?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Rice blast is one of the most destructive diseases of rice and is caused by the infection with rice blast fungus, Magnaporthe oryzae. In the present study, we obtained 12 bacterial isolates from Sichuan basin neutral purplish soil. Among them, the strain named ZW-10 showed significant activity against M. oryzae. Morphological and biochemical studies, along with 16S rDNA sequencing analysis, proved that the strain belongs to Bacillus velezensis. The cell-free culture filtrate of ZW-10 inhibited the growth of M. oryzae, suggesting the presence of active enzymes and secondary metabolites. The experimental results showed that ZW-10 produced cellulase, protease, and peroxidase. The temperature experiment indicated that the secondary metabolisms of ZW-10 remained active at high temperature. In addition, this strain had good acidity resistance. As shown in leaf experiments in vitro as well as field trials, the liquid culture and the cell-free culture filtrate of ZW-10 had significant inhibitory effects on rice blast. In conclusion, ZW-10 can be used as a potential biological agent in the control of rice blast.
Introduction
Rice (Oryza sativa), one of the world’s most important crops, is a staple food to about a half of the world’s population [Citation1]. Rice blast, a disease caused by the ascomycete fungus Magnaporthe oryzae, is one of the most widely distributed and serious fungal diseases in cultivated crops [Citation2,Citation3]. The disease could attack in different rice growth stages and affect different tissues of rice simultaneously [Citation4]. Annually, rice blast leads to 10–30% loss of the rice grain yield [Citation5–7]. M. oryzae can cause disease on a wide range of crops, such as barley, oats, ryegrass, and millets [Citation8]. At present, it is controlled mainly by spraying chemical fungicides and breeding resistant varieties. Nevertheless, this phytopathogenic fungus is causing enormous reductions in rice yield and quality [Citation9]. Chemical fungicides have many advantages, such as low cost and high effectiveness; however, overuse of chemical control agents could cause not only environmental pollution and pathogen resistance, but also other problems [Citation10]. Biological control is valued progressively more because of its advantages of environmental friendliness, low residual effect, high selectivity, and long-lasting prevention and control [Citation11].
In the process of rice blast prevention and treatment, biological control is a modern trend in microbial pesticides to seek effective agricultural antibiotics based on microbial metabolites. The applications of Bacillus are quite widespread. Actinomycetes, Agrobacterium spp., Bacillus spp., Pseudomonas spp. and so on secrete secondary metabolites, such as antifungal proteins and peptides [Citation12,Citation13]. Antibiotics are produced by diverse antagonistic microbes [Citation14]. For example, B. subtilis FZB24 and B. subtilis MBI600, QST713, and GB03 are the subject of the production and application license of United States’ Environment Protection Agency [Citation15]. B. subtilis RB14 and NB22 isolated by Japanese scholars have a strong inhibitory effect on Rhizoctonia solani and Fusarium oxysporum, pathogens of tomato [Citation16]. Cavaglieri et al. [Citation17] isolated Bacillus RC8, RC9, and RC11 and recorded significant antagonistic effects against F. verticillioides in maize.
In the study presented here, 12 microbial strains from Sichuan basin neutral purplish soil were isolated and screened. Among them, one strain showed strong inhibition against M. oryzae; we named it ZW-10. Through characterization of morphological, biochemical, and physiological properties and 16S rDNA sequence analysis, the strain was identified as belonging to B. velezensis. In addition, we researched the preventive and therapeutic effects of ZW-10 and its fermentation metabolites against rice blast. Furthermore, the biocontrol effect of antagonism on M. oryzae was evaluated on leaves in vitro and in field trials. Understanding the underlying mechanisms, may provide a possibility of developing new biological pesticides.
Materials and methods
Isolation and screening of antagonistic strains from soil
The Sichuan basin neutral purplish soil sample (Wenjiang district, Chengdu, Sichuan, PR China) was provided by the College of Resource Science and Technology of Sichuan Agricultural University. A total of 12 strains were isolated from the soil.
Rice blast ascomycete fungus M. oryzae (Guy11) was provided by Plant Pathology Laboratory of Sichuan Agricultural University. Fungal spores were inoculated on PDA and incubated at 28 °C for 7 days. The bacterial isolates were screened for their anti-fungal potential by a dual culture plate assay. The plates were incubated at 28 °C. After incubation for 2–3 days, the growth inhibition of M. oryzae was compared by a degree of radian. The strongest antagonistic bacterial isolate was named ZW-10 and was studied further.
Enzyme production analysis and identification
The identification of isolate ZW-10 was carried out by routine biochemical and physiological tests and 16S rDNA sequence analysis. The biochemical and physiological indices of ZW-10 were measured based on Bergey’s Manual of Systematic Bacteriology [Citation18]. The method of determining chitinase production was described by Agrawal and Kotasthane [Citation19]. The measurements of cellulose, hydrocyanic acid, and protease production were done as described by Gopalakrishnan et al. [Citation20]. Gelatinase production was described by Bose et al. [Citation21].
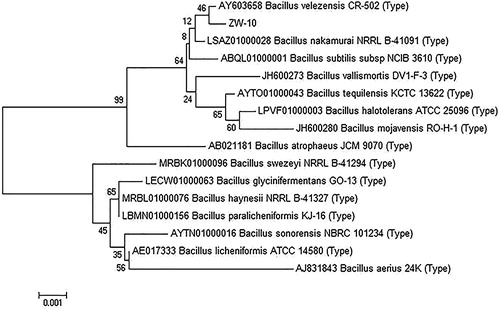
The total genomic DNA was extracted using a commercial DNA extraction kit. The 16S rDNA was amplified by PCR, using the universal forward primer P2 (5′-CAGAGTTTGATCCTGGCT-3′) and universal reverse primer P5 (5′-AGGAGGTGATCCAGCCGCA-3′). The 50 μL PCR mixture was placed in a small Eppendorf tube containing 2 μL of forward primer, 2 μL of reverse primer, 5 μL of 10× Vazyme LAmp Buffer, 0.5 μL of Vazyme LAmp DNA Polymerase, 1 μL of the extracted DNA, 1 μL of 10 mmol/L highly pure dNTPs, and 38.5 μL of sterile distilled water. The polymerase chain reaction program was run in Biometra TOne 96G, as follows: an initial denaturation for 5 min at 94 °C, a 32-cycle of denaturation (0.5 min at 94 °C), annealing (0.5 min at 55 °C), and extension (1.5 min at 72 °C), and a final extension for 7 min at 72 °C [Citation22,Citation23]. The PCR products were purified using an OMEGA Gel Extraction Kit. The amplified 16S rDNA was transformed into Escherichia coli. Positive recombinants were identified, and sent to TSINGKE (Chengdu, China) for sequencing. The similarity of 16S rDNA and gyrB sequences was compared using the BLAST search program in NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi), and phylogenetic analysis of ZW-10 based on 16S rDNA sequence analysis was constructed using MEGA 4.0.
Antibiotic susceptibility test
The single colonies of ZW-10 cultured on PDA plates were picked with an inoculation ring, and inoculated to Landy liquid medium to be incubated at 28 °C, 180 rpm on a rotary shaker for 4 days. Then, 200 μL bacterial suspension of ZW-10 was coated uniformly on PDA plates. Antimicrobial susceptibility test papers were affixed to the sides of PDA Petri dishes that were incubated for 36 h at 28 °C, to measure the size of the bacteriostatic zone.
Evaluation of antifungal activity
The strain ZW-10 was inoculated into Landy liquid medium to be incubated at 28 °C, 180 rpm on a rotary shaker. After 72 h of inoculation, the fermentation broth was taken out and centrifuged at 10,000 rpm (22,400g) for 2 min at 25 °C to pellet bacterial cells. The supernatant of the fermentation broth was mixed with PDA at different concentrations (1:10, 1:20, 1:40, 1:80, and 1:160 v/v). Then fungal plugs of M. oryzae were transferred to the centre of the mixed medium and were incubated at 28 °C [Citation23]. The M. oryzae colony diameter was measured after 7 days. The formula of relative inhibition rate was described by Boukaew [Citation24]:
where R1 is the M. oryzae colony diameter in the control, R2 is the M. oryzae colony diameter in the dual culture plates with supernatant of fermentation broth of ZW-10 mixed with PDA.
Effects of pH and temperature of filtrate on the M. oryzae growth inhibition rate
The cell-free culture filtrate of ZW-10 was prepared and divided into 13 parts. The pH value was adjusted from 1 to 13, and then refrigerated at 4 °C for 24 h. The fermentation broth with different pH values was mixed with PDA medium. The plugs of M. oryzae were transferred to the PDA plates. After incubation at 28 °C for 8 days, the growth of M. oryzae was assessed by measuring the diameter of the colony, and the growth inhibition rate was evaluated by comparison with the control plates without ZW-10.
In the temperature test, the samples of cell-free culture filtrate were exposed to 4, 28, 37, 60, 80, 100, and 121 °C for 1 h, and the test was done after the sample had cooled down to room temperature (25 °C). The specific procedures were the same as described above for the pH tests.
Correlation between cell growth and antifungal activity
Five hundred millilitres of Landy medium in a 1 L flask were inoculated with ZW-10 (1 μL/mL) and incubated at 37 °C, 180 rpm for 108 h. During incubation, 1 mL samples were taken out at intervals of 12 h for measurement of optical density at 600 nm (OD600) and antifungal activity against M. oryzae by measuring the colony diameter of M. oryzae. The specific procedure was described above.
Experiment in vitro using leaves
The experiment was divided into three groups (prevention, treatment, and control). Normal growing leaves (5 cm) were placed in Petri dishes with 6-benzylaminopurine solution. Three gentle punctures per leaf segment facilitated the penetration of treatment agents.
Prevention group: 3 μL of droplets of the cell-free culture filtrate of ZW-10 and fermentation liquid of ZW-10 were applied to punctured leaves. Then the leaves were incubated at 28 °C in the dark. After 24 h, all punctured sites of leaves were inoculated with 3 μL droplets of spore suspension of M. oryzae (1 × 105 spores/mL) [Citation25]. Under the condition of alternating light and dark, the leaves were incubated at 28 °C for 5 days. Treatment group: the method was the same as for the prevention group, but leaves were inoculated with spore suspension of M. oryzae first and then the cell-free culture filtrate and fermented liquid was inoculated. In the control group, sterile water and medium solution were used instead and the other steps were the same. After 5 days, the lesion diameters were compared.
Field trial
The bioassay was conducted in accordance with the previous methods [Citation26]. Seeds of Lijiang Xintuan Heigu (50 seeds as a treatment group) were soaked in 3% v/v H2O2 solution for 12 h to accelerate germination, and then water was used to accelerate germination until the germinated seeds became white. The seeds were sown in the divided area test field and covered with film for 1 week. The emergence of seeds was observed every 2 days and water was supplemented as needed. When the seedlings grew to the two-leaf stage (about 10 days), the film was removed, and growth continued normally according to the field culture conditions.
In the prevention experiment, the rice seedlings were sprayed to runoff with the cell-free culture filtrate of ZW-10 containing Tween 20 (1000/1, v/v). Each plot was sprayed with 20 mL of treatment solution. After 24 h, each plot was sprayed by 20 mL of a spore suspension (1 × 105 spores/mL). In the treatment group: the method was the same as in the prevention group, but leaves were inoculated with spore suspension of M. oryzae in the first, and then the cell-free culture filtrate was inoculated.
Statistical analysis
The experiments were repeated at least in three times. Statistical analysis of the data was done with SPSS 20.0 software. Differences were considered statistically significant at p < 0.05 (*) and p < 0.01 (**). All data are expressed as mean values with standard deviation (±SD).
Results and discussion
Isolation of bacterial strains with antifungal activity against M. oryzae
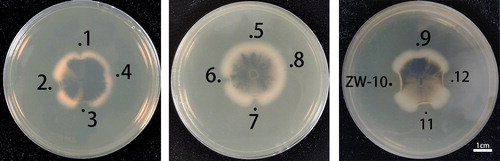
The Sichuan basin neutral purplish soil sample was provided by the College of Resource Science and Technology of Sichuan Agricultural University. Purple soil is formed by weathering of purple rock, which is rich in calcium, phosphorus, potassium, and other nutrients. Sichuan Basin is the place where purple soil is most widespread [Citation27]. More than 60 kinds of bacilli were isolated and identified at this soil sample collection site [Citation28]. Among the 12 strains isolated from the Sichuan basin neutral purplish soil sample, 9 strains in the fermentation broth showed an inhibitory effect on M. oryzae mycelial growth (). In this study, ZW-10 strain had the strongest antagonistic activity. So, we selected ZW-10 for subsequent experiments.
Figure 1. Screening of the strong antagonistic strains against M. oryzae.
Serial numbers 1–12 represent the fermentation broth filtrate of 12 bacterial isolates.
The 16S rDNA sequence analysis (Supplemental data) indicated that the strain ZW-10 showed ∼99.71% similarity to B. velezensis strain FZB42. A phylogenetic tree displaying the relationship between strain ZW-10 and another 15 strains with high homology was shown in . Bacillus is regarded as a promising strain for industrial application because it has a wide variety of antibacterial compounds as well as amylase, lipase, and other industrial enzymes [Citation29]. Many strains of Bacillus, including B. subtilis, B. siamensis, B. amyloliquefaciens, and B. atrophaeus produce antimicrobial substances and virtually >10% of antimicrobial agents reported known to be produced by Bacillus species [Citation30–33]. Many scholars have found that B. velezensis is effective in the prevention and control of plant diseases. B. velezensis was effective in controlling root rot of hydroponically grown vegetables [Citation34], and was regarded as a biocontrol agent against pear [Citation35] diseases and wheat powdery mildew [Citation36]. In addition, B. velezensis was effective against fungal pathogens such as Colletotrichum spp., C. gloeosporioides, Pestalotiopsis maculans, and F. solani [Citation37]. Importantly, a strain of B. velezensis ZSY-1 can synthesize phenol and inhibit the growth of plant pathogenic fungi [Citation38]. B. velezensis produce a variety of biologically active metabolites against plant pathogenic fungi. Cao et al. [Citation39] extracted the surfactant peptides from the fermentation broth of B. velezensis, which could effectively inhibit the growth of fungal hyphae; the activity of C. gloeosporioides (Penz.) was inhibited by bacteriomycin secreted by B. velezensis [Citation40]. In recent years, a large number of studies have been conducted on B. velezensis as a plant pathogen control agent, but there is scarcity of information on the inhibitory effect of B. velezensis on the rice blast pathogen.
The biochemical and physiological properties of strain ZW-10 are summarized in and the morphological characteristics of ZW-10 in . Scanning electron microscopy observations showed that ZW-10 was rod-shaped (), gram-positive (), spore containing (), and with good motility (), but without capsule. It could grow in 9% w/v NaCl culture. ZW-10 grew well on the peroxidase (POD) detection, protease detection, and cellulase detection media, with a clear circle generated around ZW-10 colonies, indicating the ZW-10 produced protease (), POD (), and cellulase (). The exocrine proteins of many strains, especially those belonging to Bacillus, inhibit the growth of plant pathogenic fungi [Citation41]. Protease, cellulase, and chitinase hydrolyze the mycelial cell wall of pathogenic fungi and inhibit pathogen infection by destroying the cell wall [Citation42]. Cellulase and protease might cause an abnormal hyphal morphology of pathogens [Citation43]. So, we speculated that cellulase and protease secreted by ZW-10 caused the hyphal deformation and growth suppression of M. oryzae.
Figure 3. Morphological characteristics of ZW-10.
Colony morphology (A); scanning electron microscope observation (B); gram staining (C); spore staining (D); flagella staining (E); and decidua staining (F).
Note: Magnification; 400-fold (C and D) and 1000-fold (E and F)
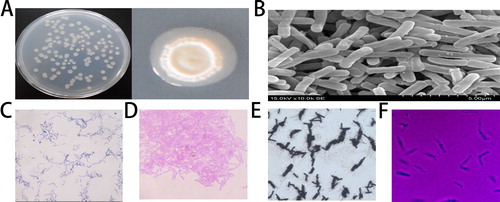
Figure 4. Production of enzymes and secondary metabolites by ZW-10.
HCN test (A); protease text (B); peroxidase test (C); chitinase test (D); gelatinase assay (E); cellulase assay (F).
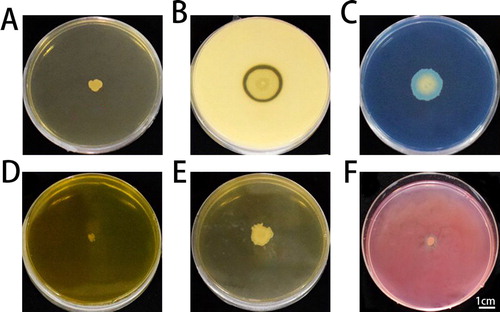
Table 1. Physiological and biochemical results of ZW-10.
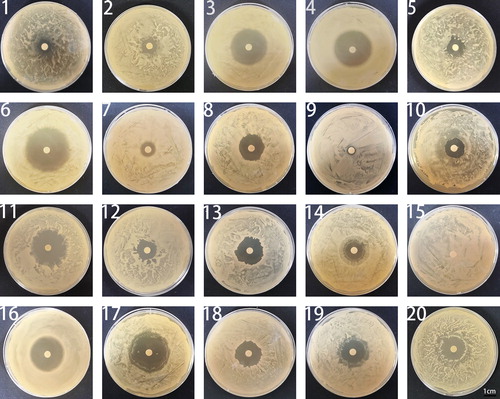
According to the standard of the antimicrobial susceptibility test by the slip method of the health industry of the People’s Republic of China, ZW-10 is susceptible to 18 antibiotics, except penicillin and neomycin (). The prevention and control by the strain ZW-10 is relatively simple and convenient. If B. velezensis ZW-10 was applied to the environment, it could be controlled by common antibiotics.
Figure 5. Sensitivity of strain ZW-10 to antibiotics.
1–20 represent doxycycline, carboxy, ceftazidime, benzillin, kanamycin, cefazolin, penicillin, cefuroxime, erythromycin, cefoperazone, neomycin, minocycline, gentamicin, tetracycline, ceftriaxone, piperacillin, cefradine, butamine, ampicillin, and benzocillin.
Evaluation of the strain ZW-10 antagonism to M. oryzae
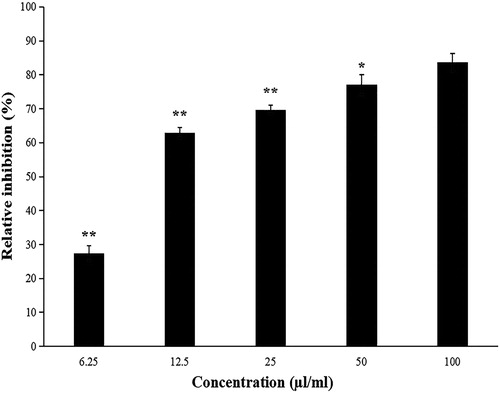
The cell-free culture filtrate of ZW-10 had an inhibitory effect of M. oryzae. At the lowest concentration of 6.25 μL/mL, the relative inhibition rate was 27.4%. When the concentration of fermentation broth reached 12.5 μL/mL, the inhibition rate was 62.9%. The inhibitory effect on M. oryzae was directly proportional to the concentration of fermentation broth (). Hence, ZW-10 has a strong antagonistic effect on M. oryzae.
Figure 6. Effect of different concentrations of fermentation broth on the inhibition of M. oryzae.
Note: Fermentation broth concentration =100 μL/mL as a reference, significant difference analysis.
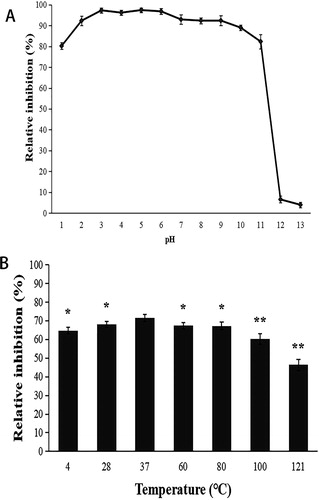
The results demonstrated that in the pH range 1–11, the cell-free culture filtrate of ZW-10 had significantly strong activity against M. oryzae. And the activity of the cell-free culture filtrate was lost after the treatment pH was 12. The results showed that the ZW-10 fermentation broth stable under acidity, but did not tolerate strong alkaline conditions (). Regarding the thermal stability, the activity of the fermentation broth remained at around 50% after treatment at 121 °C, with the inhibition rate remaining above 60% up to the temperature of 100 °C. The low temperature treatment had a small significant effect on the activity of the fermentation broth, with the highest inhibition rate of 71.56% reached at 37 °C (). In terms of thermal tolerance, the tolerance of the ZW-10 cell-free culture filtrate was stronger than that previously reported in B. amyloliquefaciens Rdx5 [Citation44]. Some previous reports showed that the antifungal metabolites produced by Bacillus spp. have thermal stability and strong acid stability [Citation45]. The results indicated that the inhibitory action of strain ZW-10 fermented liquid was relatively stable, which is advantageous for the planting practice in the process of popularization and application in crop production.
Figure 7. Effects of pH (A) and temperature (B) on the antifungal activity of the cell-fell culture filtrate of ZW-10.
Note: The pH ranged from 1 to 13 (A).
The temperature ranged from 4 to 121 °C (B).
With treatment temperature of 3 °C as a reference, significant difference analysis.
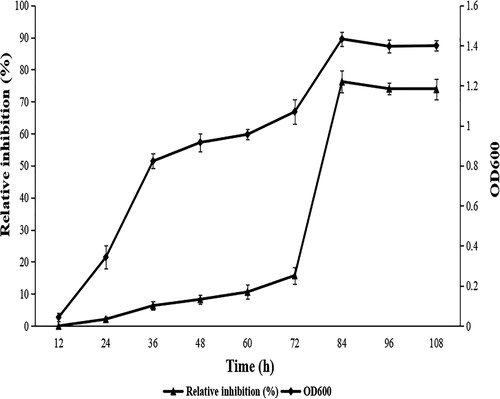
According to the growth curve of strain ZW-10, the inhibitory activity of the fermentation broth supernatant showed a positive correlation trend with the fermentation time. In the range of 12–36 h, ZW-10 was in logarithmic phase, and the number of ZW-10 cells increased rapidly. After that, the inhibitory activity of the ZW-10 broth supernatant continued to increase, suggesting that the residues and metabolites in the bacterial solution continued to accumulate. The antifungal activity of the fermentation broth supernatant increased rapidly between 60 and 84 h. At 84 h, the relative inhibitory activity of the fermentation broth supernatant reached the highest level of 76.27% ± 3.36%. After 84 h, the inhibitory activity remained approximately steady as the fermentation time progressed ().
Antifungal activity of ZW-10 against M. oryzae
In the leaf experiment in vitro, the length of the lesions on the inoculated leaves treated with fermented liquid of ZW-10 and its fermented broth were significantly smaller than that on the leaves treated with sterile water and Landy medium (). The length of the lesions on the leaves treated with the fermented liquid of ZW-10 and its fermentation broth was smaller by 58.2% and 51.8% in the prevention group, respectively, compared with those treated with sterile water. In the treatment group, the length of leaf lesions treated with ZW-10 and its fermentation broth was smaller by 53.3% and 36.1%, respectively, compared with the sterile water treatment (). Landy culture medium treatment produced a similar result to water treatment. Inoculation of ZW-10 fermentation liquid produced a better inhibitory effect against M. oryzae than that of its fermented broth (). In conclusion, ZW-10 significantly improved the severity of M. oryzae.
Figure 9. Biocontrol effect of ZW-10 illustrated by the length of leaf spots in vitro.
(A) Prevention group: (a) Sterile water was applied to slightly punctured sites of leaves first and then spore suspension of M. oryzae was inoculated. (b) Landy medium was applied to gently punctured sites of leaves first, and then spore suspension of M. oryzae was inoculated. (c) Fermentation liquid of ZW-10 was applied to gently punctured sites of leaves first, and then spore suspension of M. oryzae was inoculated. (d) The cell-fell culture filtrate of ZW-10 was applied to gently punctured sites of leaves first, and then spore suspension of M. oryzae was inoculated.
(B) Treatment group: (e) Spore suspension of M. oryzae was inoculated to gently punctured sites of leaves first, and then sterile water was applied to the same site. (f) Spore suspension of M. oryzae was inoculated to gently punctured sites of leaves first, and then Landy medium was applied to the same site. (g) Spore suspension of M. oryzae was inoculated to gently punctured sites of leaves first, and then fermentation liquid of ZW-10 was applied to the same site. (h) Spore suspension of M. oryzae was inoculated to gently punctured sites of leaves first, and then the cell-fell culture filtrate of ZW-10 was applied to the same site.
(C) Composite bar chart of the prevention and the treatment groups.
Note: Both the prevention groups and the treatment groups were treated with water as a reference, significant difference analysis.
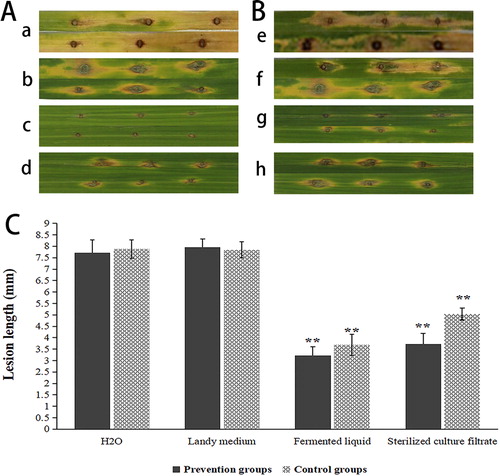
Table 2. Statistical results of experimental plaque length in isolated leaves (mm).
In the field experiment, by spraying fermented liquid of ZW-10 and its fermented broth, the number of lesions on rice leaves was significantly smaller than that in the treatments with sterile water and Landy medium (). In the prevention group, the number of leaf lesions on plants treated with the fermented liquid of ZW-10 and its fermentation broth was lower by 73.7% and 63.7%, respectively, compared with those treated with water. In the treatment group, the results were similar (). Moreover, the preventative effect was more prominent than the treatment effect, and the effect of spraying the fermented liquid of ZW-10 was most significant (). It has been reported that the secondary metabolites of bacteria not only inhibit the growth of pathogens, but also induce the plant's own defence mechanisms to resist pathogens [Citation46], such as activating enzymes superoxide dismutase, POD, polyphenol oxidase, and others [Citation47,Citation48]. Although the spraying of carbendazim also had significant inhibitory effects, carbendazim had toxic and side effects on humans, animals, and soil microbes [Citation49,Citation50]. ZW-10 can be used as a substitute for fungicides, representing the biological control of M. oryzae. We are going to further study the mechanism of ZW-10 to control rice blast disease.
Figure 10. Biocontrol efficiency of ZW-10 against M. oryzae on rice evaluated based on the number of lesions on leaves (field trial).
(A) Prevention group: (a) Spraying sterilized water after spraying spore suspension of M. oryzae. (b) Spraying Landy medium after spraying spore suspension of M. oryzae. (c) Spraying 100 mg/mL of carbendazim after spraying spore suspension of M. oryzae. (d)Spraying fermentation liquid of ZW-10 after spraying spore suspension of M. oryzae. (e) Spraying cell-fell culture filtrate of ZW-10 after spraying spore suspension of M. oryzae.
(B) Treatment group: (f) Spraying spore suspension of M. oryzae after spraying sterilized water. (g) Spraying spore suspension of M. oryzae after spraying Landy medium. (h) Spraying spore suspension of M. oryzae after spraying 100 mg/mL of carbendazim. (i) Spraying spore suspension of M. oryzae after spraying fermentation liquid of ZW-10. (j) Spraying spore suspension of M. oryzae after spraying cell-fell culture filtrate of ZW-10.
(C) Composite bar chart of the prevention and the treatment groups.
Note: Both the prevention and the treatment groups were treated with water as a reference, significant difference analysis.
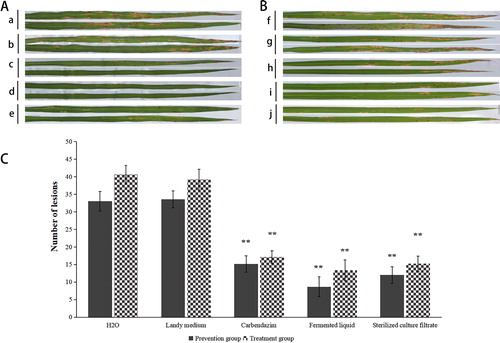
Table 3. Statistical results of the number of leaves disease spots in field experiment (mm).
Conclusions
We isolated a strain of B. velezensis (named ZW-10) from the Sichuan basin neutral purplish soil. ZW-10 had a significant inhibitory effect on rice blast fungus. A series of physiological and biochemical indicators showed that the secondary metabolites of B. velezensis have strong inhibitory effects on M. oryzae, even under acidic and high-temperature conditions. In terms of enzyme production, B. velezensis ZW-10 produced POD, protease, and cellulase, which have been shown in the literature to promote the cell wall lysis in fungi. When ZW-10 was applied to rice, the bacterial solution and fermentation broth supernatant had a significant control effect on rice blast, which was better than that of carbendazim. In conclusion, B. velezensis ZW-10 is a candidate biological control agent. In addition to describing the strain’s physiological and biochemical characteristics, this study also demonstrated its potential for application to rice blast as a bacterial biocontrol agent.
Supplemental Material
Download PDF (83.7 KB)Acknowledgements
We would like to thank the College of Resource Science and Technology of Sichuan Agricultural University for providing the Sichuan basin neutral purplish soil. We are also grateful to Plant Pathogenic Laboratory of Sichuan Agricultural University for providing the rice blast pathogenic fungus M. oryzae Guy11.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Howard RJ, Valent B. Breaking and entering: host penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu Rev Microbiol. 1996;50(1):491–512.
- Ishii H. Impact of fungicide resistance in plant pathogens on crop disease control and agricultural environment. JARQ. 2006;40(3):205–211.
- Komatsu K, Urayama S-i, Katoh Y, et al. Detection of Magnaporthe oryzae chrysovirus 1 in Japan and establishment of a rapid, sensitive and direct diagnostic method based on reverse transcription loop-mediated isothermal amplification. Arch Virol. 2016;161(2):317–326.
- Li YB, Wu CJ, Jiang GH, et al. Dynamic analyses of rice blast resistance for the assessment of genetic and environmental effects. Plant Breed. 2007;126(5):541–547.
- Dean RA, Talbot NJ, Ebbole DJ, et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434(7036):980–986.
- Talbot NJ. On the trail of a cereal killer: exploring the biology of Magnaporthe grisea. Annu Rev Microbiol. 2003;57(1):177–202.
- Sakulkoo W, Osés-Ruiz M, Garcia EO, et al. A single fungal MAP kinase controls plant cell-to-cell invasion by the rice blast fungus. Science. 2018;359(6382):1399–1403.
- Martin-Urdiroz M, Oses-Ruiz M, Ryder LS, et al. Investigating the biology of plant infection by the rice blast fungus Magnaporthe oryzae. Fungal Genet Biol. 2016;90:61–68.
- Zhang QF. Strategies for developing green super rice. Proc Natl Acad Sci USA. 2007;104(42):16402–16409.
- Raupach GS, Kloepper JW. Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology. 1998;88(11):1158–1164.
- Shan HY, Zhao MM, Chen DX, et al. Biocontrol of rice blast by the phenaminomethylacetic acid producer of Bacillus methylotrophicus strain BC79. Crop Prot. 2013;44:29–37.
- Chae K, Lee IH, Choi CS, et al. Purification and characterization of chitin-binding proteins from the hemolymph of sweet potato hornworm, Agrius convolvuli. Comp Biochem Physiol B Biochem Mol Biol. 1999;124(4):475–481.
- Newman SL, Gootee L, Gabay JE, et al. Identification of constituents of human neutrophil azurophil granules that mediate fungistasis against Histoplasma capsulatum. Infect Immun. 2000;68(10):5668–5672.
- Krishnamurthy K, Gnanamanickam S. Biological Control of Rice Blast by Pseudomonas fluorescens Strain Pf7–14: evaluation of a Marker Gene and Formulations. Biol Control. 1998;13(3):158–165.
- Baker KF. Evolving concepts of biological control of plant pathogens. Annu Rev Phytopathol. 1987;25(1):67–85.
- Asaka O, Shoda M. Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis RB14. Appl Environ Microbiol. 1996;62(11):4081–4085.
- Cavaglieri L, Passone A, Etcheverry M. Correlation between screening procedures to select root endophytes for biological control of Fusarium verticillioides in Zea mays L. Biol Control. 2004;31(3):259–267.
- Sato K, Azama Y, Nogawa M, et al. Analysis of a change in bacterial community in different environments with addition of chitin or chitosan. J Biosci Bioeng. 2010;109(5):472–478.
- Agrawal T, Kotasthane AS. Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chhattisgarh in Central India. Springerplus. 2012;1(1):73.
- Gopalakrishnan S, Pande S, Sharma M, et al. Evaluation of actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea. Crop Prot. 2011;30(8):1070–1078.
- Bose A, Chawdhary V, Keharia H, et al. Production and characterization of a solvent-tolerant protease from a novel marine isolate Bacillus tequilensis P15. Ann Microbiol. 2014;64(1):343–354.
- Jensen M, Webster J, Straus N. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl Environ Microbiol. 1993;59(4):945–952.
- Xu T, Li Y, Zeng XD, et al. Isolation and evaluation of endophytic Streptomyces endus OsiSh-2 with potential application for biocontrol of rice blast disease. J Sci Food Agric. 2017;97(4):1149–1157.
- Boukaew S, Prasertsan P. Suppression of rice sheath blight disease using a heat stable culture filtrate from Streptomyces philanthi RM-1-138. Crop Prot. 2014;61:1–10.
- Zhang HF, Zhao Q, Liu KY, et al. MgCRZ1, a transcription factor of Magnaporthe grisea, controls growth, development and is involved in full virulence. FEMS Microbiol Lett. 2009;293(2):160–169.
- Zhang YL, Li S, Jiang DH, et al. Antifungal activities of metabolites produced by a termite-associated Streptomyces canus BYB02. J Agric Food Chem. 2013;61(7):1521–1524.
- Pang S, Li TX, Wang YD, et al. Spatial distribution pattern of soil nitrogen in croplands at county scale and related affecting factors. Ying Yong Sheng Tai Xue Bao. 2010;21(6):1497–1503.
- Liu B, Liu GH, Sengonca C, et al. Bacillus praedii sp. nov., isolated from purplish paddy soil. Int J Syst Evol Microbiol. 2017;67(8):2823–2828.
- Gurung N, Ray S, Bose S, et al. A broader view: microbial enzymes and their relevance in industries, medicine, and beyond. Biomed Res Int. 2013;2013:329121.
- Baruzzi F, Quintieri L, Morea M, et al. Antimicrobial compounds produced by Bacillus spp. and applications in food. Sci Microb Pathog. 2011;2:1102–1111.
- Jeong H, Jeong DE, Kim SH, et al. Draft genome sequence of the plant growth-promoting bacterium Bacillus siamensis KCTC 13613T. J Bacteriol. 2012;194(15):4148–4149.
- Sonenshein AL. Control of sporulation initiation in Bacillus subtilis. Curr Opin Microbiol. 2000;3(6):561–566.
- Urdaci M, Pinchuk I. Antimicrobial activity of Bacillus probiotics. In Bacterial spore formers–Probiotics and emerging applications. Norfolk, UK: Horizon Bioscience; 2004. p. 171–182.
- Kanjanamaneesathian M, Wiwattanapatapee R, Rotniam W, et al. Application of a suspension concentrate formulation of Bacillus velezensis to control root rot of hydroponically grown vegetables. N Z Plant Prot. 2013;66:229–234.
- Sun PP, Cui JC, Jia XH, et al. Complete genome sequence of Bacillus velezensis L-1, which has antagonistic activity against pear diseases. Genome Announc. 2017;5(48):e01271.
- Cai XC, Liu CH, Wang BT, et al. Genomic and metabolic traits endow Bacillus velezensis CC09 with a potential biocontrol agent in control of wheat powdery mildew disease. Microbiol Res. 2017;196:89–94.
- Reyes-Estebanez M, Sanmartín P, Camacho-Chab JC, et al. Characterization of a native Bacillus velezensis-like strain for the potential biocontrol of tropical fruit pathogens. Biol Control. 2020;141:104127.
- Gao Z, Zhang B, Liu H, et al. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol Control. 2017;105:27–39.
- Cao LN, Pan LF, Gong L, et al. Interaction of a novel Bacillus velezensis (BvL03) against Aeromonas hydrophila in vitro and in vivo in grass carp. Appl Microbiol Biotechnol. 2019;103(21-22):8987–8999.
- Jin PF, Wang HN, Tan Z, et al. Antifungal mechanism of bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum gloeosporioides Penz. Pestic Biochem Physiol. 2020;163:102–107.
- Yuan WM, Crawford DL. Characterization of Streptomyces lydicus WYEC108 as a potential biocontrol agent against fungal root and seed rots. Appl Environ Microbiol. 1995;61(8):3119–3128.
- Öztopuz Ö, Pekin G, Park RD, et al. Isolation and evaluation of new antagonist Bacillus strains for the control of pathogenic and mycotoxigenic fungi of fig orchards. Appl Biochem Biotechnol. 2018;186(3):692–711.
- Shrestha A, Sultana R, Chae JC, et al. Bacillus thuringiensis C25 which is rich in cell wall degrading enzymes efficiently controls lettuce drop caused by Sclerotinia minor. Eur J Plant Pathol. 2015;142(3):577–589.
- Dong YL, Li H, Rong SH, et al. Isolation and evaluation of Bacillus amyloliquefaciens Rdx5 as a potential biocontrol agent against Magnaporthe oryzae. Biotechnol Equip. 2019;33(1):408–418.
- Gul SA, Ashraf SAES, JaimimS P, et al. Ex vivo application of secreted metabolites produced by soil-inhabiting Bacillus spp. efficiently controls foliar diseases caused by Alternaria spp. Appl Environ Microbiol. 2016;82(2):478–490.
- Tahir HAS, Gu Q, Wu H, et al. Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci Rep. 2017;7:40481.
- Rais A, Shakeel M, Malik K, et al. Antagonistic Bacillus spp. reduce blast incidence on rice and increase grain yield under field conditions. Microbiol Res. 2018;208:54–62.
- Li WT, Zhu ZW, Chern M, et al. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell. 2017;170(1):114–126.e115.
- Smith ME, Lewandrowski JK, Uri ND. Agricultural chemical residues as a source of risk. Rev Agric Econ. 2000;22(2):313–325.
- Feola G, Rahn E, Binder CRJA. Suitability of pesticide risk indicators for less developed countries: a comparison. Agric Ecosyst Environ. 2011;142(3–4):238–245.