Abstract
The present study aims at the use of transcriptomic data of wild barley (Hordeum spontaneum) under salt stress in detecting regulated metabolites and crosstalking signal transduction pathways that help plants withstand harsh conditions. The studied pathways involved those emphasizing the roles of phytohormones auxin and abscisic acid (ABA) in conferring stress tolerance and adaptation. Of which, tryptophan-core pathways include metabolites that trigger auxin-responsive genes towards downstream signal transduction cascades and better plant growth. Analysis of ‘MAPK signalling’ pathway indicated active participation of several MAPK modules in receiving and delivering signals under salt stress towards appropriate downstream biological decisions. The results indicated that one enriched MEKK1 module, e.g. MEKK1-MKK2-MPK4/6, and two MKK3 modules, e.g. MEKK17/18-MKK3-MPK1/2/7/14 and ?-MKK3-MPK8, in this wild plant can effectively participate in salt stress adaptation and tolerance as well as homeostasis of reactive oxygen species (ROS). ABA seems to be a main player in the leaves of H. spontaneum in triggering signal transduction via MAPK modules. MKK7 was shown to be repressed under salt stress. This MAPK disturbs normal production, signalling and distribution of auxin, thus, enforces the plant to perform better under salt stress. In conclusion, the present study addresses signal transduction pathways that might justify the ability of this wild plant to tolerate salt stress.
Introduction
The problem of soil salinization, desertification and continual scarcity of water used for agriculture is growing worldwide [Citation1]. Salinity is a sort of physiological drought in which plant roots become unable to absorb water from soil. Salinity induces toxic effects owing to undesirable minerals like sodium and chlorine [Citation2]. Wild plant species are considered donors of raw material in improving the genetic makeup of cultivated crops either via genetic transformation or metabolic engineering approaches. Wild barley (Hordeum spontaneum) is a promising candidate donor of salt-related genes especially in agriculture of cereal crops [Citation3]. This wild species was extensively studied and has proven to be highly tolerant to abiotic stresses [Citation4]. Nowadays, genome-wide analysis became the approach of choice in breeding programs as it allows fine tuning of gene expression even for genes of small magnitude [Citation5–9].
Auxin, like other phytohormones, participates in almost all processes of plant development and responses to either biotic or abiotic stress. There are two major groups of proteins that respond to auxin signalling in the cell. They are auxin response factors (ARFs) and their cognate molecules termed auxin/indole-3-acetic acid (Aux/IAA) repressors [Citation10]. ARFs bind to auxin response elements (AuxREs) in promoters of responsive genes whose transcriptional activity is required under certain environmental conditions. The action of ARFs can be inhibited by heterotypic dimerization with Aux/IAA proteins [Citation10]. Cell proteasomes help in degrading Aux/IAA towards liberation of ARFs for downstream action [Citation11]. The spatio-temporal expression of these two protein families predicts the fate of downstream signal responses and subsequent metabolic processes [Citation12,Citation13].
Another important protein family termed mitogen-activated protein kinase (MAPK) acts under mineral stress mainly at the interface between H2O2 production and auxin signalling. MAPKs comprise a large group of transferases existing in both cytoplasm and nucleus with vital roles in signal transduction, plant development and response to stimuli [Citation14–22]. Nonetheless, MAPK signalling can also act as a regulator of polar auxin transport (PAT) and spatio-temporal distribution of auxin in certain plant tissues [Citation23–25]. Therefore, signal transduction under abiotic stress is thought to involve several upstream and downstream steps of auxin, and other phytohormones, production and signalling that provoke a cascade of events towards making a response to a given stimulus. The present study attempted to predict metabolites acting as major players in this time-dependent cascade of events from a transcriptomic dataset collected from leaves of the wild barley (H. spontaneum) under severe (500 mmol/L NaCl) salt stress.
Materials and methods
Seeds of H. spontaneum were germinated and salt stress treatment (500 mmol/L NaCl) was conducted in a replicated experiment on 14-day-old seedlings for 0 (control), 2, 12 and 24 h as previously described [Citation26]. Total RNAs were extracted from emergent leaves using Trizol (Invitrogen, Life Tech, Grand Island, NY, USA) and treated with RNase-free DNase (Promega Corporation, Madison, WI, USA). Polymerase chain reaction (PCR) was done using primers specific for actin gene (Supplemental Table S1) in order to prove that original RNAs were not contaminated with DNAs. Part of collected total RNAs from plant leaves was kept for validation (1 mg) and the other part (30 µg) was shipped to Beijing Genomics Institute (BGI), Shenzhen, China, for deep sequencing. Next-generation sequencing was done using the Illumina Miseq platform and generated raw data were submitted by the University of Texas at Austin, USA to NCBI in FASTQ format, with accession number of PRJNA227211 for the experiment (https://www.ncbi.nlm.nih.gov/bioproject?LinkName=sra_bioproject&from_uid=537429) and individual accession numbers for different samples (https://www.ncbi.nlm.nih.gov/sra?LinkName=bioproject_sra_all&from_uid=227211). Raw data were assembled using genome-guided Trinity de novo transcriptome assembly program (https://github.com/trinityrnaseq/trinityrnaseq/wiki/Genome-Guided-Trinity-Transcriptome-Assembly) with Hordeum vulgare genome used as the guide (https://plants.ensembl.org/Hordeum_vulgare/Info/Index, Taxonomy ID 112509). Principle component analysis was done using Perl-to-R script (PtR part of trinity RNA toolkit). Coding DNA sequences (CDS) of assembled transcripts were identified using the online tool ORF-Predictor with default parameters [Citation27]. Differential expression analysis was done by EdgeR (version 3.0.0, R version 2.1.5) with fold change of ≥2 measured against actin house-keeping gene. Generated clusters with appropriate algorism were analyzed for GO terms using Blast2GO (http://www.blast2go.org/). Protein Information Resource (PIR) was used to retrieve UniProt IDs. PIR includes PSD (protein sequence database), UniProt, SwissProt, TrEMBL, RefSeq, GenPept and PDB (protein data bank) databases. Predicted CDSs were annotated against protein databases and detected CDSs were mapped to reference canonical pathways in Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.ad.jp/kegg/) with fold change of ≥5 in order to detect regulated metabolites in biological pathways.
Assembled dataset was validated via qRT-PCR for four selected transcripts whose encoded metabolites, detected by KEGG analysis, were enriched. Expression levels of transcripts were detected using the Agilent Mx3000P qPCR Systems (Agilent technology, USA). First-strand cDNA was synthesized using 1 mg of total RNA, 0.5 mg of reverse primer of each gene (Supplemental Table S1) and Superscript II reverse transcriptase (Invitrogen). All cDNA-synthesized samples were diluted (1:10) prior to amplification. The reaction (25 mL) components included 12.5 mL Maxima™ SYBR Green/ROX qPCR master mix, 0.2 mmol/L of each gene’s forward and reverse primers (Supplemental Table S1) and PCR-grade water added up to 22.5 mL. Finally, 2.5 mL of diluted cDNA template were added to the reaction mix. Forty PCR cycles for each gene were performed with denaturation at 94 °C for 15 s, annealing at 55 °C for 30 s and extension at 72 °C for 45 s. Amplification of each gene was carried out in triplicate along with a no-template control (NTC, PCR-grade water). Data were collected and amplification plots of DRn versus cycle number were generated. Calculations were made to detect the expression level of each gene under a given treatment relative to its expression level under control conditions and barley actin gene was used as the house-keeping gene.
Results and discussion
Salt stress experiment in seedlings of H. spontaneum was done and leaf transcriptomes were harvested at 0, 2, 12 and 24 h time points. Principle component analysis (PCA) based on raw data generated from deep sequencing successfully separated replicated samples of each time point (). qRT-PCR of four upregulated MAPK transcripts showed perfect alignment with RNA-Seq dataset (). These results reflect the accuracy of material harvesting approach, data assembly and RNA-Seq analysis. The results of cluster analysis referring to differentially expressed transcripts under salt stress are shown in Supplemental Table S2, whilst those of upregulated and downregulated KEGG pathways are shown in Supplemental Tables S3 and S4, respectively. Amongst the introduced data, focus was given to those that are related to signal transduction processes as this wild plant is well-known for its ability to survive under harsh environmental stresses. Exposure of plants to salt or drought stress provokes several signal transduction pathways that crosstalk in order to sort out the best processes of adaptation to stress conditions. Selected pathways for further characterization are those emphasizing the roles of phytohormones auxin and abscisic acid (ABA) in conferring stress tolerance and adaptation.
Figure 1. Principal component analysis (PCA) based on transcriptomic data of control (C) and salt stressed (500 mmol/L NaCl) samples across different time points (e.g. 2, 12 and 24 h) in leaves of H. spontaneum.
Tryprophan-core analysis
The main crosstalking signal transduction pathways in leaves of H. spontaneum under salt stress were selected for further analysis. These pathways include ‘Phenylalanine, tyrosine and tryptophan biosynthesis’ (Supplemental Figures S2 and S3, respectively), ‘Tryptophan metabolism’ (Supplemental Figures S4 and S5, respectively), ‘plant hormone signal transduction’ (, respectively) and ‘MAPK signalling’ (Supplemental Figures S8 and S9, respectively). Upregulated steps of the selected KEGG pathways refer to metabolites that were either enriched at 2 h or, in most cases, at 2 and 12 h time points of salt stress. The first two, out of the four, pathways likely crosstalk towards production of tryptophan, and consequently production of auxin or indole acetic acid (IAA). Auxin, then, provokes downstream ‘Plant hormone signal transduction’ pathway, whilst phytohormones auxin and ABA provoke ‘MAPK signalling’ pathway. The latter processes initiate signal transduction cascades that strengthen plants’ ability to survive and maintain proper growth rates under stress conditions. The major player in ‘Phenylalanine, tyrosine and tryptophan biosynthesis’ and ‘Tryptophan metabolism’ pathways is the recovered rate of tryptophan biosynthesis that determines the tryptophan-dependent level of the phytohormone auxin, which is subsequently involved in the downstream cascade of events.
Figure 2. Upregulated (a) and downregulated (b) transcripts in ‘Phenylalanine, tyrosine and tryptophan biosynthesis’ pathway across time of salt stress (0, 2, 12 and 24 h) in leaves of H. spontaneum.
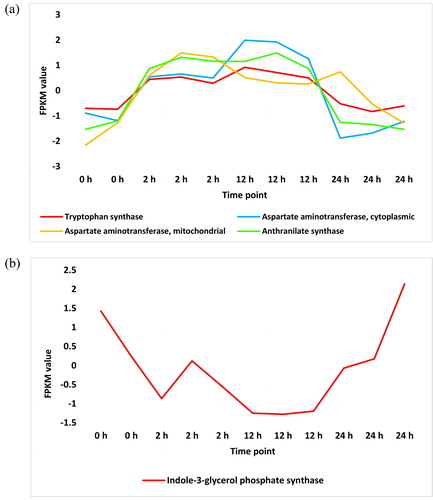
Figure 3. Upregulated (a) and downregulated (b) transcripts in ‘Tryptophan metabolism’ pathway across time of salt stress (0, 2, 12 and 24 h) in leaves of H. spontaneum.
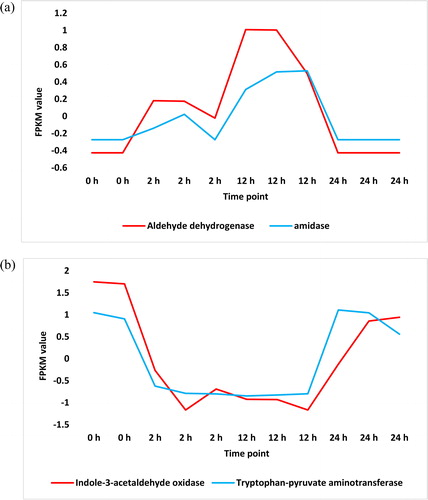
Figure 4. Upregulated (a) and downregulated (b) transcripts in ‘Plant hormone signal transduction’ pathway across time of salt stress (0, 2, 12 and 24 h) in leaves of H. spontaneum. IAA = AUX/IAA protein, ARF = auxin response factor, GH3.8 = Gretchen Hagen 3.8.
Figure 5. Upregulated (a) and downregulated (b) transcripts in KEGG pathway ‘MAPK signalling’ across time of salt stress (0, 2, 12 and 24 h) in leaves of H. spontaneum. CaM4 = calmodulin-4, MPK = mitogen-activated protein kinase, MKK = MPK kinase, MEKK = MKK kinase, YODA = MEKK4, ER/ERL1 = LRR receptor-like serine/threonine-protein kinase 1, FLS2 = LRR receptor-like serine/threonine-protein kinase FLS2, FRK1 = Senescence-induced receptor-like serine/threonine-protein kinase, XRN = 5′-3′ exoribonuclease 2, RTE1 = Protein REVERSION-TO-ETHYLENE SENSITIVITY1, EIN = ethylene-insensitive proteins.
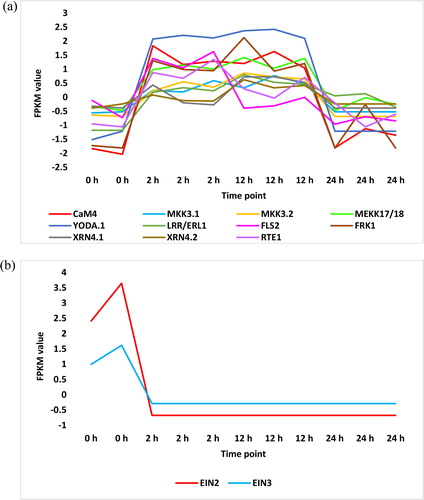
Figure 6. Regulated MPKs (a), MKK (b) and MEKK (c) transcripts in KEGG ‘MAPK signalling’ pathway or in cluster analysis across time of salt stress (0, 2, 12 and 24 h) in leaves of H. spontaneum. MPK = mitogen-activated protein kinase, MKK = MPK kinase, MEKK = MKK kinase, YODA = MEKK4.
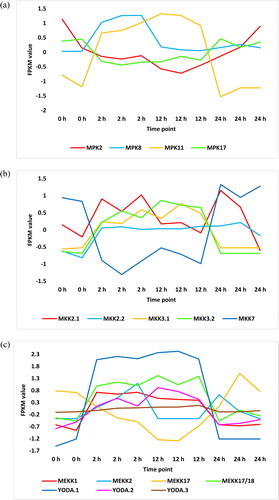
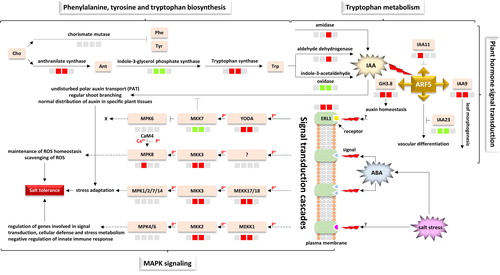
Figure 7. Crosstalking signal transduction related pathways in H. spontaneum under salt stress indicating sources of signals, signal transduction cascades and biological process responses. Cho = chorismate, Phe = phenylalanine, Tyr = tyrosine, Ant = anthranilate, Trp = tryptophan, IAA = indole acetic acid, ARF = auxin response factor, GH3.8 = Gretchen Hagen 3.8, ABA = abscisic acid, ERL1 = LRR receptor-like serine/threonine-protein kinase 1, MPK = mitogen-activated protein kinase, MKK = MPK kinase, MEKK = MKK kinase, YODA = KEKK4, CaM4 = calmodulin-4. The four-box configuration, from left to right, refers to regulation at 0 (control), 2, 12 and 24 h time points of salt stress, respectively. Upregulated transcripts at a given time point are shown in red boxes, whilst downregulated transcripts are shown in green boxes. Unregulated transcripts are shown in grey boxes.
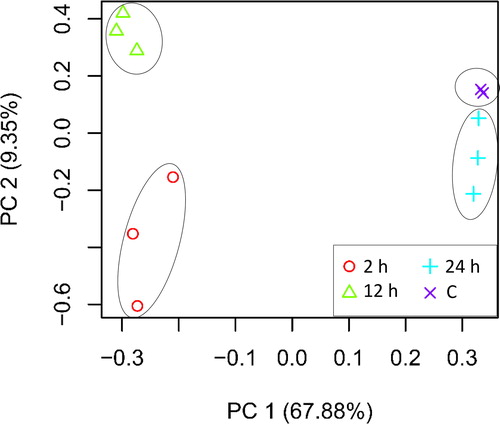
In ‘Phenylalanine, tyrosine and tryptophan biosynthesis’ pathway, two enzymes, acting like master switches, compete towards production of the three amino acids phenylalanine, tyrosine and tryptophan. The first enzyme acting towards production of tryptophan is termed anthranilate synthase (EC:4.1.3.27), whilst that acting towards production of the other two amino acids is termed chorismate mutase (EC:5.4.99.5). The results indicated that anthranilate synthase was highly enriched in leaves of H. spontaneum due to salt stress, whilst regulation of chorismate mutase was not detected (, and Supplemental Figure S2 and Table S3). Expected absence of the latter enzyme in leaves of this wild plant makes the route towards production of phenylalanine and tyrosine under salt stress unlikely. On the other hand, indole-3-glycerol phosphate synthase (EC:4.1.1.48), also acting as an intermediate step towards production of tryptophan, is unexpectedly repressed (, and Supplemental Figure S3 and Table S4). However, the expression level of this enzyme under salt stress in the leaves of H. spontaneum might not considerably affect tryptophan production, as another downstream enzyme termed tryptophan synthase (EC:4.2.1.20), participating in the last step of tryptophan production, was highly enriched in KEGG dataset (, and Supplemental Figure S2 and Table S3).
The fate of synthesized tryptophan is to participate as a substrate towards IAA biosynthesis. In ‘Tryptophan metabolism’ pathway, three enzymes were highly enriched in the leaves of H. spontaneum under salt stress (, and Supplemental Figure S4 and Table S3). Of which, aldehyde dehydrogenase (EC:1.2.1.3) and amidase (EC:3.5.1.4) participate, via two different branches, in the last step of IAA biosynthesis. On the other hand, two other enzymes were repressed under salt stress (, and Supplemental Figure S5 and Table S4). Of which, the enzyme indole-3-acetaldehyde oxidase (EC:1.2.3.7) also participates in the last step of IAA synthesis. Fortunately, enriched aldehyde dehydrogenase (, and Supplemental Figure S4 and Table S1) might compensate the adverse response of indole-3-acetaldehyde oxidase as the two enzymes act on the same step of a single branch of the pathway. Earlier studies indicated occurrence of tryptophan-independent biosynthesis of IAA due to the action of cytosolic enzyme indole synthase [Citation19]. The latter enzyme does not exist within the list of highly enriched metabolites of KEGG dataset under salt stress in H. spontaneum. Thus, tryptophan-independent biosynthesis of IAA is unlikely to take place under salt stress in this wild plant.
The pathway ‘Plant hormone signal transduction’ lies upstream of many biological processes due to the action of many phytohormones. In this pathway, synthesized auxin acts in signalling a battery of downstream metabolites some of which were proven to be highly enriched in leaves of H. spontaneum (, and Supplemental Figure S6 and Table S3). They are auxin response factors (ARFs), auxin/indole acetic acid protein (AUX/IAA) and Gretchen Hagen 3 (or GH3). GH3 family participates in maintaining homeostasis and regulation of the active form of auxin in the cell, whilst regulation of ARF family likely stimulates cell enlargement and improves growth of plants subjected to salt stress. AUX/IAA transcription factors generally function as repressors of auxin-responsive genes through binding to promoter elements of ARFs. However, Aux/IAA genes were previously reported to be expressed in distinct spatial and temporal patterns [Citation28]. These differential expression patterns contribute to the diverse responses of auxin in different plant tissues and developmental stages with or without abiotic stresses [Citation29]. In the present study, genes encoding five AUX/IAA proteins, namely IAA1, IAA6, IAA9, IAA11 and IAA23, were regulated in cluster analysis under salt stress (Supplemental Table S2). However, KEGG analysis indicated that only IAA9 and IAA11 were highly enriched (, and Supplemental Figure S6 and Table S3), whilst IAA23 was repressed (, and Supplemental Figure S7 and Table S4) under salt stress. IAA9 was previously reported to positively participate in leaf morphogenesis and fruit development in tomato [Citation28], whilst no exact roles of IAA11 and IAA23 are known in the literature. Genes encoding a number of five ARFs, namely ARF3, ARF5, ARF8, ARF15 and ARF18, were regulated in cluster analysis in leaves of H. spontaneum under salt stress (Supplemental Table S2). Of which, only ARF5 was highly enriched in KEGG analysis (, and Supplemental Figure S6 and Table S3). Although, general roles of ARFs are well-defined, influence of ARFs on auxin-responsive genes is not straightforward. It was reported that some ARFs participate in transcriptional activation or repression of auxin-responsive genes [Citation30]. In Arabidopsis, some ARFs act as transcriptional activators (ARF5-ARF8 and ARF19), whereas others act as repressors (ARF2, ARF4 and ARF9). Interestingly, ARF5 was recently reported to participate in vascular cambium regulation via attenuating the expression of WOX4 gene due to auxin signalling in Arabidopsis [Citation31,Citation32]. Cambium in plants is a layer of partially undifferentiated cells between xylem and phloem. A general role of ARF5 in promoting vascular differentiation was also recently argued [Citation32], but its exact role under salt stress is not justified. Genes encoding two GH3s were regulated in leaves of H. spontaneum under salt stress (Supplemental Table S2) of which only GH3.8 was highly enriched (, and Supplemental Figure S6 and Table S3). No role was given to this particular member of GH3 family in the literature, but its general role, as a class of auxin-induced conjugating enzymes, is participation in auxin homeostasis and regulation as well as alleviation of excessive auxin in the cell [Citation33]. GH3 also allows the activated form of ARFs to improve downstream growth parameters, such as cell and shoot elongation [Citation33,Citation34]. Based on analysis of tryptophan-core pathways, we can conclude that salt stress in the leaves of H. spontaneum induces auxin biosynthesis. This process can help the plant to grow properly due to enrichment of important metabolites in the three crosstalking pathways ‘Phenylalanine, tyrosine and tryptophan biosynthesis’, ‘Tryptophan metabolism’ and ‘Plant hormone signal transduction’.
MAPK-core analysis
Another pathway of regulated signal transduction in leaves of H. spontaneum under salt stress is ‘MAPK signalling’ pathway. The published KEGG pathway of ‘MAPK signalling’ includes seven MAPK modules. They are MEKK1-MKK1/2-MPK4, ?-MKK3-MPK1/2/7/14, ?-MKK3-MPK6, MEKK1-MKK2-MPK4/6, MEKK17/18-MKK3-MPK1/2/7/14, ?-MKK3-MPK8 and YODA(MEKK4)-MKK4/5-MPK3/6. KEGG analysis in the present study indicated high enrichment of three MAPKs namely YODA (MEKK4), MEKK17/18 and two isoforms of MKK3 (, and Supplemental Figure S8 and Table S3), whilst no MAPK metabolites were repressed (, and Supplemental Figure S9 and Table S4) under salt stress in H. spontaneum. Other non-MAPK metabolites in this pathway were also regulated of which LRR receptor-like serine/threonine-protein kinase 1 (ER or ERL1), REVERSION-TO-ETHYLENE SENSITIVITY1 (RTE1) and calmodulin-4 (CaM4) were highly enriched (, and Supplemental Figure S8 and Table S3), whilst ethylene-insensitive proteins 2 and 3 (EIN2 and EIN3) were highly repressed (, and Supplemental Figure S9 and Table S4). Although ethylene signal seems to penetrate its signal transduction cascade due to enrichment of RTE1 and repression of both EIN2 and EIN3, there is no explanation for the defense response being triggered under salt stress (Supplemental Figures S8 and S9). Cluster analysis of RNA-Seq dataset of H. spontaneum indicated upregulation of genes encoding two other MPKs, namely MPK8 and MPK11 ( and Supplemental Table S2), one MKK, namely MKK2 ( and Supplemental Table S2), and three MEKKs, namely MEKK1 and two isoforms of YODA ( and Supplemental Table S2). There is no consistent pattern of expression of MEKK2 () and other regulated isoform of MKK2 (). Cluster analysis also indicated downregulation of genes encoding two other MPKs namely MPK2 and MPK17 ( and Supplemental Table S2), one MKK namely MKK7 ( and Supplemental Table S2), and one MEKK namely MEKK17 ( and Supplemental Table S2).
As a prerequisite for considering a given module as an effective participant in the leaves of H. spontaneum under salt stress, we decided to choose modules enriched in at least two consecutive, out of the three, positions of different MAPK modules for further analysis. We assume that the occurrence of two consecutive MAPKs in a given module sufficiently gives a strong prediction on the origin and fate of signal transduction cascade, especially when the third MAPK in the module, which is usually a MEKK, is unknown. Modules that fit within this criterion are MEKK1-MKK2-MPK4/6, MEKK17/18-MKK3-MPK1/2/7/14 and ?-MKK3-MPK8. As MKK4/5 in YODA(MEKK4)-MKK4/5-MPK3/6 module can be replaced with MKK7 in activating MPK3/6 [Citation35], then the module YODA(MEKK4)-MKK7-MPK3/6 was further studied for downstream signalling and consequent biological decisions under salt stress.
MEKK1-MKK2-MPK4/6 module indicates enrichment at both MEKK and MKK positions ( and Supplemental Table S3), whilst no enrichment was shown at MPK position. This module was reported to negatively regulate the innate immune responses in plants [Citation36]. However, under salt stress, a report indicated that MKK2 in this module confers salt and cold tolerance via regulating the expression of genes involved in signal transduction, cellular defense and stress metabolism [Citation37]. Another report indicated that MPK5 is the candidate MAPK involved in regulating cold tolerance in banana (Musa sp.) [Citation38]. As the non-regulated MPK4/6 in this module is likely activated due to the action of enriched MKK2, then we assume downstream signal fate and response, involving salt tolerance, will occur in H. spontaneum, especially that the pathway shows no clear obstacles or blockage of the signal cascade downstream this MAPK cascade (Supplemental Figure S8).
The two MKK3-containing modules namely MEKK17/18-MKK3-MPK1/2/7/14 and ?-MKK3-MPK8 showed enrichment in MEKK position of the first module (e.g. MEKK17/18) and in MPK position of the second (e.g. MPK8). Aligning with the condition of MPK4/6 in MEKK1-MKK2-MPK4/6 module, MPK1/2/7/14 signal can proceed downstream the MAPK cascade with no known blockage in the pathway to retrieve the expected fate and response involving stress adaptation (Supplemental Figure S8). Although we lack information of the preceding MEKK of ?-MKK3-MPK8 module, we see that enrichment of CaM4 (Supplemental Figure S8 and Table S3) can enlarge the activation level of MPK8 originally caused by MKK3. These high phosphorylation-dependent and -independent activation levels of MPK8 strengthen our prediction of the influence of this module in the leaves of H. spontaneum under salt stress and the expected downstream signal cascade and biological response. The pathway indicates that the response to this MAPK cascade involves scavenging or homeostasis of reactive oxygen species (ROS) (Supplemental Figure S8) generated due to salt stress.
YODA is shown to be the only enriched MAPK in the YODA(MEKK4)-MKK4/5-MPK3/6 module in the leaves of H. spontaneum (Supplemental Figure S8). A preceding enriched metabolite namely ER/ERL1 (Supplemental Figure S8) seems to enhance the activation of YODA; however, our speculation that MKK7 can replace MKK4/6 does not support occurrence of downstream signalling for two reasons. The first is that MKK7 is repressed in the leaves of H. spontaneum ( and Supplemental Table S2), which makes the step upstream MKK7 activation a dead-end. The second reason is that the position of MPK in the module can be filled with either MPK2, MPK12 or MPK15 as recently detected from yeast-two-hybrid [Citation35] (simplified in Supplemental Table S5) or with either MPK3 or MPK6 as described by Wang et al. [Citation39]. Of which, the gene encoding MPK2 was downregulated under salt stress () and the other MPKs were not proven either in the present study or in the literature, to be regulated under salt stress. This information suggests that it is not likely for the module involving YODA-MKK7-MPK3/6 to be properly induced in H. spontaneum under salt stress.
MAPKs are amongst the largest group of transferases existing in the cytoplasm and nucleus and play vital roles in signal transduction, plant development and response to stimuli [Citation15,Citation17–22,Citation40,Citation41]. At a structural level, MAPK involves three types of kinases functioning stepwise. They are MAP kinase kinases (MAPKKKs, MAP3Ks or MEKKs), MAP kinase kinases (MKKs, MAP2Ks or MEKs) and MAP kinases (MAPKs or MPK). In MAPK cascades, MEKKs are first activated via stimulation of plasma membrane receptors. MEKKs, in turn, initiates signal transduction cascades by activating downstream MKKs by phosphorylation. MKKs subsequently behave as a dual-specificity kinases and phosphorylate downstream MPKs [Citation42,Citation43]. Phosphorylated MPKs, then, trigger different biological responses based on type of preceding signal or stimulus. This stepwise phosphorylation is termed MAPK cascade. In Arabidopsis, there are 80 MEKK, 10 MKK and 20 MPK genes. Each category is comprised of four groups namely A-D. MPKs are characterized by the conserved threonine and tyrosine (T-X-Y) motif of which X is any amino acid. This motif is a target of phosphorylation by MKKs during signal transduction. Based on X amino acid, the 20 Arabidopsis MPKs have been divided into two subtypes, TEY or Thr-Glu-Tyr (12 amino acids) and TDY or Thr-Asp-Tyr (8 amino acids) [Citation44]. In either structure, the motif exists almost at the middle of the MPK sequence.
Hormones, like auxin, abscisic acid (ABA), jasmonic acid (JA), salicylic acid (SA), ethylene (ET), brassinosteroids (BR) and gibberellins (GA), can differentially select distinct MAPK module structures (or cascade) that subsequently trigger a required biological process [Citation20,Citation21,Citation24,Citation42,Citation43,Citation45–51]. MAPK normally exchanges signalling with auxin. However, module MKK7-MPK6 was proven to negatively regulate auxin signalling, especially at early stages of plant development. We indicated that MKK7 activation does not occur due to repression of the encoding gene under salt stress. MKK7 disturbs polar auxin transport (PAT), shoot branching and distribution of auxin in specific plant tissues [Citation12,Citation23–25,Citation52,Citation53]. Thus, repression of this MKK in H. spontaneum under salt stress allows normal production, signalling, transport and distribution of auxin in different tissues that might help in reducing or alleviating the detrimental effects of salt stress. However, current knowledge of MAPK cascade involvement in auxin signalling is still fragmentary and requires further experimentation and support.
As indicated in ‘MAPK signalling’ pathway (, and Supplemental Figure S8 and Table S3) and yeast-two-hybrid (Supplemental Table S5), MKK3 is implicated in several modules, which highlight distinct signalling types towards a variety of downstream biological processes. Although Colcombet et al. [Citation54] indicated in an earlier yeast-two-hybrid experiment that MKK3 does not react with MPK6 or MPK8, Jagodzik et al. [Citation35] indicated, in a similar experiment, that MKK3 in Arabidopsis reacts with MPKs 1, 2, 6, 7, 8 and 14 (reactions simplified in Supplemental Table S5). The first discovery of MKK3 indicated a role during plant-pathogen interaction [Citation55]. In leaves of H. spontaneum, MKK3 was highly enriched under salt stress and the resulting functional module with MPK1/2/7/14 at MPK position was reported to be induced by ABA towards salt/drought/osmotic stress adaptation [Citation17,Citation56]. The targeted functions of MKK3-MPK1/2/7/14 module differ based on the original signal type determined by the type of the preceding MEKK. For example, ?-MKK3-MPK1/2/7/14 module is activated by H2O2 and leads to pathogen defense, whereas MEKK17/18-MKK3-MPK1/2/7/14 is induced by ABA and/or osmotic/salt/drought stress and leads to stress adaptation. The latter condition likely occurs in H. spontaneum due to salt stress in alignment with Danquah et al. [Citation17] who showed that plants impaired in the MKK3 module are less able to stand drought stress in a long-term mild drought experiment. The ?-MKK3-MPK8 module is originally induced by wounding towards homeostasis of reactive oxygen species (ROS) [Citation17]. Jasmonic acid production was also shown to be amongst the several wound responses [Citation57,Citation58]. As plants in the present study are not wounded, then the occurrence of the MKK3-MPK8 module can be due to ABA accumulation under salt stress as previously described [Citation59]. Stress-induced signalling of ABA and ROS was previously proven to coincide at the MAPK level. Additionally, ROS was reported to activate several MAPKs that crosstalk during the post-harvest senescence of horticultural products [Citation60]. In the present study, the gene encoding MPK8 does not seem to be highly upregulated to make this metabolite displayed in KEGG pathway of the present study. Phosphorylation independent activation of MPK8 in the present study, occurring from the highly enriched calcium/calmodulin 4 (CaM4), can compensate for insufficient expression of this MPK. Then, activation of MPK8 is both Ca2+-dependent and MKK3-dependent [Citation61]. The two alternative signalling processes maximize the possibility of leaf cells in H. spontaneum to restrict ROS more efficiently. Referring to MKK3 results in the present study, we claim that salt stress can induce elevated ABA signalling levels that activate MEKK17/18 or the unknown MEKK preceding MKK3 to generate different MAPK signals targeting distinct biological processes as recently described [Citation62]. However, induction of MAPK-like (MPKL), a type of proteins with kinetic activities that contains MAPK signature TxY motif, abolishes drought tolerance in maize (Zea mays) due to the reduction of ABA production [Citation63]. This negative influence was compensated by the application of exogenous ABA.
Stepwise cascade of signal transduction events in leaves of H. spontaneum is briefly described in . The figure refers to the occurrence of tryptophan biosynthesis that leads to the production of auxin, important in promoting plant growth under abiotic stress. also indicates enrichment of a number of MAPK modules that exist within several signal transduction cascades to eventually drive important biological decisions under salt stress.
Conclusions
We conclude that Hodeum spontaneum likely favours the approach of tryptophan biosynthesis, which is an important substrate for auxin production and subsequent signalling towards improving plant growth under salt stress. Enrichment of MEKK1 module, e.g. MEKK1-MKK2-MPK4/6, and the two MKK3 modules, e.g. MEKK17/18-MKK3-MPK1/2/7/14 and ?-MKK3-MPK8, in H. spontaneum successfully results in receiving and delivering signals under salt stress towards downstream biological responses. Repression of MKK7 under salt stress secured normal production, signalling and distribution of auxin to help plants perform appropriately under harsh conditions. Our transcriptome analysis adds to our knowledge of some of the mechanisms that can be adopted by the wild barley (H. spontaneum) to cope with salt stress.
Supplemental Material
Download Zip (3.6 MB)Acknowledgements
The author thanks Professor Dr. Ahmed Bahieldin (Department of Biological Sciences, Faculty of Science, King Abdulaziz University) for providing aliquots of total RNA collected from wild barley at different time points of salt stress for validating RNA-Seq dataset.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Ashraf W, Abd El-Shafi MA, Gheith EMS, et al. Using different statistical procedures for evaluation drought tolerance indices of bread wheat genotypes. Adv Agric Biol. 2015;4:19–30.
- Zhihui L, Moxin X, Daowei Z, et al. Effects of salinity, temperature, and polyethylene glycol on the seed germination of sunflower (Helianthus annuus L.). Sci World J. 2014;2014:Article ID:170418.
- Wu D, Qiu L, Xu L, et al. Genetic variation of HvCBF genes and their association with salinity tolerance in Tibetan annual wild barley. PloS One. 2011;6(7):e22938.
- Gupta S, Rupasinghe T, Callahan DL, et al. Spatio-temporal metabolite and elemental profiling of salt stressed barley seeds during initial stages of germination by MALDI-MSI and µ-XRF spectrometry. Front Plant Sci. 2019;10:1139.
- Mwando E, Han Y, Angessa TT, et al. Genome-wide association study of salinity tolerance during germination in barley (Hordeum vulgare L.). Front Plant Sci. 2020;11:118.
- Shi Y, Gao L, Wu ZC, et al. Genome-wide association study of salt tolerance at the seed germination stage in rice. BMC Plant Biol. 2017;17(1):92.
- Hazzouri KM, Khraiwesh B, Amiri KMA, et al. Mapping of HKT1;5 gene in barley using GWAS approach and its implication in salt tolerance mechanism. Front Plant Sci. 2018;9:156.
- Naveed SA, Zhang F, Zhang J, et al. Identification of QTN and candidate genes for salinity tolerance at the germination and seedling stages in rice by genome-wide association analyses. Sci Rep. 2018;8(1):6505.
- Yu J, Zhao W, Tong W, et al. A Genome-wide association study reveals candidate genes related to salt tolerance in rice (Oryza sativa) at the germination stage. IJMS. 2018;19(10):3145.
- Chandler JW. Auxin response factors. Plant Cell Environ. 2016;39(5):1014–1028.
- Chapman EJ, Estelle M. Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet. 2009;43:265–285.
- Tanaka H, Dhonukshe P, Brewer PB, et al. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell Mol Life Sci. 2006;63(23):2738–2754.
- Vernoux T, Brunoud G, Farcot E, et al. The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol. 2011;7:508.
- Hirt H. MAP kinases in plant signal transduction. Results Probl Cell Differ. 2000;27:1–9.
- Ligterink W, Hirt H. Mitogen-activated protein [MAP] kinase pathways in plants: versatile signaling tools. Int Rev Cytol. 2001;201:209–275.
- Seguí-Simarro JM, Testillano PS, Jouannic S, et al. Mitogen-activated protein kinases are developmentally regulated during stress-induced microspore embryogenesis in Brassica napus L. Histochem Cell Biol. 2005;123(4-5):541–551.
- Danquah A, de Zélicourt A, Boudsocq M, et al. Identification and characterization of an ABA-activated MAP kinase cascade in Arabidopsis thaliana. Plant J. 2015;82(2):232–244.
- Danquah A, de Zélicourt A, Colcombet J, et al. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv. 2014;32(1):40–52.
- Wang B, Chu J, Yu T, et al. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc Natl Acad Sci USA. 2015;112(15):4821–4826.
- de Zelicourt A, Colcombet J, Hirt H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 2016;21(8):677–685.
- Raja V, Majeed U, Kang H, et al. Abiotic stress: interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017;137:142–157.
- Xu C, Liu R, Zhang Q, et al. The diversification of evolutionarily conserved MAPK cascades correlates with the evolution of fungal species and development of lifestyles. Genome Biol Evol. 2017;9(2):311–322.
- Petrasek J, Friml J. Auxin transport routes in plant development. Development. 2009;136(16):2675–2688.
- Leyser Q. Auxin signaling. Plant Physiol. 2018;176(1):465–479.
- Dory M, Hatzimasoura E, Kállai BM, et al. Coevolving MAPK and PID phosphosites indicate an ancient environmental control of PIN auxin transporters in land plants. FEBS Lett. 2018;592(1):89–102.
- Bahieldin A, Atef A, Sabir JSM, et al. RNA-Seq analysis of the wild barley (H. spontaneum) leaf transcriptome under salt stress. C R Biol. 2015;338(5):285–297.
- Min XJ, Butler G, Storms R, et al. OrfPredictor: predicting protein-coding regions in EST-derived sequences. Nucleic Acids Res. 2005;33(Web Server issue):W677–W680.
- Wang H, Jones B, Li Z, et al. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell. 2005;17(10):2676–2692.
- Abel S, Nguyen MD, Theologis A. The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol. 1995;251(4):533–549.
- McSteen P. Auxin and monocot development. Cold Spring Harb Perspect Biol. 2010;2(3):a001479.
- Ji J, Strable J, Shimizu R, et al. WOX4 promotes procambial development. Plant Physiol. 2010;152(3):1346–1356.
- Brackmann K, Qi J, Gebert M, et al. Spatial specificity of auxin responses coordinates wood formation. Nat Commun. 2018;9(1):875.
- Paponov IA, Paponov M, Teale W, et al. Comprehensive transcriptome analysis of auxin responses in Arabidopsis. Mol Plant. 2008;1(2):321–337.
- Knauss S, Rohrmeier T, Lehle L. The auxin-induced maize gene ZmSAUR2 encodes a short-lived nuclear protein expressed in elongating tissues. J Biol Chem. 2003;278(26):23936–23943.
- Jagodzik P, Tajdel-Zielinska M, Ciesla A, et al. Mitogen-activated protein kinase cascades in plant hormone signaling. Front Plant Sci. 2018;9:1387.
- Gao M, Liu J, Bi D, et al. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res. 2008;18(12):1190–1198.
- Teige M, Scheikl E, Eulgem T, et al. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell. 2004;15(1):141–152.
- Tak H, Negi S, Rajpurohit YS, et al. MusaMPK5, a mitogen activated protein kinase is involved in regulation of cold tolerance in banana. Plant Physiol Biochem. 2020;146:112–123.
- Wang H, Ngwenyama N, Liu Y, et al. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 2007;19(1):63–73.
- Gupta R, Chakrabarty SK. Gibberellic acid in plant, still a mystery unresolved. Plant Signal. Behav. 2013;8(9):e25504.
- Sheikh AH, Raghuram B, Jalmi SK, et al. Interaction between two rice mitogen activated protein kinases and its possible role in plant defense. BMC Plant Biol. 2013;13:121.
- Rodriguez MC, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol. 2010;61:621–649.
- Hettenhausen C, Schuman MC, Wu G. MAPK signaling: a key element in plant defense response to insects. Insect Sci. 2015;22(2):157–164.
- Bigeard J, Hirt H. Nuclear signaling of plant MAPKs. Front Plant Sci. 2018;9:469.
- Smekalova V, Doskocilova A, Komis G, et al. Crosstalk between secondary messengers, hormones and MAPK modules during abiotic stress signalling in plants. Biotechnol Adv. 2014;32(1):2–11.
- Mohanta TK, Arora PK, Mohanta N, et al. Identification of new members of the MAPK gene family in plants shows diverse conserved domains and novel activation loop variants. BMC Genomics. 2015;16:58.
- Simonini S, Deb J, Moubayidin L, et al. A noncanonical auxin-sensing mechanism is required for organ morphogenesis in Arabidopsis. Genes Dev. 2016;30(20):2286–2296.
- Enders TA, Frick EM, Strader LC. An Arabidopsis kinase cascade influences auxin-responsive cell expansion. Plant J. 2017;92(1):68–81.
- Corredoira E, Cano V, Bárány I, et al. Initiation of leaf somatic embryogenesis involves high pectin esterification, auxin accumulation and DNA demethylation in Quercus alba. J Plant Physiol. 2017;213:42–54.
- Wójcikowska B, Gaj MD. Expression profiling of AUXIN RESPONSE FACTOR genes during somatic embryogenesis induction in Arabidopsis. Plant Cell Rep. 2017;36(6):843–858.
- Kamada M, Miyamoto K, Oka M, et al. Regulation of asymmetric polar auxin transport by PsPIN1 in endodermal tissues of etiolated Pisum sativum epicotyls: focus on immunohistochemical analyses. J Plant Res. 2018;131(4):681–692.
- Dai Y, Wang H, Li B, et al. Increased expression of MAP KINASE KINASE7 causes deficiency in polar auxin transport and leads to plant architectural abnormality in Arabidopsis. Plant Cell. 2006;18(2):308–320.
- Jia W, Li B, Li S, et al. Mitogen-activated protein kinase cascade MKK7-MPK6 plays important roles in plant development and regulates shoot branching by phosphorylating PIN1 in Arabidopsis. PLoS Biol. 2016;14(9):e1002550.
- Colcombet J, Sözen C, Hirt H. Convergence of multiple MAP3Ks on MKK3 identifies a set of novel stress MAPK modules. Front Plant Sci. 2016;7:1941.
- Dóczi R, Brader G, Pettkó-Szandtner A, et al. The Arabidopsis mitogen-activated protein kinase kinase MKK3 is upstream of group C mitogen-activated protein kinases and participates in pathogen signaling. Plant Cell. 2007;19(10):3266–3279.
- Umezawa T, Sugiyama N, Takahashi F, et al. Genetics and phosphoproteomics reveal a protein phosphorylation network in the abscisic acid signaling pathway in Arabidopsis thaliana. Sci Signal. 2013;6(270):rs8.
- Sözen C, Schenk ST, Boudsocq M, et al. Wounding and insect feeding trigger two independent MAPK pathways with distinct regulation and kinetics. Plant Cell. 2020;32(6):1988–2003.
- Hõrak H. Defense, fast and slow: activation of different MAPK pathways in response to wounding. Plant Cell. 2020;32(6):1788–1789.
- Shang Y, Dai C, Lee MM, et al. BRI1-associated receptor kinase 1 regulates guard cell ABA signaling mediated by open stomata 1 in Arabidopsis. Mol Plant. 2016;9(3):447–460.
- Gouda MHB, Zhang C, Wang J, et al. ROS and MAPK cascades in the post-harvest senescence of horticultural products. J. Proteomics Bioinform. 2020;13(1):1–7.DOI:10.35248/0974-276X.1000508.
- Takahashi F, Mizoguchi T, Yoshida R, et al. Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol Cell. 2011;41(6):649–660.
- Wang P, Zhao Y, Li Z, et al. Reciprocal regulation of the TOR kinase and ABA receptor balances plant growth and stress response. Mol Cell. 2018;69(1):100–112.
- Zhu D, Chang Y, Pei T, et al. The MAPK‐like protein 1 positively regulates maize seedling drought sensitivity by suppressing ABA biosynthesis. Plant J. 2020;102(4):747–760.
