Abstract
The hemibiotrophic oomycetes are significant threats to a wide range of Cucurbitaceae species, causing substantial losses of plant productions. Particularly, Phytophthora melonis Katsura evokes severe symptoms, thus dramatically limiting the yield in cucumber. However, the information about cucumber–P. melonis interaction is still limited. This study explored the changes in the activities of phenylalanine ammonia-lyase (PAL), peroxidase (POX), catalase (CAT), superoxide dismutase (SOD) and polyphenol oxidase (PPO) in cucumber roots of two resistant genotypes (Soheil and Ramezz), one moderately resistant genotype (Baby) and three highly susceptible genotypes (Extrem, Mini 6–23 and Yalda), over the time courses of 7, 14 and 21 days after inoculation (DAI). The results indicated that the activities of defense-related enzymes differed between the resistant and highly susceptible genotypes. Although the defense-related enzymatic activities were elevated sharply in the resistant and moderately resistant genotypes after inoculation, no significant correlations were present between the activity trends of PPO, SOD and CAT and resistance characteristics. Moreover, no significant changes in enzyme activities were found in the control plants, non-inoculated plants of the six genotypes during the testing period. Altogether, the resistance of cucumber to P. melonis is related to POX and PAL activities, but does not show relationship with PPO, SOD and CAT activities. Studying the physiological metabolic pathways of POX and PAL appears to be an important direction in research to elucidate the resistance to P. melonis in cucumber genotypes.
Introduction
Phytophthora melonis Katsura, a hemibiotrophic organism, belongs to Oomycota phylum and is one of the causal agents of damping-off disease. It is considered a potentially destructive disease of cucumber (Cucumis sativus L.; 2n = 2x = 14) across Iran and other parts of the world, as it causes economic losses [Citation1,Citation2]. Therefore, it is vital to elucidate the mechanisms of plants in response to damping-off. Typical symptoms of the disease are root and root collar rot, stem lesions, foliar blight, fruit rot and plant death [Citation3,Citation4]. Although current control measures for P. melonis are based on the use of fungicides [Citation5], identification of disease-resistant genotypes may be an effective and environmentally friendly strategy in modern crop production [Citation6–9]. To date, analyses of the interaction between P. melonis and C. sativus have been limited on screening resistant genotypes to damping-off [Citation1,Citation6] and no cucumber genotype immune to P. melonis has been reported. In the never ending struggle against pathogens, plants gradually developed a series of complex defense mechanisms involving several factors like defense-related enzymes and inhibitors which lead to prevent infection of pathogens [Citation10]. However, the plant response during the P. melonis–C. sativus interactions or, more specifically, the underlying factors that increase susceptibility and/or resistance of the cucumber plant is not fully understood. So, identification and application of candidate resistance genes and defense enzymes associated with P. melonis responses can be an efficient method to build up the host resistance in cucumber genotypes. In 2019, three defense-related genes of CsWRKY20, CsLecRK6.1 and LOX1 genes were reported to be involved in the resistance to the P. melonis infection in cucumber [Citation6,Citation11]. A complex network of defense-related enzymes, such as peroxidase (POX; EC1.11.1.7), superoxide dismutase (SOD; EC1.15.1.1), catalase (CAT; EC1.11.1.6) and phenylalanine ammonia lyase (PAL; EC4.3.1.5), promote the scavenging of ROS and are related to resistance inducement in plants [Citation12–14]. PAL has a crucial role in flavonoid productions and lignin biosynthesis, which play a key role in the phenylpropanoid biosynthetic pathway [Citation15]. PAL is one of the most extensively studied enzymes with respect to plant responses to environmental stresses, including pathogen infection [Citation16,Citation17]. Polyphenol oxidase (PPO) and peroxidase (POX) are important oxidative enzymes found in most plant species. PPO and POX catalyze the formation of lignin and other oxidative phenols, thereby contributing to the formation of defense barriers by structural reinforcement, to protect against pathogens [Citation18,Citation19]. In cucumber the activities of peroxidase increased following the inoculation with Fusarium oxysporum [Citation20]. [Citation21] reported that high activities of antioxidant enzymes played a major role in both basal and induced resistance of cabbage to black rot. Moreover, regarding the oxidative burst, CAT and SOD are useful biochemical indicators of disease resistance [Citation22]. The activities of different defense-related enzymes vary in different plants after pathogen attack and are highly complex [Citation23,Citation24]. To the best of our knowledge, no comprehensive enzymic analyses have yet been performed on cucumber plants to compare defense-related enzymes in resistant and susceptible cucumber genotypes upon inoculation with P. melonis. Therefore, the present study determined the activities of PAL, POX, CAT, SOD and PPO quantitatively and investigated their roles in response to P. melonis inoculation in the cucumber genotypes. A better insight in cucumber defense responses can help to establish biochemical characteristics for the selection of resistant cucumber sources and provide a theoretical basis for disease control and breeding of cucumber plants with higher resistance toward damping-off.
Materials and methods
Plant material
Two cucumber genotypes showing different levels of resistance to damping-off (Soheil and Ramezz), one moderately resistant genotype (Baby) and three susceptible genotypes (Extrem, Mini 6–23 and Yalda) were selected among a collection of thirty-eight commercial genotypes of domestic and exotic hybrids, and inbred lines from different seed companies. They were recently investigated and were screened in another study to identify resistant and susceptible genotypes [Citation6,Citation25,Citation26]. The six genotypes are listed from least to most susceptible according to the disease severity index in greenhouse investigations in . One hundred seeds per genotype were grown in seedling nursery trays filled with forced-air oven sterilized mix of sand-peat moss in equal parts at 200 °F for 30 min. Cucumber seedlings were grown in a greenhouse (26 ± 2 °C), provided with 16 h photoperiod and 65% relative humidity. The experiment was arranged in a completely randomized factorial design with three replications [Citation27,Citation28].
Table 1. List of Cucumis sativus genotypes, properties, breeding company, method of cultivation, mortality rate and reaction to damping-off at transplanting stage used in this study.
Pathogen culture and plant inoculation
An aggressive isolate of P. melonis obtained from naturally infected cucumber plants exhibiting post-emergence damping-off and root rot symptoms and identified as P. melonis (MH924841) was used. Inoculum was obtained by growing the P. melonis isolate in 500-mL flasks containing Water-soaked wheat seeds, which had been autoclaved at 120 °C for 30 min on 2 consecutive days. The flasks were inoculated with 5 cm disks cut from a 10-day-old culture of the P. melonis isolate, and placed in the dark for 2 weeks at 25 ± 2 °C. then, 45-day-old seedlings were inoculated by 10 g of wheat seed (106 sporangia mL−1) and incubated for two days under saturated moist conditions in the greenhouse [Citation6,Citation29–31]. In the seedling stage, inoculated and control root samples were harvested in three biological replicates over the time courses of 7, 14 and 21 days after inoculation (DAI) and then immediately transferred to liquid nitrogen and kept at −80 °C. All experiments were repeated three times.
Antioxidant enzyme extraction and activity assays
The pre-weighed infected and non-infected roots were homogenized in 4 mL buffer (50 mmol L−1 Tris pH = 8.5, 14.4 mmol L−1 2-mercaptoethanol) and 1% (w/w) insoluble polyvinylpolypyrrolidone and centrifuged at 6000 g for 15 min at 4 °C to determine the SOD, CAT, POX, PPO and PAL activities [Citation32]. The total protein content of each enzyme extract was determined using bovine serum albumin as the standard [Citation33]. The CAT activity was determined by measuring the rate of H2O2 conversion to O2 at 240 nm and expressed as U mg−1 protein [Citation34]. The POX activity was determined by the method described by [Citation35]. The photometric intensity of the reaction was measured using a spectrophotometer (470 nm) in a 40 mmol L−1 hydrogen peroxide solution and expressed as units of enzyme activity per milligram of soluble protein per minute (U mg−1 protein). The activity of PPO was determined using the method of Constabel & Ryan [Citation36]. Supernatant was added to substrate consisting of 5 mg mL−1 L-3,4-dihydroxyphenylalanine (L-Dopa). The assay solution consisted of 100 mmol L−1 NaPO4 (pH 7), 0.015% (w/v) sodium dodecyl sulfate, and catalase (280 U mL−1). The absorbance was measured at 490 nm and the PPO activity was expressed as U mg−1 protein. The activity of SOD was assayed by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) following the method of [Citation37] and expressed as units of enzyme activity per milligram of soluble protein per minute (U mg−1 protein). The PAL activity was assayed following the method of [Citation38]. The photometric intensity of the reaction was measured using a spectrophotometer (290 nm) with 15 mmol L−1 phenylalanine as the substrate. PAL specific activity was expressed in U mg−1.
Statistical analysis
The data collected from all experiments were analyzed separately for each parameter and subjected to two-way analysis of variance (ANOVA) using SAS (ver. 9.4, SAS Institute, Cary, NC). The means were compared for significance using least significant differences (L.S.D.) at p ≤ 0.05 and the values presented are means of three replicates [Citation39]. The Spearman correlation coefficients were determined between cucumber genotypes susceptibility to damping-off and activity of defense-related enzymes after inoculation by P. melonis.
Results and discussion
Disease response
P. melonis-inoculated plants of all tested genotypes revealed a wide range of symptoms depending on their genetic makeup and none of the inoculated plant was symptomless. The genotypes that responded as resistant showed minor symptoms, moderately resistant ones indicated moderate, while highly susceptible genotypes revealed severe symptoms as earlier reported by [Citation6]. The defense responses in the resistant genotypes were able to stop pathogen growth and infection, whereas in the susceptible genotypes, necrosis continued to progress along the roots and stem until the plant died ().
Defense enzymes activities
Early and elevated levels of the expression of various defense enzymes are an important feature of plant disease resistance to different pathogens [Citation10,Citation40]. Although defense-related enzymes constitute an important protective system for plants against pathogen invasion, the underlying mechanism of defense reactions and their relationships with damping-off diseases in cucumber remain unclear. Using uninoculated cucumber genotypes and time points before inoculation as controls, this study analyzed the changes in the activities of PPO, PAL, SOD, POX and CAT in the roots of six cucumber genotypes with different susceptibilities to damping-off to identify indices that provide relevant information on damping-off resistance. Data suggest that diverse mechanisms contribute to the specific levels of resistance to P. melonis in different genotypes. Although oxidative stress caused by P. melonis led to increased activities of defense-related enzymes, the overall enzyme activity patterns were distinct by enzyme and genotype. Correlation analysis was performed between cucumber genotypes susceptibility to damping-off and the activity of defense-related enzymes, including PAL, PPO, POX, SOD and CAT, after inoculation with P. melonis ().
Table 2. Spearman correlations (correlation index) between cucumber genotypes susceptibility to damping-off and activity of defense-related enzymes, including PAL, PPO, POX, SOD and CAT, after inoculation by P. melonis.
Peroxidase activity (POX)
Analysis of variance and changes in POX activity in the roots of the six cucumber genotypes with different levels of resistance after inoculation with P. melonis was represented in and and . In Ramezz and Baby, POX activity indicated a significant increase in 14 DAI, and increased to maximum at 21 DAI (). Highest POX activity of 80.8 units was recorded in resistant Ramezz after 21 DAI, which was 6 times higher than that of the untreated control, which showed 13.8 units POX activity after 21 DAI (). POX activity in Soheil increased at 7 and 14 DAI, decreased at 21 DAI and showed no significant difference between control and inoculated plants at 28 DAI (). Pattern study of POX activity in inoculated susceptible samples of Mini 6-23 and Extrem were similar to the uninoculated samples, but, the enzyme activity was significantly higher in inoculated susceptible samples of Yalda at 21 DAI. Interestingly, the POX activity in infected plants of Extrem was lower than that in controls (). The increase in POX activity caused by inoculation varied among the genotypes and had a strong negative correlation with damping-off susceptibility at 14 DAI (). Generally, POX activity in plant tissues is induced by pathogen infection and a greater increase was recorded in resistant plants compared to the susceptible ones [Citation41,Citation42]. The increase in POX activity observed in the resistant genotypes in this study was similar to that previously observed in castor infected with Fusarium oxysporum [Citation41], cucumber infected by Pythium aphanidermatum [Citation43], and zucchini and cucumber infected by mosaic virus [Citation44]. The increased activity of POX in the resistant genotypes after inoculation at 14 DAI was correlated with damping-off susceptibility in the cucumber genotypes and related to plant disease resistance. [Citation24] found that the POX activity levels could potentially be used as a genetic marker for resistant evaluation during the early phase of infection. Our results support this indication that POX may also play an important role in cucumber resistance to damping-off. POX and POD are widely distributed in higher plants and protect cells against the damaging effects of H2O2 by catalyzing its decomposition [Citation45].
Figure 2. Trends of changes in peroxidase (POX) activity in the six cucumber genotypes after inoculation with P. melonis. Uninoculated (control) genotypes (A) vs. inoculated genotypes (B) from day 7 to day 21.
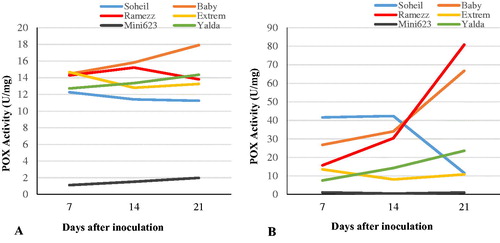
Table 3. Analysis of variance in relation to quantification of enzyme activities in inoculated resistant, moderately resistant and susceptible cucumber genotypes as compared to controls (non-inoculated ones).
Table 4. Peroxidase (POX) activities in inoculated resistant, moderately resistant and susceptible cucumber genotypes as compared to controls (non-inoculated ones).
Superoxide dismutase (SOD)
Analysis of variance and the activities of SOD in the roots of susceptible and resistant cucumber genotypes subjected to pathogen infection are presented in and and . The SOD activity in the control of each genotype showed little change from day 7 to day 21 (). However, the SOD activity in the inoculated cucumbers changed dramatically. Before inoculation, the SOD activities of all genotypes were relatively low. In resistant and moderately resistant genotypes, the SOD activity increased after inoculation by P. melonis at 14 DAI, remained high in Soheil and Baby and decreased in Ramezz at 24 DAI (). The level of SOD in inoculated Soheil ranged from 0.42 U mg−1 (7 DAI) to 1.68 U mg−1 (21 DAI); in Ramezz, it ranged from 0.21 U mg−1 (7 DAI) to 0.35 U mg−1 (21 DAI) and in Baby from 2.19 U mg−1 (7 DAI) to 0.79 U mg−1 (21 DAI). The genotype with the highest activities was the moderately resistant Baby. The SOD activity in ‘Baby’ was 4.09 times greater compared to the control at 14 DAI. The induction of SOD in all genotypes found little to no significant differences compared to the control plants at 7 DAI, except Baby and Yalda (). The SOD activity of the susceptible Mini 6–23 also showed an increase of 1.47 U mg−1 on day 21 after inoculation, but the susceptible Yalda began to decline on day 21 after inoculation. The maximum amount of SOD activity between the three susceptible genotypes in inoculated plants was observed in Mini 6–23 at 21 DAI (). The increase in SOD activity caused by inoculation did not correlate with damping-off susceptibility (, varied between −0.56 and −0.14). After inoculation with P. melonis, the SOD activity increased in the resistant genotypes. Although the SOD activity also increased in the highly susceptible genotypes Mini 6–23 and Yalda, the response time and range of increase were far lower than those in the resistant genotypes. Therefore, the differences in the activity of SOD between the six genotypes may be due to the complex activity patterns of SOD and appeared to be an important physiological basis for resistance to disease. However, the SOD activities did not correlate with damping-off susceptibility. Other researchers have indicated an increase in the activity of SOD after inoculation in cucumber [Citation46,Citation47] and other plants in a way similar to our study [Citation22].
Figure 3. Trends of changes in superoxide dismutase (SOD) activity in the six cucumber genotypes after inoculation with P. melonis. Uninoculated (control) genotypes (A) vs. inoculated genotypes (B) from day 7 to day 21.
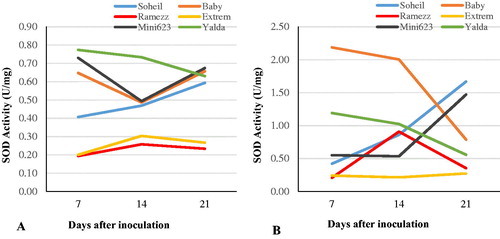
Table 5. Superoxide dismutase (SOD) activities in inoculated resistant, moderately resistant and susceptible cucumber genotypes as compared to controls (non-inoculated ones).
Catalase activity (CAT)
The changes in CAT activity of the six cucumber genotypes after inoculation with P. melonis are shown in and and . Before inoculation, the CAT activities in resistant and moderately resistant genotypes (Soheil and Baby), were significantly higher than in the other resistant genotype (Ramezz) (). After inoculation, the CAT activity in Soheil increased sharply to 20.3 U mg−1 at 14 DAI and then decreased at 21 DAI (). A similar pattern was also observed in Ramezz. However, the CAT activity in another moderately resistant genotype (Baby) showed a different reaction, with a non-significant decrease after inoculation at 14 DAI and a significant increase at 21 DAI. The maximum CAT activity in roots of inoculated plants was observed in Ramezz and Soheil (). No significant difference was found in two susceptible genotypes, Extrem and Mini 6–23 ( and ). In another susceptible genotype (Yalda), CAT activity never exceeded the activity before inoculation at 14 and 21 DAI (). However, no correlation was observed between damping-off susceptibility and CAT activity changes (). The CAT enzyme activity displayed different patterns in different genotypes under stress and mainly affects plant disease resistance through two physiological pathways [Citation18]. This is an important H2O2-scavenging enzyme in plants and is localized in the peroxisomes. Catalase can be induced and then be consumed as a result of oxidative stress [Citation48]. In this study, there was a difference in the CAT activity between the resistant and susceptible genotypes, suggesting that CAT showed intraspecific genotypic variation in response to P. melonis inoculation and that altered CAT activity also had an important effect on damping-off resistance. However, the physiological pathway by which CAT affects the resistance to P. melonis remains unclear and further investigations are needed. This result is consistent with other studies for Lilium hybrids [Citation22], Juglans [Citation18] and Brassica juncea [Citation49].
Figure 4. Trends of changes in catalase (CAT) activity in the six cucumber genotypes after inoculation with P. melonis. Uninoculated (control) genotypes (A) vs. inoculated genotypes (B) from day 7 to day 21.
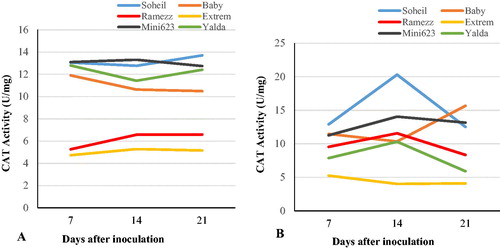
Table 6. Catalase (CAT) activities in inoculated resistant, moderately resistant and susceptible cucumber genotypes as compared to controls (non-inoculated ones).
Activity of phenylalanine ammonia-lyase (PAL)
In each of the six cucumber genotypes, the PAL activities of the control plants indicated no significant changes from day 7 to day 21 (). In the resistant and moderately resistant genotypes, PAL activities showed significant difference from the control at the same time point after inoculation. After inoculation, PAL activity in the resistant genotype Soheil increased, reaching its first peak on day 7, then it decreased on day 14 and increased to 1.86 U mg−1 on day 21 after inoculation (). The CAT activity in resistant Ramezz increased sharply to 2.17 U mg−1 at 7 DAI and then decreased at 14 and 21 DAI (). The moderately resistant genotype, Baby, showed behavior similar to that of Soheil, but it increased sharply and reached its maximum on day 21 (5.20 U mg−1). The two highly susceptible genotypes Extrem and Mini 6–23 showed increased PAL activity on day 7 and 14 and then decreased on day 21, which was different from the patterns of PAL activity in the other genotypes (). In contrast, in the other genotype, Yalda, PAL activity increased on day 7 and then decreased on day 14 and 21 (). Therefore, the changes in PAL activity showed differences among different genotypes and it was significantly higher in inoculated plants of both moderately resistant and resistant genotypes, as compared to susceptible genotypes. Significant negative correlations between the activity of PAL and damping-off susceptibility were only observed at 7 and 14 DAI (, r = −0.94 and r = −0.82, respectively); in the highly susceptible genotypes, the increase of PAL activity was lower than in the resistant genotypes. Due to the importance of PAL in the phenylpropanoid pathway and the relationship between PAL and plant disease resistance, it has always been a hot research topic. Several previous studies using different plant species have shown that the PAL activity is increased after fungal infection and played an important role in plant defense against pathogenic fungi [Citation50,Citation51]. In this study, significant changes in the PAL activity occurred after inoculation with P. melonis in resistant and moderately resistant genotypes. There were also some differences among the genotypes. Numerous studies also revealed that the activation of PAL and subsequent increase in phenolic content in plants is a general response associated with disease resistance. In black rice, PAL contributes to the resistance mechanism against Xanthomonas oryzae pv. oryzae [Citation52]. In tomato, PAL activity was enhanced in roots by a biotic elicitor Fusarium mycelium extract [Citation53]. In cucumber roots, high levels of PAL were induced after inoculation with P. aphanidermatum [Citation54]. In transgenic soybean, overexpression of GmPAL2.1 increases resistance to Phytophthora sojae [Citation51]. Therefore, an increase in the level of PAL in the infected root tissue of cucumber genotypes showed that the phenyl propanoid pathway accumulated phenolics might have prevented the pathogen invasion, and thus, the activity maintained at higher levels during the infection period.
Figure 5. Trends of changes in phenylalanine ammonia-lyase (PAL) activity in the six cucumber genotypes after inoculation with P. melonis Uninoculated (control) genotypes (A) vs. inoculated genotypes (B) from day 7 to day 21.
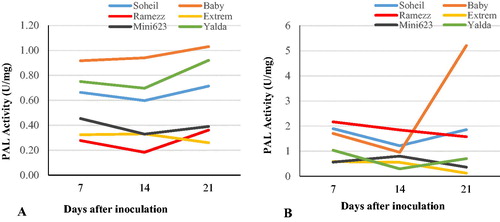
Table 7. Phenylalanine ammonia-lyase (PAL) activities in inoculated resistant, moderately resistant and susceptible cucumber genotypes as compared to controls (non-inoculated ones).
Activity of polyphenol oxidase (PPO)
As shown in , comprehensible difference in PPO activity was not observed in the control of each genotype from day 7 to day 21. After inoculation with P. melonis, the PPO activity in the cucumber plants changed notably. Changes in the PPO activity of the roots of the six cucumber genotypes with different levels of resistance after inoculation with P. melonis are shown in and . The PPO activities in the resistant and moderately resistant genotypes were significantly higher than those in the three highly susceptible genotypes. In Soheil and Baby, the activity of PPO continuously displayed high activity until 21 DAI (). In the resistant genotype Ramezz, the significant increase in the activity of POX occurred at 7 DAI and then began to decrease at 14 and 21 DAI after inoculation (). The maximum amount of PPO activity was found to be 6-fold higher in the roots of inoculated Ramezz at 7 DAI than the respective control plants. The CAT activity of the three highly susceptible genotypes Extrem, Mini 6–23 and Yalda revealed a similar trend after inoculation: a slow increase at 7 DAI followed by a decrease at 14 and 21 DAI (). In the present study, PPO activity showed no significant differences in inoculated susceptible genotypes, as compared to non-inoculated plants. There was no correlation between damping-off susceptibility and PPO activity in the cucumber genotypes (). Furthermore, the activity of PPO was correlated significantly with the POX (r = 0.23) and PAL (r = 0.53) activities in the six genotypes of cucumber with different susceptibilities to damping-off (). Our findings show that, although the PPO activity increased in the roots of infected plants in comparison to the control in the resistant and moderately resistant genotypes, the activities varied among the three genotypes. Several studies have indicated induction of PPO in plants in response to infection by different pathogens [Citation55,Citation56]. In the present study, systemic induction of PPO expression in the resistant and moderately resistant genotypes in response to P. melonis might provide an additional line of defense to protect cucumber plants against further attack by pathogens. PPOs appear to play a role in the resistance to P. melonis, since the PPO activity was considerably higher in the roots of inoculated plants of resistant genotypes, which is consistent with observations made for cucumber, walnut and common bean plants in which PPO was demonstrated to be induced by Fusarium oxysporum, Xanthomonas arboricola and arbuscular mycorrhizal fungi, respectively [Citation18,Citation57,Citation58]. Additionally, a significant correlation between the antioxidant enzymes indicates the role of the antioxidant enzymes system under P. melonis infection. The role of antioxidant enzymes in eliciting systemic resistance against Fusarium oxysporum, Pyricularia oryzae, Alternaria sp. and Sclerotium sp. has been reported earlier [Citation59]. These findings indicated the complex interaction between both oxidative-burst and response to damping-off. It can be speculated that oxidative burst and H2O2 accumulation also happened in resistant genotypes as an adaptive response to P. melonis infection, and that resistant genotypes may handle stress better than susceptible genotypes during the infection due to other mechanisms. However, the results focused on the changes in defensive enzyme activities are far from sufficient to explain damping-off resistance in cucumber. To understand cucumber resistance physiology more clearly, other physiological indicators should be considered.
Figure 6. Trends of changes in polyphenol oxidase (PPO) activity in the six cucumber genotypes after inoculation with P. melonis. Uninoculated (control) genotypes (A) vs. inoculated genotypes (B) from day 7 to day 21.
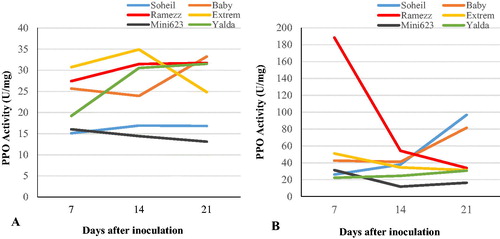
Table 8. Polyphenol oxidase (PPO) activities in inoculated resistant, moderately resistant and susceptible cucumber genotypes as compared to controls (non-inoculated ones).
Conclusions
The results of this study indicated that the disease defense response is more pronounced in the resistant genotypes as demonstrated by an earlier defense response through increasing the content of defense-related enzymes. In addition, the results suggest that PAL and POX have active roles in disease resistance against damping-off; however, there was no direct connection between damping-off resistance and PPO, SOD and CAT activities. Meanwhile, the varied response of defense-related enzymes suggests that oxidative-burst response occurred after inoculation by P. melonis, and the response may have differed depending on the genotype and inoculation period. These findings can help us to understand the resistance physiology of damping-off and provide indicators for cucumber breeding. However, diverse mechanisms contribute to the specific levels of resistance to P. melonis in genotypes and the mechanisms by which defense-related enzymes accumulation contributes to resistance in cucumber remain to be explored in future studies.
Acknowledgments
The authors are thankful to Plant Protection Research Division, Isfahan Center for Agricultural and Natural Resources Research and Education (AREEO), Isfahan, Iran and also, Plant Protection Research Institute, Tehran, Iran, for providing facilities to run the project.
Disclosure statement
There is no conflict of interest relating to this article.
References
- Nazavari K, Jamali F, Bayat F, et al. Evaluation of resistance to seedling damping-off caused by Phytophthora drechsleri in cucumber cultivars under greenhouse conditions. Biol Forum. 2016;8:54–60.
- Wu T, Wang R, Xu X, et al. Cucumis sativus L-type lectin receptor kinase (CsLecRK) gene family response to Phytophthora melonis, Phytophthora capsici and water immersion in disease resistant and susceptible cucumber cultivars. Gene. 2014;549(2):214–222.
- Bagheri LM, Nasr-Esfahani M, Abdossi V, et al. Analysis of candidate genes expression associated with defense responses to root and collar rot disease caused by Phytophthora capsici in peppers Capsicum annuum. Genomics. 2020;112(3):2309–2317.
- Hatami N, Aminaee MM, Zohdi H, et al. Damping-off disease in greenhouse cucumber in Iran. Arch Phytopathol Plant Prot. 2013;46(7):796–802.
- Lamichhane JR, Dürr C, Schwanck AA, et al. Integrated management of damping-off diseases. A review. Agron Sustain Develop. 2017;37:10.
- Hashemi L, Golparvar AR, Nasr Esfahani M, et al. Correlation between cucumber genotype and resistance to damping-off disease caused by Phytophthora melonis. Biotechnol Biotechnol Equip. 2019;33(1):1494–1504.
- Moghaddam GA, Rezayatmand Z, Nasr Esfahani M, et al. Genetic defense analysis of tomatoes in response to early blight disease, Alternaria alternata. Plant Physiol Biochem. 2019;142:500–509.
- Moghaddam GA, Rezayatmand Z, Nasr Esfahani M, et al. Bio-genetic analysis of resistance in tomato to early blight disease. Alternaria alternata. Phytochem. 2020.
- Tehrani MM, Nasr Esfahani M, Mousavi A, et al. Regulation of related genes promoting resistant in Iris against root rot disease, Fusarium oxysporum f. sp. gladioli. Genomics. 2020;112(5):3013–3020.
- Andersen EJ, Ali S, Byamukama E, et al. Disease resistance mechanisms in plants. Genes. 2018;9(7):339.
- Hashemi L, Golparvar AR, Nasr Esfahani M, et al. Regulation of novel candidate genes resistant in response to Phytophthora melonis in cucumber Cucumis sativus. Mol Biol Rep. 2020;47:4933–4944.
- Khatediya NK, Parmar DV, Mahatma MK, et al. Increased accumulation of phenolic metabolites in groundnut (Arachis hypogaea L.) genotypes contribute to defense against Sclerotium rolfsii infection. Arch Phytopathol Plant Prot. 2018;51(9–10):530–549.
- Prasannath K, De Costa DM. Induction of peroxidase activity in tomato leaf tissues treated with two crop management systems across a temperature gradient. Proceedings of the International Conference on Dry Zone Agriculture 2015; 2015 October 15th & 16th; Sri Lanka: Faculty of Agriculture, University of Jaffna; 2015. p. 34–35.
- Xie J-H, Chai T-T, Xu R, et al. Induction of defense-related enzymes in patchouli inoculated with virulent Ralstonia solanacearum. Electron J Biotechnol. 2017;27:63–69.
- Yusuf CYL, Abdullah JO, Shaharuddin NA, Abu Seman, et al. Characterization of promoter of EgPAL1, a novel PAL gene from the oil palm Elaeis guineensis Jacq. Plant Cell Rep. 2018;37(2):265–278.
- Huang J, Gu M, Lai Z, et al. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010;153(4):1526–1538.
- Kim DS, Hwang BK. An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic aciddependent signalling of the defense response to microbial pathogens. J Exp Bot. 2014;65(9):2295–2306.
- Jiang S, Han S, He D, et al. The accumulation of phenolic compounds and increased activities of related enzymes contribute to early defense against walnut blight. Physiol Mol Plant Pathol. 2019;108:101433.
- Li L, Steffens J. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta. 2002;215(2):239–247. pmid: 12029473.
- Zhao S, Du CM, Tian CY. Suppression of Fusarium oxysporum and induced resistance of plants involved in the biocontrol of cucumber Fusarium wilt by Streptomyces bikiniensis HD-087. World J Microbiol Biotechnol. 2012;28(9):2919–2927.
- Amaral LS, Debona D, Costa LC, et al. Biochemical insights into basal and induced resistance in cabbage to black rot. J Phytopathol. 2019;167(7–8):390–403.
- Su X, Guan L, Hu F. Comparison of defensive enzyme activities in the leaves of seven oriental lily hybrids after inoculation with Botrytis elliptica. J Am Soc Hortic Sci. 2019;144(1):55–62.
- Siddique Z, Akhtar KP, Hameed A, et al. Biochemical alterations in leaves of resistant and susceptible cotton genotypes infected systemically by cotton leaf curl Burewala virus. J Plant Interact. 2014;9:702–711.
- Su Y, Wang Z, Xu L, et al. Early selection for smut resistance in sugarcane using pathogen proliferation and changes in physiological and biochemical indices. Front Plant Sci. 2016;7:1133.
- Nasr Esfahani M, Ansari Pour B. The variable differences in onion cultivars to pink root rot disease in Iran. J Gen Plant Pathol. 2008;74(1):46–244.
- Nasr Esfahani M. Morphological, virulence and genetic variability of Ulocladium atrum causing potato leaf blight disease in Iran. J Plant Prot Res. 2019;59:41–49.
- Nasr Esfahani M, Chatraee M, Shafizadeh S, et al. Evaluation of resistance of cucurbit and cucumber cultivars to Phytophthora drechsleri in Greenhouse. Iran Seed Plant Improv J. 2012;28:407–417.
- Nasr Esfahani M, Nasehi A, Rahmanshirazi P, et al. Susceptibility assessment of bell pepper genotypes to crown and root rot disease. Arch Phytopathol Plant Protect. 2014;47(8):944–953.
- Ghasemi A, Golparvar A, Isfahani M. Analysis of genetic diversity of sugar beet genotypes using random amplified polymorphic DNA marker. Genetika. 2014;46(3):975–984.
- Nasr Esfahani M. Analysis of virulence and genetic variability of Alternaria alternata associated with leaf spot disease in potato plants in Iran. Acta Mycol. 2018a;53(1):9.
- Nasr Esfahani M. Genetic variability and virulence of some Iranian Rhizoctonia solani isolates associated with stem canker and black scurf of potato (Solanum tuberosum L.). J Plant Prot Res. 2020;60:21–30.
- Jung W-J, Mabood F, Souleimanov A, et al. Induction of defense-related enzymes in soybean leaves by class IId bacteriocins (thuricin 17 and bacthuricin F4) purified from Bacillus strains. Microbiol Res. 2011;167(1):14–19.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254.
- Gratão PL, Monteiro CC, Carvalho RF, et al. Biochemical dissection of diageotropica and Never ripe tomato mutants to Cd-stressful conditions. Plant Physiol Biochem. 2012;56:79–96.
- Dazy M, Jung V, Férard JF, et al. Ecological recovery of 712 vegetation at a former coke-factory industrial wasteland: role of plant 713 antioxidant enzymes and possible implications in site restoration. Chemosphere. 2008;74(1):57–63.
- Constabel CP, Ryan CA. A survey of wound-and methyl jasmonate-induced leaf polyphenol oxidase in crop plants. Phytochemistry. 1998;47(4):507–511.
- Van Rossum MW, Alberda M, van der Plas LH. Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci. 1997;130(2):207–216.
- Beaudoin-Eagan LD, Thorpe TA. Shikimate pathway activity during shoot initiation in tobacco callus cultures. Plant Physiol. 1983;73(2):228–232.
- Nasr Esfahani M. Genetic and virulence variation in Fusarium oxysporum f. sp. cepae causing root and basal rot of common onion in Iran. J Phytopathol. 2018b;166(7–8):572–580.
- Vanitha SC, Umesha S. Variations in defense related enzymeactivities in tomato during the infection with bacterial wilt pathogen. J Plant Interact. 2008;3(4):245–253..
- Bharathi E, Santha Lakshmi Prasad M, Yadav P, et al. Defense responses to Fusarium oxysporum f. sp. ricini infection in castor (Ricinus communis L.) cultivars. Indian Phytopathology. 2019;72(4):647–656.
- Mydlarz LD, Harvell CD. Peroxidase activity and inducibility in the see fan coral exposed to a fungal pathogen. Comparative Biochemistry & Physiology. 2006;10:1016.
- Sabbaghi E, Sabbagh SK, Panjehkek N, et al. Jasmonic acid induced systemic resistance in infected cucumber by Pythium aphanidermatum. J Agric Sci. 2018;24:143–152.
- Riedle-Bauer M. Role of reactive oxygen species and antioxidant enzymes in systemic virus infections of plants. J Phytopathol. 2000;148(5):297–302.
- Fernandes CF, Moraes VCP, Vasconcelos LM, et al. Induction of an anionic peroxidase in cowpea leaves by exogenous salicylic acid. J Plant Physiol. 2006;163(10):1040–1048.
- Moradi N, Rahimian H, Dehestani A, et al. Cucumber response to sphaerotheca fuliginea: differences in antioxidant enzymes activity and pathogenesis-related gene expression in susceptible and resistant genotypes. J Plant Mol Breed. 2016;4(2):33–40.
- Nostar O, Ozdemir F, Bor M, et al. Combined effects of salt stress and cucurbit downy mildew (Pseudoperospora cubensis Berk. and Curt. Rostov.) infection on growth, physiological traits and antioxidant activity in cucumber (Cucumis sativus L.) seedling. Physiol Mol Plant Pathol. 2013;83:84–92.
- Garg N, Manchanda G. ROS generation in plants: boon or bane? Plant Biosystems. 2009;143(1):81–96.
- Pandey V, Tewari AK, Saxena D. Activities of defensive antioxidant enzymes and biochemical compounds induced by bioagents in Indian mustard against alternaria blight. Proc Nat Acad Sci. 2017;2:1–10.
- Saunders J, O’neill N. The characterization of defense responses to fungal infection in alfalfa. Biol Control. 2004;49:715–728.
- Zhang CZ, Wang X, Zhang F, et al. Phenylalanine ammonia-lyase2.1 contributes to the soybean response towards Phytophthora sojae infection. Sci Rep. 2017;7(1):7242.
- Solekha R, Susanto FA, Joko T, et al. Phenylalanine ammonia lyase (PAL) contributes to the resistance of black rice against Xanthomonas oryzae pv. oryzae. J Plant Pathol. 2019;102:359–365..
- Mandal S, Mitra A. Reinforcement of cell wall in roots of Lycopersicon esculentum through induction of phenolic compounds and lignin by elicitors. Physiol Mol Plant Pathol. 2007;71(4–6):201–209.
- Chen C, Belanger RR, Benhamou N, et al. Defense enzymes induced in cucumber roots by treatment with plant growth promoting rhizobacteria (PGPR). Physiol Mol Plant Pathol. 2000;56(1):13–23.
- Khodadadi F, Tohidfar M, Vahdati K, et al. Functional analysis of walnut polyphenol oxidase gene (JrPPO1) in transgenic tobacco plants and PPO induction in response to walnut bacterial blight. Plant Pathol. 2020;69(4):756–764.
- Vanitha SC, Niranjana SR, Umesha S. Role of phenylalanine ammonia lyase and polyphenol oxidase in host resistance to bacterial wilt of tomato. J Phytopathol. 2009;157(9):552–557.
- Abdel-Fattah GM, El-Haddad SA, Hafez EE, et al. Induction of defense responses in common bean plants by Arbuscular mycorrhizal fungi. Microbiol Res. 2011;166(4):268–281.
- Moghbeli E, Nemati SH, Aroiee H, et al. Evaluation of resistance, enzymatic response, and phenolic compounds in roots of F1 cucumber hybrids to Fusarium oxysporium f. sp. radicis-cucumerium. Journal of Horticultural Research. 2017;25(1):117–124.
- Rais A, Jabeen Z, Shair F, et al. Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PLoS One. 2017;12(11):e0187412. journal. pone.0187412.
- Erwin DC, Ribeiro OK. Phytophthora disease worldwide. St. Paul, MN: APS Press; 1996. p. 562.

