Abstract
This study utilized a renewable energy source, agricultural waste, in anaerobic digestion (AD) at appropriate conditions to obtain biogas and biomethane as an energy carrier. Maize stalks underwent ultrasound (US) pre-treatment for better accessibility for microorganisms, as lignocelluloses have a stable structure, insoluble in water and resist both mechanical and enzymatic attack. The digestate after an anaerobic digestion process was used for cultivation of algae after adsorption with activated carbon for clarification. Photosynthetic microalgae have industrial and economic perspectives, so their low-cost cultivation has a great potential for many applications. The results showed the impact of US pre-treatment of maize stalks as a sole substrate and co-digested with algal biomass. The total yields were 1116 cm3/L, 1350.5 cm3/L and 1293.25 cm3/L for the untreated, ultrasonically pre-treated and microwaved maize stalks. The possibility of accumulating algal biomass using anaerobic digestate as a medium was demonstrated. US pre-treatment (400 W) showed high efficiency with respect to the extractives obtained per unit of energy input. Addition of 4 g/L of microalgal biomass as a co-substrate led to an increase in the biogas yield compared to native stalks. A small closed circle system, starting from anaerobic digestion of lignocellulosic substrates followed by microalgae cultivation in the digestate and subsequent return of microalgal biomass back in the bioreactor as a co-substrate was realized, encouraging circular economy. The suggested scheme is a simple and low-cost technology, as the substrate used is freely available and renewable, and algae proved to grow in a waste effluent as medium.
Introduction
Environmental protection and renewable energy sources have become important global concerns in recent years. The fossil fuels are constantly depleting while the available renewable sources possess a great economical potential. Energy produced from biogas after its purification and concentration can be used for electricity generation, heat production or as direct automobile fuel [Citation1]. Biogas can generate electricity by using a combustion engine, fuel cell or gas turbine, with the resulting electricity being used on-site or sold. The production of biogas through anaerobic digestion offers significant advantages over other ways of agricultural waste treatment [Citation2]. The technologies for biogas production are widely known and are the most convenient way of recycling bio-waste. The application of the obtained biogas depends on the quantity of methane in it. As a raw material for the production of heat and electrical energy, biogas may serve in many biogas plants to cover part of their electrical needs. Biomass has the advantage to be considered as carbon neutral because the quantity of CO2 released during combustion is the same as that absorbed by the plant during photosynthesis [Citation3]. The biomethane production from lignocellulosic wastes is a widely studied process because of the broad spread and easy access to materials such as wheat straw, maize stalks, etc. Harvesting residues and other agricultural waste represent available source of lignocellulosic material useful for energy production. Lignocellulosic biomass is one of the most abundant renewable resources in the world and can be degraded by microorganisms. The increase in the effectiveness of these technologies leads to increased methane quantity in the biogas, sustainable waste management and to the transition from a linear to a circular economy [Citation4]. The structure of lignocellulosic residues still presents technological barriers due to their limited bioavailability so the pre-treatment of substrates that are difficult for attack is a key to the improved performance of anaerobic digestion technology [Citation5]. For the delignification of lignocellulosic substrates, various mechanical, thermal, and chemical pre-treatment methods have been reported in literature [Citation6]. Different pre-treatment techniques can offer particle size reduction, solubilization and biodegradability enhancement [Citation7]. Diminishing the substrate particles size and thus increasing the substrate surface availability for enzymatic degradation is a common step which, together with other pre-treatment techniques, leads to enhanced biodegradation [Citation8]. To increase the enzymatic and microbial accessibility, pre-treatment is an obligatory step before the whole complex biotechnological process [Citation9–11]. One of the various pre-treatment techniques for the fractionation of lignocellulosic biomass to intensify the production of biofuels is the use of ultrasound. Ultrasound technology is considered a green technique for use in chemical processes as it can reduce reaction times and chemical loading and has low energy consumption and low environmental impact [Citation7]. Ultrasound-assisted approaches result in the reduction in treatment time and also limit the amount of required enzyme. Overall, the ultrasound-assisted approach is considered to be a novel and environmentally friendly green technique, giving a significant degree of intensification [Citation12]. Ultrasound provides physical augmentation by shear forces, mass transfer and surface erosion as well as chemical effects of producing oxidizing radicals. These effects facilitate homolytic cleavage within the lignin macromolecule, to enhance the cleavage of linkages between the lignin and the hemicellulose and degrade lignin compounds through hydroxyl attack of the phenolic ring. Furthermore, ultrasound tends to aid in the separation and depolymerization of polysaccharides [Citation6]. Lignocellulosic biomass is difficult for microorganisms to degrade because of the combination of cellulose, hemicellulose and lignin. Therefore, using vital microorganisms in microbial consortia is more efficient than free enzymes, as microorganisms can regenerate and produce a variety of useful enzymes at the same time. To achieve effective biogas production and enhance the biomethane yield, it is possible to couple the appropriate pre-treatment type with using mixed cellulolytic consortia [Citation13]. Anaerobic digestion is a waste-towards-energy technology employing consortia of anaerobic microorganisms and resulting in continuous production of methane-rich biogas, together with intermittent release of effluent digestate rich in undigested solids, organic and inorganic compounds and metal salts that could be further utilized.
The systems for microalgae cultivation often involve the use of fresh-water, fertilizers as nutrients, and the injection of CO2 for growth. These factors raise the cost of production and diminish its attractiveness as an alternative. In order to overcome these problems, some methods use wastewater or digestate from anaerobic biodegradation for cultivation. This implementation allows treatment of the wastewater and obtaining biomass [Citation14]. The study of this integrated approach leads to the comparative assessment among cultures grown in wastewater or digestate versus ones grown in an enriched medium. The perspective is accumulation of biomass rich in valuable products, as well as nutrients removal as a form of remediation. On the other hand, selected algal strains could support the absorption of carbon wastes for the production of biogas [Citation15, Citation16].
Materials and methods
Pre-treatment
Three types of pre-treatment were performed: US 400 W – pre-treatment with ultrasound with power 400 W; pre-treatment with microwaves (MW) with power 400 W and pre-treatment with microwaves with power 2 kW. Maize stalks were physically pre-treated using ultrasound sonication in a Lab750 for pipe (SinapTec, Lezennes, France) apparatus, at constant extraction temperature (70 °C) and a solid/solvent (distilled water) ratio 1:30 (w/v) for 1 h. After pre-treatment, the liquid phase was discharged and the solid fraction was dried in an Escalibur® Food dehydrator at 70 °C. Microwaves pre-treatment was carried out using the same apparatus.
Anaerobic digestion
Anaerobic digestion was done in a laboratory-scale bioreactor with automatic control of temperature − 37 °C and working volume of 2 L. The cultivation was in a batch operation and the duration of each process was 28 days. The concentration of the substrate was 5 g/L working volume for the substrate maize stalks. Digestate from a laboratory methane-generating digester fed with native maize stalks was used as inoculum, containing the microbial consortium involved in the process. During the experiments, the pH was maintained in the range from 6.5 to 8.5. All experiments were conducted at least in triplicate.
Decolorization of digestate
The digestate was sieved trough a 0.25-mm laboratory sieve. A definite quantity − 100 mL, of the digestate was centrifuged (Universal centrifuge Z 306, Hermle, Germany) at 15,000 rpm and the supernatant was subjected to decolorization. Adsorption was performed with introducing active carbon − 8 g in 100 mL of liquid digestate after the end of the process of biomethane production. Active carbon (Fluka) was used for clarification of the obtained digestate to become suitable for cultivation of algae. After 24 h at room temperature, another centrifugation was carried out and the supernatant was used as a cultivation medium.
Microalgae cultivation
Green algae Scenedesmus acutus and Klebsormidium flaccidum, yellow-green alga Trachydiscus minutus, red alga Porphyridium aerugineum and blue green alga Synechocystis salina deposited in the collection of the Laboratory of Experimental Algology, Institute of Plant Physiology and Genetics, BAS, Sofia, Bulgaria, were used. All extensive culture experiments were conducted at day light in special vessels (20 mL) at room temperature. pH was 7.0. Intensive cultivation was performed with initial algal culture density of 0.8 mg/mL dry weight (DW) used for all experiments. Cultivation was carried out at 25 °C and continuous illumination with cool-white fluorescent lamps at a photon flux density of 132 μmol photons/(m2 s). A carbon source was provided by bubbling sterile 2% CO2 (v/v) in air through the cultures. Growth of the algal culture was estimated following the increase in its weight. For this purpose, 10 mL of the algal suspension were centrifuged at 6000 g for 20 min (Rotofix 32 A, Hettich). The supernatant was removed and the cells were dried at 105 °C for 16 h. For the control samples, standard culture medium was used [Citation17].
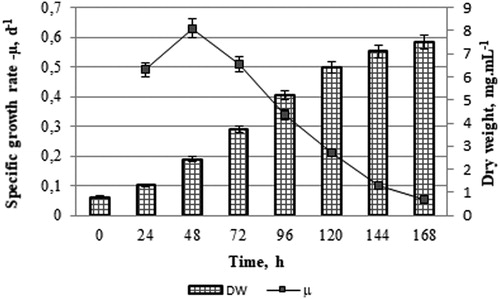
The growth was evaluated by the increase in algal biomass. The DW (mg/mL) was determined gravimetrically. The specific growth rate [μ] was calculated using the following formula: μ=ln(mt2/mt1)/(t2–t1), where mt are the dry weights at different days (t1 = 0 and t2 = 7), [Citation18].
Cell count was carried out to evaluate the growth and development of the investigated species, using a Burker counting chamber.
Pigments - chlorophyll a, chlorophyll b and carotenoids, were measured spectrophotometrically at 665 nm, 645 nm and 460 nm, respectively, using a T70 UV/Vis (PG Instruments Ltd, Leicester, UK) spectrophotometer after extraction with boiling methanol. Using the absorptions, the pigment content was calculated, employing the Mackiney formulas [Citation19].
Total phenolics
Total phenolic content in extracts were determined using Folin-Ciocalteau reagent [Citation20]. The liquid extracts were diluted and mixed with Folin-Ciocalteau reagent (2 N) and 20% sodium carbonate solution. The mixture was incubated in the dark for 2 h. After incubation, the absorbance of the mixture was measured at 765 nm using an UVmini1240 spectrophotometer (Shimadzu, France). The results were expressed as equivalent of gallic acid (GAE) in mg per 100 g maize stalks on a dry weight basis.
Cellulose concentration
Cellulose concentration was measured spectrophotometrically after lignin, hemicellulose and xylosans extractions with acetic acid/nitric acid reagent. The remaining cellulose was dissolved in 67% H2S04 and determined by the anthrone reagent [Citation21].
Total solids (TS)
TS and volatile solids (VS) were measured according to standard methods [Citation22].
Volatile fatty acids
The concentration of VFAs was analyzed using a Thermo Scientific Focus GC gas chromatograph equipped with a TG-WAXMS A column (L: 30 m, I.D.: 0.25 mm, Film: 0.25 µm), Split/Splitless injector and FID.
Biogas quantity and quality
Daily biogas yield was analyzed using graduated gasholder working on a water displacement method.
Biogas composition: The main components of biogas mixture – CH4 and CO2, were analyzed using Dräger X-am7000 (Germany), equipped with infra-red sensors.
Biodegradation degree
Biodegradation degree was calculated according to the following formula: biodegradation degree: BD = [ODMi – ODMo/ODMi] x 100, where BD is the biodegradation degree, ODMi is the input organic dry matter, ODMo is the output organic dry matter.
Data analysis
The experiments were conducted in triplicate. The data were presented as the mean values with standard deviation (±SD). The difference between the treatments was statistically analyzed by one-way analysis of variance (ANOVA) followed by the Bonferroni’s post hoc test at significance level of p < 0.05, using GraphPAD InStat Software (San Diego, CA, USA).
Results and discussion
Pre-treatment as tool for increasing substrate accessibility
In our study, we focussed on the task of making cellulose more accessible to microorganism attack. Three types of pre-treatment were tested: US 400 W – pre-treatment with ultrasound with power 400 W; pre-treatment with microwaves (MW) with power 400 W and pre-treatment with microwaves with power 2 kW. When US and MW pre-treatments were applied, the US showed higher efficiency with respect to the extractives obtained per unit of energy input ( and ). Phenoloics and VFAs were present after treatment. The increased quantity of released phenolics and VFAs established is probably as a result of some chemical alterations in the lignocellulosic structure of the substrate.
Figure 1. Effect of ultrasound (US) and microwave (MW) pre-treatment on extractives and energy input during the pre-treatment process (A) and alteration in cellulose concentration in pre-treated maize stalks vs. native material (B).
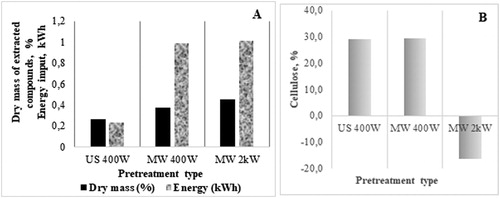
Table 1. Profile of extracted compound.
The pre-treatments affected the composition of the lignocellulosic substrate (). The energy input in the system is higher with the microwaves, or more energy is used, but the quantity of extracted substances is slightly increased. the data of the analysis of the cellulose content is presented compared to the not pre-treated substrate (0 point). In the first two cases (US 400 W and microwaves with power 400 W), the cellulose content increased, but this increase was only in relation to the total solids, so the pre-treatment had affected the substrate in a way to release other components in the liquid fraction, while the cellulose remained intact. Using higher power (2 kW) led to partial breakage of the cellulose chains. The released components in the liquid fraction are presented in . With increase of the energy input, the total phenols extracted were increased, which could indicate lignin destruction. Measurement of the VFAs showed that acetate and butyrate mainly prevailed, although the quantities were small and no serious chemical changes were provoked. The energy input for the pre-treatment of maize stalks is presented in . The data show the energy input with application of US − 0.2 kWh for pre-treatment of 25 g of the lignocellulosic material. The energy input was 0.024 kWh for pre-treatment of 3 g maize stalks used in the co-digestion process with Scenedesmus acutus. It is difficult to make an energy balance for small laboratory bioreactors. However, the energy value for the co-digestion anaerobic process, leading to 54% methane in the biogas released was comparable to the energy value for pre-treatment when the substrate loading of the bioreactor was 5 g/L. More realistic measurements require a pilot scale process with higher volumes. In previous investigations, we reached the conclusion that energy balance makes sense for bioreactors with higher working volumes [Citation23]. In this case we could not report extra energy obtained but equalized energy balance in this first step.
Ultrasonic irradiation can intensify various methods of biomass pre-treatment and could be an efficient approach for intensifying the bioconversion of a range of lignocellulosic materials. Maize stalks were the substrate on which pre-treatments were applied. There is an increased demand for biogas production, which calls for participation of renewable resources in order for the demand to be met [Citation24]. Nevertheless, the direct use of lignocellulosic biomass has a drawback of longer residence time necessary to complete the AD process because of the hemicellulose and lignin cross-linked matrix, [Citation25, Citation26]. The effective treatment of biomass with ultrasound lies on the principle of cavitation. It is a spontaneous reaction in which ultrasonic waves create small vacuum bubbles. When they can no longer absorb energy, they collapse violently. The action of ultrasonic waves in liquid medium leads to creation of a microenvironment of high temperature and pressure responsible for substantial alterations in the surface morphology - increased surface areas favouring higher transfer rates and alteration processes [Citation12, Citation27]. Thus, the effect is to facilitate cleavage within the lignin macromolecule and enhance the disruption of linkages between hemicellulose and lignin.
Biogas generation utilizing pre-treated maize stalks
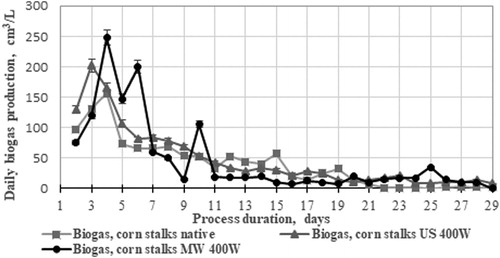
The dynamics in the variation of daily biogas yields showed differences between the yields obtained from untreated and ultrasonically treated and microwaved maize stalks, where the total yields achieved using the three independent biodegradation processes were 1116 cm3/L, 1350.5 cm3/L and 1293.25 cm3/L, respectively (). There was higher biogas yield depending on the substrate and loading − 142.7 cm3/g of biomass, when US pre-treated maize stalks were used as feedstock, compared to the native stalks − 98 cm3/g biomass. The difference may be due to the peculiarities of the chosen treatment method, and one possible explanation is that at this power the ultrasound helps not only to destroy the lignocellulosic matrix, but also to facilitate the passage into the aqueous fraction of soluble and easily digestible components of the biomass by the microorganisms (). During the process with US pre-treated maize stalks, 46% methane was measured in the released biogas on the 10th day, while the microwave pre-treatment led to 38% methane in the biogas released. Improvement in methane yield by ultrasonic pre-treatment was achieved at different conditions by several researchers [Citation28]. Ultrasound was applied as a sole technique or in combination with other pre-treatments to different substrates to improve biodegradability of substrates in the anaerobic digestion process.
Digestate as a medium for microalgae cultivation
After the biomethane generating process, part of the digestate was used as nutrient medium for microalgae cultivation. The use of active carbon is an easy procedure. The operating parameters dose, contact time, temperature and pH have to lead to reaching equilibrium for the adsorption process. We did not have to raise temperature or change the pH of the digestate for decolorization. Adsorption took place overnight and the decolorized digestate was ready to be used as a medium for cultivation of microalgae. Additionally, working with active carbon is safe, and recently it is being used as a detoxifying agent. It is also low-cost as active carbon can be obtained from waste [Citation29]. The anaerobic digestate was clarified and used as a medium for microalgae in batch experiments for a period of 14 days. Different microalgal cultures were tested, relying on their short growth cycle and ability to photosynthetically fix carbon dioxide to produce various biologically active substances and accummulate biomass. Microalgae can colonize very different environments, so this group of organisms represents one of the most promising sources for different applications [Citation30, Citation31]. That is why we tried to use the digestate after a methane-generating anaerobic process as a cultivation medium for two representatives of green algae. Scenedesmus acutus and Klebsormidium flaccidum; a representative of yellow-green algae, Trachydiscus minutus; a red alga, Porphyridium aerugineum; and a blue green alga, Synechocystis salina. They were tested for ability to grow and actively deplete substances present in the obtained digestate. The liquid fraction of the digestate obtained by utilizing pre-treated agricultural wastes for methane synthesis is rich in macro- and micronutrients that are valuable and proved to be appropriate for algae cultivation. Large-scale production of microalgae exists but is associated with high costs. That is why the selection of a cheap and promising medium for microalgae production is of great importance [Citation32]. With the suggested laboratory- scale technology, a low-cost and environmentally friendly production could be achieved. Without addition of fresh water and involving the ability of algae to utilize nutrients from the digestate, the amount of wastes released in the environment is reduced, promoting sustainable waste management. The microalgal growth was determined based on the increase in the number of algal cells of the investigated cultures ().
Figure 3. Growth of different algal species in the anaerobic decolorized digestate from the biomethane generating process with ultrasound pre-treated maize stalks as a medium.
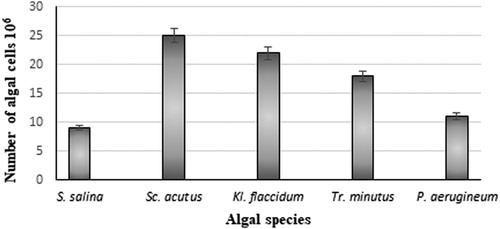
Most of the algae grew well on this waste product (). Аn essential condition for algal growth and development was ensuring light penetration. Decolorization with active carbon proved its favourable effect (). The representatives of green microalgae showed the highest increase in cell counts. The microalgae that showed maximal growth were Scenedesmus acutus and Klepsormidium flaccidum.
Figure 4. Images visualizing the suspension of Scenedesmus acutus grown in a standard medium as a control – 1; grown in digestate from a process with ultrasound (US) pre-treated maize stalks – 2 and decolorized with active carbon digestate from the same anaerobic digestion process – 3.

To confirm the growth and development of the algal culture, the presence of pigments, chlorophyll ‘a’ and ‘b’ and carotenoids, were established in the methanol extracts of the algal culture (Sc. acutus) grown in the digestate and compared to the control (). There were no pigments in the digestate without algae ().
Figure 5. Specrophotometric measurement of absorption of pigments at the end of cultivation. 1 - Scenedesmus acutus grown in a standard medium (control); 2 - Scenedesmus acutus cultivated in a digestate that had undergone adsorption with active carbon; 3 - only digestate.
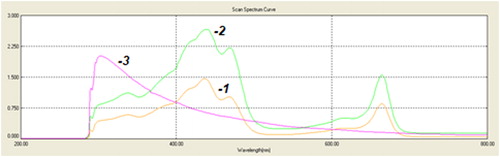
The calculated pigment quantities are presented in , demonstrating the possibility for growth and development of the algal strains in the digestate. In addition, microalgal biomass can have great potential as a source of novel bioactive compounds with health promoting applications in human, animal and aquatic lives. One of the valuable properties of green algae is their use for the production of natural pigments [Citation33, Citation34].
Table 2. Pigment content (% of DW).
The dry weight and specific growth rate of Scenedesmus acutus grown in the anaerobic digestate using US pre-treated maize stalks were estimated to characterize its growth ().
Enhanced biogas/methane yield at co-digestion
We added green algal biomass as a substrate and co-substrate in the anaerobic digestion process to observe its effect on the daily biogas/methane yields ().
Figure 7. Dynamics in the variation of daily biogas/methane yields for Scenedesmus acutus biodegradation as a sole substrate and as an adjunct to maize stalks.
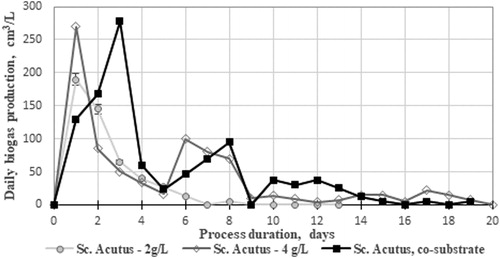
Anaerobic biodegradation of Scenedsmus acutus biomass produced a significantly higher yield of biogas per unit of biomass added compared to the same process for maize feedstocks, with a double increase in feed quality resulting in a nearly double increase in the final product. Maize stalks treated with 400 W US (3.75 g/L) with added suspension of Sc. acutus (1.25 g/L) gave an yield of 205.5 cm3/g biomass added; Sc. acutus 2 g/L gave an yield of 495 cm3/L; Sc. acutus 4 g/L gave an yield of 860 cm3/L. In the co-digestion process of maize stalks with Sc. acutus, the total volume was 1088 cm3/L for a period of 20 days. The process was shorter, the initial yields were higher and an increase in the methane yield was calculated to be 54% in the biodegradation process with addition of algal biomass as a co-substrate.
Algae contain polysaccharides with zero lignin and low cellulose content, which makes them a material more easily utilized and converted to methane by an anaerobic digestion processes [Citation35]. Addition of algal biomass which is easily consumed leads to enhancement in biomethane yield [Citation36]. The same trend was reported by Rincon et al. [Citation27]. Introducing the microalgal biomass back into the reactor on the one hand increases the biomethane yield and, on the other, closes the circle of full utilization.
For comparison of data, the biodegradation degree was calculated. The biodegradation degree was 72% for the process with co-digestion, 60% and 48% for the pre-treated substrates with US and MW, respectively.
The increase in the effectiveness and the efficiency of such a technology leads to an increase in methane quantity in the biogas, to sustainable waste management and to transition from a linear to a circular economy [Citation37]. Furthermore, microalgae are fast-growing phototrophic microorganisms which utilize light energy and inorganic nutrients (carbon dioxide, nitrogen, phosphorus etc.) and in turn are able to synthesize valuable biomass compounds, such as lipids, proteins, carbohydrate and pigments, which can find various applications [Citation38–40].
Conclusions
Anaerobic digestion of lignocellulosic agricultural waste can sustainably produce renewable energy, thus coping with unwanted waste accumulation in the environment. Finding the most appropriate pre-treatment technique for the substrate leads to faster utilization and enhanced biomethane yield. We demonstrated that anaerobic co-digestion of pre-treated maize stalks with algal biomass is a promising technology to improve digester performance and achieve a circular process. Anaerobic biorefinery is an emerging approach that not only generates bioenergy, but can also accumulate valuable algal biomass that can give high-value products.
Disclosure statement
The authors declare that they do not have potential conflict of interests.
Data availability statement
The authors confirm that the data supporting the findings of the study are available within the article.
Additional information
Funding
References
- Bochiwal C, Malley C. Chong JPJ. Biomethane as anenergy source. In: Timmis KN, editor. Handbook of hydrocarbon and lipid microbiology. Berlin: Springer; 2010. p. 2810–2815. doi:10.1007/s40362-014-0019-x
- Ward AJ, Hobbs PJ, Holliman PJ, et al. Optimisation of the anaerobic digestion of agricultural resources. Bioresour Technol. 2008;99(17):7928–7940.
- Ibarra-Gonzalez P, Rong B-G. A review of the current state of biofuels production from lignocellulosic biomass using thermochemical conversion routes. Chin J Chem Eng. 2019;27(7):1523–1535.
- Dinova N, Peneva K, Belouhova M, et al. FISH analysis of microbial communities in a full-scale technology for biogas production. Eng Life Sci. 2018;18(12):914–923.
- Hernández-Beltrán JU, Hernández-De Lira IO, Cruz-Santos MM, et al. Insight into pretreatment methods of lignocellulosic biomass to increase biogas yield: current state, challenges, and opportunities. Appl Sci. 2019;9(18):3721.
- Saritha M, Arora A. Lata Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J Microbiol. 2012;52:122–130.
- Čater M, Logar RM, Zorec M. Methods for improving anaerobic lignocellulosic substrates degradation for enhanced biogas production. Springer Sci Rev. 2014;2(1-2):51–61.
- Bussemaker MJ, Zhang D. Effect of ultrasound on lignocellulosic biomass as a pre-treatment for biorefinery and biofuel applications. Ind Eng Chem Res. 2013;52(10):3563–3580.
- Monlau F, Barakat A, Trably E, et al. Lignocellulosic materials into biohydrogen and biomethane: impact of structural features and pretreatment. Crit Rev Environ Sci Technol. 2013;43(3):260–322.
- Baruah J, Nath BK, Sharma R, et al. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res. 2018;6:141.
- Simeonov IS, Denchev DD, Kabaivanova LV, et al. Different types of pre-treatment of lignocellulosic wastes for methane production. Bulg Chem Commun. 2017;49(2):430–435. http://www.bcc.bas.bg/BCC_Volumes/Volume_49_Number_2_2017
- Subhedar PB, Gogate PR. Use of ultrasound for pretreatment of biomass and subsequent Hydrolysis and Fermentation. In: Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery-chapter 6. Amsterdam, Netherlands: Elsevier. 2016. p. 127–149.
- Parawira W. Enzyme research and applications in biotechnological intensification of biogas production. Crit Rev Biotechnol. 2012;32(2):172–186.
- Sacristan De Alva M, Luna-Pabello VM, Cadena E, et al. Green microalga Scenedesmus acutus grown on municipal wastewater to couple nutrient removal with lipid accumulation for biodiesel production. Bioresour Technol. 2013;146:744–748.
- Dalrymple OK, Halfhide T, Udom I, et al. Wastewater use in algae production for generation of renewable resources: a review and preliminary results. Aquat Biosyst. 2013;9(1):2. PMCID: PMC3561657
- Gentili FG, Fick J. Algal cultivation in urban wastewater: an efficient way to reduce pharmaceutical pollutants. J Appl Phycol. 2017;29(1):255–262.
- Burlew JS. Algal culture from laboratory to pilot plant. Washington., D. C: Carnegie Institution of Washington Publication; 1976. http://publicationsonline.carnegiescience.edu/publications_online/algal_culture.pdf
- Levasseur M, Thompson PA, Harrison PJ. Harrison PJ Physiological acclimation of marine phytoplankton to different nitrogen sources. J Phycol. 1993;29(5):587–595.
- Mackinney G. Criteria for Purity of Chlorophyll Preparations. J Biol Chem. 1940;132:91–109. https://www.jbc.org/content/132/1/91.full.pdf
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidantsby means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178.
- Updegraff DM semimicro determination of cellulose in biological materials. Analytl Biochem. 1969;3(2):420–424.
- American Public Health Association. Standard methods for the examination of waste and wastewater APHA. Washington, DC: American Public Health Association; 2005. just.edu.jo
- Georgiev R, Peychev K, Simeonov I. Determination of minimum heat insulation thickness for an experimental methane fermentor. Agric Sci Technol. 2010;2(3):160–162. http://www.uni-sz.bg/ascitech/7_2010/10.
- Matsakas L, Rova U. Christakopoulos P Strategies for enhanced biogas generation through anaerobic digestion of forest material-An overview. BioResources. 2016;11(2):5482–5499. https://www.scopus.com/record/display.uri?eid=2-s2.0-84965121644&origin
- Mason TJ, Cobley AJ, Graves JE, et al. New evidence for the inverse dependence of mechanical and chemical effects on the frequency of ultrasound. Ultrason Sonochem. 2011;18(1):226–230.
- Zheng Y, Zhao J, Xu F, et al. Pre-treatment of lignocellulosic biomass for enhancedbiogas production. Prog Energy Combust Sci. 2014;42:35–53.
- Castrillón L, Fernández-Nava Y, Ormaechea P, et al. Optimization of biogas production from cattle manure by pre-treatment with ultrasound and co-digestion with crude glycerin. Bioresour Technol. 2011;102(17):7845–7849.
- Cesaro A, Naddeo V, Amodio V, et al. Enhanced biogas production from anaerobic codigestion of solid waste by sonolysis. Ultrason Sonochem. 2012;19(3):596–600.
- El –Sheikh A, Newman AH, Al-Daffaee P, et al. Characterization of activated carbon prepared from a single cultivar of Jordanian Olive stones by chemical and physicochemical techniques. J Anal Appl Pyrolysis. 2004;71(1):151–164. https://pubmed.ncbi.nlm.nih.gov.
- Chia MA, Lombardi AT, Melão MdGG. Melão MDGG Growth and biochemical composition of Chlorella vulgaris in different growth media. An Acad Bras Cienc. 2013;85(4):1427–1438.
- Ivanova J, Kabaivanova L, Petrov P. Optimization strategies for improved growth, polysaccharide production and storage of the red microalga Rhodella reticulata. Bulg Chem Commun. 2015;47(1):167–174. https://pdfs.semanticscholar.org/35fd/f215049df96969
- Ivanova J, Kabaivanova L, Petkov G. Temperature and irradiance effects on Rhodella reticulata growth and biochemical characteristics. Russ J Plant Physiol. 2015;62(5):647–695.
- Imai M, Ikari K, Suzuki I. High-performance hydrolysis of cellulose using mixed cellulase species and ultrasonication pre-treatment. Biochem Eng J. 2004;17(2):79–83.
- Beale SI. Photosynthetic pigments: perplexing persistent prevalence of 'superfluous' pigment production. Curr Biol. 2008;18(8):R342–343.
- Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta. 2005;1740(2):101–107. https://www.ncbi.nlm.nih.gov/pubmed/15949675
- Vergara-Fernandez A, Vargas G, Alarcon N, et al. Evaluation of marine algae as a source of biogas in a two-stage anaerobic reactor system. Biomass Bioenergy. 2008;32(4):338–344.
- Rincón B, Fernández-Rodríguez MJ, de la Lama-Calvente D, et al. Influence of microalgae addition as co-substrate in anaerobic digestion processes. In: Microalgal Biotechnology. London: Intech Open; 2018.
- Markou G, Georgakakis D. Cultivation of filamentous cyanobacteria (bluegreen algae) in agro-industrial wastes and wastewaters: a review. Appl Energy. 2011;88(10):3389–3401.
- Gour RS, Chawla A, Singh H, et al. Characterization and screening of native scenedesmus sp. isolates suitable for biofuel feedstock. PLoS One. 2016;11(5):e0155321.
- Kobayashi N, Noel EA, Barnes A, et al. Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour Technol. 2013;150:377–386.