization domains. J Mol Biol. 2005;352(3):585–596.
- Yamasaki K, Kigawa T, Inoue M, et al. A novel zinc-binding motif revealed by solution structures of DNA-binding domains of Arabidopsis SBP-family transcription factors. J Mol Biol. 2004;337(1):49–63.
- Klein J, Saedler H, Huijser P. A new family of DNA binding proteins includes putative transcriptional regulators of the Antirrhinum majus floral meristem identity gene SQUAMOSA. Mol Gen Genet. 1996;250(1):7–16.
- Riese M, Hohmann S, Saedler H, et al. Comparative analysis of the SBP-box gene families in P. patens and seed plants. Gene. 2007;401(1–2):28–37.
- Xie K, Wu C, Xiong L. Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiol. 2006;142(1):280–293.
- Li J, Hou H, Li X, et al. Genome-wide identification and analysis of the SBP-box family genes in apple (Malus × domestica Borkh.). Plant Physiol Biochem. 2013;70:100–114.
- Zhou Q, Zhang S, Chen F, et al. Genome-wide identification and characterization of the SBP-box gene family in Petunia. BMC Genomics. 2018;19(1):193.
- Cai C, Guo W, Zhang B. Genome-wide identification and characterization of SPL transcription factor family and their evolution and expression profiling analysis in cotton. Sci Rep. 2018;8(1):762.
- Manning K, Tör M, Poole M, et al. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet. 2006;38(8):948–952.
- Jung JH, Lee HJ, Ryu JY, et al. SPL3/4/5 integrate developmental aging and photoperiodic signals into the FT-FD module in Arabidopsis flowering. Mol Plant. 2016;9(12):1647–1659.
- Song S, Zhou H, Sheng S, et al. Genome-wide organization and expression profiling of the SBP-box gene family in Chinese jujube (Ziziphus jujuba Mill.). Int J Mol Sci. 2017;18(8):1734.
- Han YC, Gao HY, Chen HJ, et al. The involvement of papaya CpSBP1 in modulating fruit softening and carotenoid accumulation by repressing CpPME1/2 and CpPDS4. Sci Hortic. 2019;256:108582.
- Wei J, Wang C, Jiang J, et al. Cloning and expression analysis of BplSPL2, a novel SBP-box gene from Betula Platyphylla. Biotechnol Biotechnol Equip. 2010;24(2):1824–1827.
- Chao LM, Liu YQ, Chen DY, et al. Arabidopsis transcription factors SPL1 and SPL12 confer plant thermotolerance at reproductive stage. Mol Plant. 2017;10(5):735–748.
- Zhang HX, Jin JH, He YM, et al. Genome-wide identification and analysis of the SBP-Box family genes under phytophthora capsici stress in pepper (Capsicum annuum L.). Front Plant Sci. 2016;7:504.
- Kropat J, Tottey S, Birkenbihl RP, et al. A regulator of nutritional copper signaling in Chlamydomonas is an SBP domain protein that recognizes the GTAC core of copper response element. Proc Natl Acad Sci USA. 2005;102(51):18730–18735.
- Zhang Y, Schwarz S, Saedler H, et al. SPL8, a local regulator in a subset of gibberellin-mediated developmental processes in Arabidopsis. Plant Mol Biol. 2007;63(3):429–439.
- Xu M, Hu T, Zhao J, et al. Developmental Functions of miR156-Regulated SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) Genes in Arabidopsis thaliana. PLoS Genet. 2016;12(8):e1006263.
- Ferreira e Silva GF, Silva EM, Azevedo Mda S, et al. microRNA156-targeted SPL/SBP box transcription factors regulate tomato ovary and fruit development. Plant J. 2014;78(4):604–618.
- Kelly PJ, Bones A, Rossiter JT. Sub-cellular immunolocalization of the glucosinolate sinigrin in seedlings of Brassica juncea. Planta. 1998;206(3):370–377.
- Weng PF, Wu ZF, Shen XQ, et al. A new cleaner production technique of pickle mustard tuber at low salinity by lactic acid bacteria. J Food Process Eng. 2011;34(4):1144–1155.
- Shi H, Wang LL, Sun LT, et al. Cell division and endoreduplication play important roles in stem swelling of tuber mustard (Brassica juncea Coss. var. tumida Tsen et Lee). Plant Biol (Stuttg). 2012;14(6):956–963.
- Sun Q, Zhou G, Cai Y, et al. Transcriptome analysis of stem development in the tumourous stem mustard Brassica juncea var. tumida Tsen et Lee by RNA sequencing. BMC Plant Biol. 2012;12:53.
- Altschul SF, Madden TL, Schäffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402.
- Gasteiger E, Gattiker A, Hoogland C, et al. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31(13):3784–3788.
- Kumar S, Nei M, Dudley J, et al. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinformatics. 2008;9(4):299–306.
- Chen C, Chen H, Zhang Y, et al. TBtools - an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020. doi: 10.1016/j.molp.2020.06.009
- Bailey TL, Boden M, Buske FA, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server issue):W202–W208.
- Wang Y, Tang H, Debarry JD, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49.
- Lescot M, Dehais P, Thijs G, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–327.
- Conesa A, Gotz S, Garcia-Gomez JM, et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–3676.
- Li MY, Xie FJ, He Q, et al. Expression analysis of XTH in stem swelling of stem mustard and selection of reference genes. Genes. 2020;11(1):113.
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45.
- Rose AB. The effect of intron location on intron-mediated enhancement of gene expression in Arabidopsis. Plant J. 2004;40(5):744–751.
- Kong H, Landherr LL, Frohlich MW, et al. Patterns of gene duplication in the plant SKP1 gene family in angiosperms: evidence for multiple mechanisms of rapid gene birth. Plant J. 2007;50(5):873–885.
- Semon M, Wolfe KH. Consequences of genome duplication. Curr Opin Genet Dev. 2007;17(6):505–512.
- Yamasaki H, Hayashi M, Fukazawa M, et al. SQUAMOSA promoter binding protein-like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell. 2009;21(1):347–361.
- Wu G, Park MY, Conway SR, et al. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell. 2009;138(4):750–759.
- Kavas M, Kızıldoğan AK, Abanoz B. Comparative genome-wide phylogenetic and expression analysis of SBP genes from potato (Solanum tuberosum). Comput Biol Chem. 2017;67:131–140.
- Nagaharu U. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. J Jpn Bot. 1935;7:389–452.
- Panjabi P, Jagannath A, Bisht NC, et al. Comparative mapping of Brassica juncea and Arabidopsis thaliana using intron polymorphism (IP) markers: homoeologous relationships, diversification and evolution of the A, B and C Brassica genomes. BMC Genomics. 2008;9:113.
- Li M, Zhao SZ, Zhao CZ, et al. Cloning and characterization of SPL-family genes in the peanut (Arachis hypogaea L.). Genet Mol Res. 2016;15(1).
- Hou H, Li J, Gao M, et al. Genomic organization, phylogenetic comparison and differential expression of the SBP-Box family genes in grape. PLoS One. 2013;8(3):e59358.
- Zhang L, Wu B, Zhao D, et al. Genome-wide analysis and molecular dissection of the SPL gene family in Salvia miltiorrhiza. J Integr Plant Biol. 2014;56(1):38–50.
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227.
- Okabe Y, Yamaoka T, Ariizumi T, et al. Aberrant stamen development is associated with parthenocarpic fruit set through up-regulation of gibberellin biosynthesis in tomato. Plant Cell Physiol. 2019;60(1):38–51.
- Huang WJ, Wang YR, Li X, et al. Biosynthesis and regulation of salicylic acid and N-Hydroxypipecolic acid in plant immunity. Mol Plant. 2020;13(1):31–41.
- Tan HW, Song XM, Duan WK, et al. Genome-wide analysis of the SBP-box gene family in Chinese cabbage (Brassica rapa subsp. pekinensis). Genome. 2015;58(11):463–477.
- Pan F, Wang Y, Liu H, et al. Genome-wide identification and expression analysis of SBP-like transcription factor genes in Moso Bamboo (Phyllostachys edulis). BMC Genomics. 2017;18(1):486.
- Unte US, Sorensen AM, Pesaresi P, et al. SPL8, an SBP-box gene that affects pollen sac development in Arabidopsis. Plant Cell. 2003;15(4):1009–1019.