Abstract
Aphids are sap-sucking insects and are pests that cause substantial damage to many crops worldwide. Spodoptera litura is a leaf-chewing insect and poses a severe threat to many species of vegetable and cotton plants. In this paper, we studied an ethylene-responsive factor (ERF) that enhances plant resistance to sap-sucking and leaf-chewing insects. ERF11-like was obtained from Chinese cabbage via reverse transcription-polymerase chain reaction and was termed BrERF11b. The putative protein encoded by the BrERF11b gene includes 168 amino acid residues and contains an EAR motif. The plasmid pBI121-BrERF11b was constructed and transformed into tobacco plants (Nicotiana tabacum cv. K326 and N. benthamiana) by the Agrobacterium-mediated transformation method. The function of the BrERF11b gene in the resistance of the transgenic plants to Myzus persicae and S. litura was assayed. The results showed that the weights of the S. litura larvae fed the transgenic plant line were significantly reduced from 28 to 76% compared to those of the S. litura larvae fed the control plants (wild type), and the transgenic plant line KLine12 significantly reduced the survival rate of S. litura by 62.5%. The colony growth of the apteral aphids fed the transgenic plant line was significantly reduced from 22.5 to 63.4% compared to that of the apteral aphids fed the control plants. Compared to control plants, the transgenic plant line significantly reduced the survival rate of the aphids from 46 to 55%. These results first indicate that the BrERF11b gene significantly enhances plant resistance to S. litura and M. persicae.
Introduction
Aphids belong to the Aphidoidea superfamily of Hemiptera and are sap-sucking insects. Aphids are a class of pests that cause substantial damage to many crops worldwide. For example, the green aphid (Myzus persicae) is a major pest of vegetables [Citation1], and the cotton aphid (Aphis gossypii) is a major pest of cotton crops worldwide [Citation2].
Spodoptera litura belongs to the family Noctuidae of Lepidoptera. It is an omnivorous and ravenous leaf-chewing insect that feeds on 300 species of plants from more than 100 families. Owing to the persistent application of insecticides, this pest has developed resistance to conventional and new chemistries. It poses a severe threat to many species of vegetable and cotton plants [Citation3].
Some genes increase the resistance of plants to aphids and other insects of Hemiptera, for example, the lectin gene [Citation4], TPS46 gene [Citation5] and RAP1 gene [Citation6]. The TPS46 gene encodes a rice terpene synthase conferring natural resistance to Rhopalosiphum padi. The RAP1 gene confers effective resistance to the pea aphid in Medicago truncatula. However, these genes do not function to increase the resistance of plants to lepidopteran insects. Some genes, such as Cry1Ac [Citation7], Vip3AcAa [Citation7] and OsLRR-RLK1 [Citation8], increase the resistance of plants to lepidopteran insects, but these genes do not function to increase the resistance of plants to Hemipteran insects. Cry1Ac and Vip3AcAa gene are from Bacillus thuringensis (Bt). The Cry1Ac gene encodes an insecticidal crystal protein. The Vip3AcAa gene encodes a chimeric protein with the N-terminal part of Vip3Ac and the C-terminal part of Vip3Aa, and is an insecticidal protein that is secreted into the culture medium. OsLRR-RLK1 protein is an early responsive leucine-rich repeat receptor-like kinase, initiating rice defense responses against a chewing herbivore [Citation8]. Strikingly, the Cry1Ac gene from Bt bacteria increases the resistance of cotton plants to the major pest Helicoverpa armigera with great success, but secondary lepidopteran pests S. litura are tolerant to Cry1Ac [Citation7]. Therefore, S. litura is an important pest in the field when planting transgenic Bt-Cry1Ac cotton plants [Citation9]. A few genes, such as AtMYB44 [Citation10] and Hvt [Citation11,Citation12], have been found to increase the resistance of plants to the insects of the Lepidoptera and Hemiptera orders. AtMYB44 is a transcription factor of the MYB family, and Hvt is a peptide from spiders.
The functions of ethylene-responsive factors (ERFs) have been reported. There are more than 100 ERF transcription factors in every species of plant [Citation13]. ERF transcription factors function in plants to regulate their growth and development, resistance to biotic stresses and resistance to abiotic stresses [Citation13]. Resistance to biotic stresses includes resistance to insects, fungi, bacteria, viruses, etc. [Citation13,Citation14]. The number of ERF transcription factors that enhance plant resistance to microorganisms or abiotic stresses is more than the number of ERF transcription factors that enhance plant resistance to insects. OsERF3 [Citation15], Pti5 [Citation16] and JRE4 [Citation17] encode ERF transcription factors that reportedly function in insect resistance. OsERF3 functions in the resistance to rice borers [Citation15], and Pti5 functions in the resistance to aphids [Citation16]. JRE4 functions in increasing plant defenses against leaf-chewing insects [Citation17]. Taken together, many studies have confirmed that ERF transcription factors function in the resistance of plants to leaf-chewing insects or sap-sucking insects.
The EST tab of ERF transcription factors from Brassica rapa plants has been uncovered [Citation18], and genome analyses of Brassica napus are available in the NCBI database. An ERF gene (BrERF11) from the Brassica rapa plant has been reported to function in the resistance of tobacco to Ralstonia solanacearum [Citation19]. In this paper, we reported that an ERF transcription factor from Brassica rapa (Chinese cabbage) functions in the resistance of plants to green aphids and S. litura. The ERF gene has been termed BrERF11b because it has 99% nucleotide sequence identity and 99% deduced protein identity to ERF11 from Brassica napus and a deduced protein identity of 60% to BrERF11 reported by Lai et al. [Citation19].
Materials and methods
Construction of the expression vector carrying the BrERF11b gene
The ORF sequence of the BrERF11b gene was searched in the NCBI database (gene ID: XM_009104664). Total RNA was extracted from the Chinese cabbage Peking variety. The ORF of the BrERF11b gene was obtained from total RNA by RT-PCR. The RT-PCR primers were as follows: forward primer: cCCCGGGatggcgccgacagttaaaac; reverse primer: CGAGCTCttagtcttcaggctttggcg. The reverse transcription enzymes MLV and rTaq were purchased from Takara Bio Inc. (Beijing). RT-PCR was performed according to the instructions for the MLV enzyme. The RT-PCR products were inserted into the pMD19 vector. Three samples of the plasmid pMD19-BrERF11b were sequenced by Thermo Fisher Scientific Company (China). Then, the binary vector pBI121 was used to construct the plasmid that expressed the BrERF11b gene in the plants Nicotiana tabacum K326 and N. benthamiana. The plasmid pBI121-BrERF11b was constructed as follows: the ORF sequence of the BrERF11b gene and the pBI121 vector were digested with Sma I and Sac I, and the digestion products were ligated by DNA ligation enzyme (Takara Bio Inc., Beijing).
Transformation of the tobacco plants
The plasmid pBI121-BrERF11b was transformed into Agrobacterium tumefaciens strain GV3101 by electrotransformation. Then, the Agrobacterium cells harboring the plasmids (pBI121-BrERF11b/GV3101) were used for the transformation of tobacco plants (Nicotiana tabacum cv. K326 and N. benthamiana) via the Agrobacterium-mediated leaf disc transformation method [Citation20]. Regenerated shoots from the calli were transferred to MS medium with 100 mg/L kanamycin to select the transformants. The plantlets were grown for 2 weeks in the culture bottle and then transplanted to soil in pots in a greenhouse maintained at 25 °C with 16 h of light each day. The seeds of T0 plants were collected and screened via culture on MS medium with 100 mg/L kanamycin and then transplanted to soil in pots in a greenhouse. The seeds of the T1 and T2 plants were cultured according to the same method.
PCR, reverse transcription-polymerase chain reaction (RT-PCR) and Southern blot detection
Genomic DNA was extracted from the fresh leaves of transformed plants (T0) and control plants (wild type, WT) by the CTAB method [Citation21]. PCR analysis was conducted by using the genomic DNA as a template and the following primers: forward: gccgacagctaaaacgac; reverse: gactacgatccatcaccacagacg. The PCR products were separated by electrophoresis in a 0.8% agarose gel.
Southern blotting was conducted according to the instructions of the DIG High Prime DNA Labeling and Detection Starter Kit II (Roche Applied Science Company, USA). Individually, the genomic DNA was extracted from the fresh leaves of transformed plants (T1) and control plants (WT) by the CTAB method. Approximately 10 µg of genomic DNA was digested with restriction enzyme and separated in a 0.8% agarose gel, and then transferred to the nylon membrane (Hybond N+, Thermo-Amersham, USA) in 0.4 mol/L NaOH transfer buffer. The PCR product from the ORF of the BrERF11b gene was used as the DNA template for the probe. The probe was labeled with DIG-11-dUTP according to the instructions from the DIG High Prime DNA Labeling. Individual lanes were hybridized with dioxigenin (DIG)-11-dUTP–labeled cDNA probes specific for the segment of the BrERF11b gene (the putative open reading frame regions). Hybridization was conducted in a rotary hybridization oven (Thermo Scientific Company, China) for 16 h at 45 °C. Membrane washing and the detection of the hybridization signal were conducted according to the instructions of Detection Starter Kit II.
Total RNA was extracted from the experimental plants. RT-PCR was conducted. Individually, reverse transcription was performed using total RNA as a template. The primers for RT-PCR were designed according to the ORF of the BrERF11b gene, and are as follows: forward: gccgacagctaaaacgac; reverse: gactacgatccatcaccacagacg. The reverse transcription enzymes MLV and rTaq were purchased from Takara Bio Inc. (Beijing). PCR products were separated by electrophoresis in a 0.8% agarose gel.
Myzus persicae and Spodoptera litura
The apterous aphids were reared in a acclimatized room with a relative humidity of 60–70%, a temperature of 20 °C and a 16:8 h (light:dark) photoperiod. Ten first-day instar aphids of Myzus persicae were selected and placed into each culture dish with experimental plant leaves. In the experiment, the plants from the transgenic plant line were set as treatment groups, wild-type plants (WT, non-transformed) were set as the control group. The transgenic plant lines are as follows: Kline12, 13, 15; Bline25, 26. The experiment was done in triplicate. The aphids of each treatment were monitored daily over the course of the experiments until the aphid number of the control (WT) was reduced by more than 20%.
Four first-instar larvae of S. litura were selected and placed into each culture dish with treated plant leaves under a photoperiod of 14:10 h (light:dark), 70% relative humidity and 25 °C. Eight repeats (total of 32 larvae) were set up for each treatment. In another experiment, four second-instar larvae of S. litura were selected and placed into each culture dish. The larvae of each treatment were monitored daily over the course of the experiment until the larval number of the control was reduced by more than 20%.
Data analysis
In sequence analysis, the unrooted phylogenetic tree of ERF transcription factor was constructed using the neighbor-joining method with 1000 bootstrap replicates. Duncan's or Student’s method from the Data Procession System (DPS) [Citation22] was used to analyze the data of the experiments with aphids and Spodoptera litura.
Results and discussion
Sequence analysis
The ORF of the BrERF11b gene is 507 bp and encodes a putative protein (termed BrERF11b-P) of 18.733 kDa that is composed of 168 amino acid (aa) residues. BrERF11b-P contains a conserved AP2/ERF domain with two highly conserved amino acid residues characteristic of the ERF protein [Citation23] and a conserved EAR motif (). BLAST search of the protein sequences showed that 30 protein sequences had similarities (E value < e−50) to the putative BrERF11b-P, 20 protein sequences of which are ethylene-responsive factors 11-like.
Figure 1. Multiple alignment of the deduced amino acid sequences of the putative protein encoded by the BrERF11b gene with those of selected ERF transcription factors. The unrooted phylogenetic tree of ERF transcription factor was constructed using neighbor-joining method with 1000 bootstrap replicates. Bootstrap values are indicated at the branches. The GenBank accession number is shown following the abbreviation of the ERF transcription factor in the unrooted phylogenetic tree.
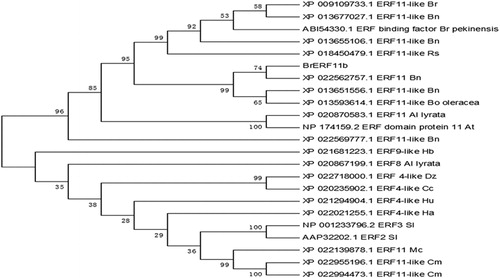
A phylogenetic tree was constructed with BrERF11b-P and the other 23 related protein sequences by multiple alignment (). The results showed that BrERF11b-P was grouped into a small clade with the ethylene-responsive transcription factor 11-like (ERF11-like) from Brassica napus. Moreover, BrERF11b-P was grouped into a large clade with ERF11 from Brassica oleracea var. oleracea, Brassica rapa, Brassica rapa subsp. Pekinensis and Raphanus sativus.
Pairwise sequence comparisons showed that BrERF11b-P had more than 98% identity with the amino acid sequence of the ERF11-like proteins XP0022562757.1 and XP 013651556.1 of Brassica napus and XP 013593614.1 of Brassica oleracea var. oleracea; additionally, it had more than 60% identity with the amino acid sequence of the ERF11-like proteins XP 009109733.1 of Brassica rapa, XP 018450479.1 of Raphanus sativus, XP 013677027.1 of Brassica napus, XP 013655106.1 of Brassica napus, ABI 54330.1 of Brassica rapa subsp. pekinensis, XP 022569777.1 of Brassica napus and XP 020870583.1 of Arabidopsis lyrata subsp. lyrata (Supplemental Table S1).
Identity and expression of the BrERF11b gene in transgenic plants
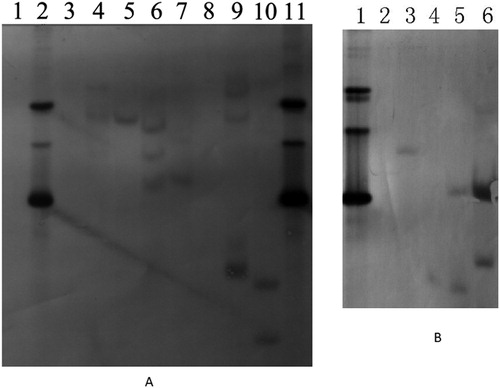
Twenty transgenic tobacco plants of N. tabacum cv. K326 and fifteen transgenic tobacco plants of N. benthamiana were generated. To confirm that the BrERF11b gene was transformed into the tobacco plants, Southern blots were conducted with five lines of transgenic tobacco plants (T1 generation), including KLine12, KLine15, KLine13, BLine 25 and BLine 26. The results are shown in . The results showed that the lines KLine12 and KLine13 were single-copy transgenic plants; the lines BLine25, BLine26 and KLine15 were multicopy transgenic plants.
Figure 2. Southern blot analysis of the transgenic plants. (A) lane 1, WT Nicotiana bethamiana; lane 2, plasmid with BrERF11b gene; lane 3, WT N, tabacum variety K326; lane 4, BLine25; lane 5, KLine13; lane 6, BLine26; lane 7, Kline12 (DNA in lanes 1 to 7 was digested by EcoR I); lane 8, WT Nicotiana bethamiana; lane 9, BLine25; lane 10, BLine26; lane 11, the plasmid with BrERF11b gene, (DNA in lanes 8 to 11 was digested by Xba I). (B) lane 1, the plasmid with BrERF11b gene; lane 2, WT N. tabacum variety K326;lane 3, KLine12; lane 4, KLine13; lane 5, KLine15; lane 6, KLine18 (DNA in lanes 1 to 6 was digested by Pst I). Note: Genomic DNA extracted from the transgenic plant using CTAB reagent was digested by restriction enzyme, and separated in an 0.8% agarose gel, and then transferred onto Hybond-N + membrane (Amerasham). Individual lanes were hybridized with dioxigenin (DIG)-11-dUTP–labeled cDNA probes specific for the segment of the BrERF11b gene (the putative open reading frame regions).
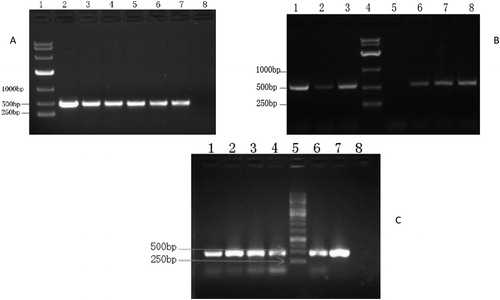
The RT-PCR analysis was conducted with the experimental plants. The results showed that the BrERF11b gene was successfully expressed in all of the experimental plants ( shows a selection of the RT-PCR results from the experimental plants).
Figure 3. RT-PCR of transgenic plants. (A) RT-PCR of plants from transgenic plant lines KLine13 and KLine15. lane1, DNA Marker (DL10000 DNA Marker, Takara Bio Inc., Beijing.); lanes 2, 3 and 4, KLine13; lanes 5, 6 and 7, KLine15; lane 8, WT N. tabacum variety K326. (B) RT-PCR of plants from transgenic plant lines BLine25 and BLine26. lanes 1, 2 and 3, BLine25; lane 4, DNA Marker (DL10000 DNA Marker, Takara Bio Inc., Beijing); lane 5, WT N. benthamiana; lanes 6,7 and 8, BLine26. (C) RT-PCR of plants from transgenic plant line KLine12. lanes 1, 2, 3, 4 and 6, KLine12; lane 5, DNA Marker (GeneRuler 1 Kb DNA Ladder, Thermo Fisher Scientific Company, China); lane 7, plasmid with BrERF11b gene; lane 8, WT N. tabacum variety K326.
Resistance of the transformed tobacco plants to S. litura
To assess the resistance of the transgenic N. tabacum variety K326 to S. litura, first-instar S. litura larvae were used to assay the resistance of the transgenic KLine12 plants. The results of the assay showed that the total weights of larvae fed the transgenic plant line KLine12 were significantly different from those of the larvae fed the controls (WT) and were reduced by 76% compared with those of the larvae fed the controls (). The survival rate of the larvae fed the transgenic plant line was significantly reduced by 62.5% compared with that of the larvae fed the controls during the feeding period of 9 days (). The results indicated that the transgenic plant line KLine12 had significant resistance to S. litura.
Figure 4. Resistance of transgenic plants to the larvae of Spodoptera litura. (A) Weight of S. litura larvae feeding on the leaves of the transgenic plant line KLine12; (B) Survival rate of S. litura feeding on the leaves of the transgenic plant line KLine12; (C): Weight of S. litura larvae feeding on the leaves of the transgenic plant lines KLine13 and KLine15; (D) Weight of S. litura larvae feeding on the leaves of the transgenic plant lines BLine 25 and BLine 26. Note: Data are mean values with standard deviation (±SD) from eight replicate experiments. Duncan's or Student’s test was used to test for significance (p < 0.05), indicated by different lowercase letters.
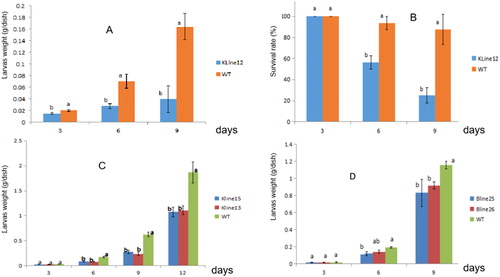
In another experiment, second-instar larvae from S. litura were used to assay the resistance of the transgenic plant lines KLine13 and KLine15. The results showed that the total weights of the living larvae fed the transgenic plant line KLine13 were significantly reduced by 40.7% compared to those of the living larvae fed the controls (WT), and the total weights of the larvae fed the line KLine15 were significantly reduced by 42.4% compared to those of the larvae fed the controls (). The survival rate of the larvae fed the transgenic plant lines KLine13 or KLine15 showed no significant difference compared to that of the larvae fed the controls during the feeding period of 12 days (Data not shown). The results indicated that the transgenic plant lines KLine13 and KLine15 also had significant resistance to S. litura.
In another experiment, the transgenic N. benthamiana plant lines Bline25 and Bline26 were used. Second-instar larvae from S. litura were used to assay the resistance of the transgenic plant lines Bline25 and Bline26. The results showed that the total weights of the larvae fed the transgenic plant line Bline25 were significantly reduced by 28.05% compared to those of the larvae fed the controls during the feeding period of 9 days, and the total weights of the larvae fed line Bline26 were significantly reduced by 20.7% compared to those of the larvae fed the controls. (). The survival rate of the larvae fed the transgenic plant lines Bline25 or Bline26 showed no significant difference compared to that of the larvae fed the controls during the feeding period of 9 days (Data not shown). It is noted that the effect of resistance to S. litura in Kline12 is better than the effects of resistance in other transgenic lines. We speculate that one of the causes may be that first-instar larvae from S. litura in the Kline12 experiment are smaller in size than the second-instar larvae from S. litura in the experiments using the other transgenic lines. Generally, the smaller the size of the larvae, the lower the survival rate of the larvae. The second cause may be that there are different effects of resistance among different transgenic lines. The effect of resistance is related to the expression level of the protein encoded by the resistance gene in different transgenic lines. Some authors have also reported that the resistance genes have different effects of resistance among a few transgenic lines. For example, the XnGroEL gene is from Xenorhabdus nematophila, and the encoded XnGroEL protein belongs to the family of molecular chaperones and is required for proper folding of cellular proteins [Citation24]. It was reported that 100% of the larvae died on plants expressing the highest level of the insecticidal XnGroEL protein (lines X3 and X4), and those plants expressing the lowest level of the protein led to 70% mortality (line X5) [Citation24]. The cry2AX1 gene encodes a chimeric Bacillus thuringiensis (Bt) toxin protein. The detached leaf bioassay demonstrated that the larvae mortality of Cnaphalocrocis medinalis was from 50% to 80% in different cry2AX1 transgenic lines [Citation25].
These results suggested that the BrERF11b gene significantly reduced the weights of living larvae feeding on the transgenic plant and significantly increased the mortality of S. litura compared to those of the larvae feeding on the controls. Some ERF transcription factors are reported to improve plant resistance against leaf-chewing insects, such as the one encoded by the OsERF3 gene from rice plants [Citation15], the ORA 59 gene from Arabidopsis thaliana [Citation26], JRE4 from tomato [Citation17], ORCA2 and ORCA3 gene from Catharanthus roseus [Citation27] and EREB58 from Zea mays [Citation28]. In this paper, the BrERF11b gene is reported to function in the resistance to leaf-chewing insects.
Resistance of transformed tobacco plants to M. persicae
To assess the resistance of the transformed tobacco plants to M. persicae, newborn apteral aphids were used. The results of the assay showed that the survival rate of aphids feeding on the transgenic plant line KLine12 was 30% during the feeding period of 21 days, and the survival rate of aphids feeding on KLine12 was significantly reduced by 55% compared to that of the aphids feeding on the controls (WT) (). The colony growth of the apteral aphids feeding on the transgenic plant line KLine12 was significantly reduced by 63.4% during the feeding period of 21 days compared to that of the apteral aphids feeding on the controls (). The results indicated that the transgenic plant line KLine12 had significant resistance to M. persicae.
Figure 5. Resistance of transgenic plants to the green aphids. (A) Survival rate of the green aphid feeding on the leaves of the transgenic plant line KLine12; (B) Colony development of the green aphid feeding on the leaves of the transgenic plant line KLine12; (C) Survival rate of the green aphid feeding on the leaves of the transgenic plant line BLine26; (D) Colony development of the green aphid feeding on the leaves of the transgenic plant line BLine26.
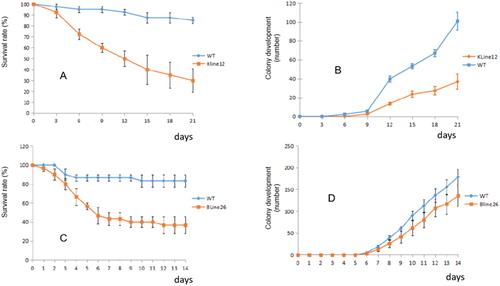
In another experiment, the survival rate of the aphids feeding on the transgenic N. benthamiana plant line BLine 26 was 36.7% during the feeding period of 14 days, and the survival rate of the aphids feeding on BLine 26 was significantly reduced by 46.7% compared to that of the aphids feeding on the controls (WT) (). The colony growth of the apteral aphids feeding on the transgenic plant line BLine 26 was significantly reduced by 22.5% during the feeding period of 14 days compared to that of the apteral aphids feeding on the controls (). The results indicated that the transgenic plant line Bline26 had significant resistance to M. persicae. It is noted that the effect of resistance to aphids in Kline12 was better than the effects of resistance in other transgenic lines. The effect of resistance was related to the expression level of the protein encoded by the resistance gene in different transgenic lines.
These results suggest that the BrERF11b gene significantly reduced the colony growth of the apteral aphids feeding on the transgenic plants and significantly increased the mortality of the aphids. Some ERF encoding genes, such as RM3 [Citation29] and Pti5 [Citation16], are reported to improve plant resistance against sap-sucking insects. In this paper, the BrERF11b gene is shown to function in plant resistance to a sap-sucking insect.
Taken together, to our knowledge, this is the first of report that the ERF11 gene can increase plant resistance to insect pests. The ERF11 gene is reported to increase plant resistance to Ralstonia solanacearum [Citation19], to promote internode elongation [Citation30], to regulate mannitol-induced growth inhibition [Citation31] and to regulate immunity to Pseudomonas syringae [Citation32]. But it has not been reported to function in plant resistance against insects yet. The BrERF11b gene has more than 50% amino acid sequence identity with ERF11-like sequences from many species of plants (Supplemental Table S1), and it is annotated as an ERF11-like gene. Therefore, it is speculated that all ERF11-like genes have similar functions to the BrERF11b gene in enhancing plant resistance to insects. The BrERF11b gene is a regulatory gene. It is important and interesting to determine the resistance genes regulated by the BrERF11b gene for future research.
Conclusions
In this paper, the BrERF11b gene is first reported as an ERF transcription factor that enhances the resistance of plants to chewing insects and sap-sucking insects, which is only the second report of a transcription factor with such a function since the discovery of the AtMYB44 transcription factor.
Supplemental Material
Download PDF (645.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data supporting this paper are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Ghodke AB, Good RT, Golz JF, et al. Extracellular endonucleases in the midgut of Myzus persicae may limit the efficacy of orally delivered RNAi. Sci Rep. 2019;9(1):1322–1326.
- Smith CM, Chuang WP. Plant resistance to aphid feeding: behavioral, physiological, genetic and molecular cues regulate aphid host selection and feeding. Pest Manag Sci. 2014;70(4):528–540.
- Ahmad M, Mehmood R. Monitoring of resistance to new chemistry insecticides in Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. J Econ Entomol. 2015;108(3):1279–1288.
- Yarasi B, Sadumpati V, Immanni CP, et al. Transgenic rice expressing Allium sativum leaf agglutinin (ASAL) exhibits high-level resistance against major sap-sucking pests. BMC Plant Biol. 2008;8:102.[cited 2020 Jul 08]
- Sun Y, Huang X, Ning Y, et al. TPS46, a rice terpene synthase conferring natural resistance to bird cherry-oat aphid, Rhopalosiphum padi (Linnaeus). Front Plant Sci. 2017;8:110. [cited 2020 Jul 08]
- Stewart SA, Hodge S, Ismail N, et al. The RAP1 gene confers effective, race-specific resistance to the pea aphid in Medicago truncatula independent of the hypersensitive reaction. Mol Plant Microbe Interact. 2009;22(12):1645–1655.
- Chen WB, Lu GQ, Cheng HM, et al. Transgenic cotton coexpressing Vip3A and Cry1Ac has a broad insecticidal spectrum against lepidopteran pests. J Invertebr Pathol. 2017;149:59–65.
- Hu L, Ye M, Kuai P, et al. OsLRR-RLK1, an early responsive leucine-rich repeat receptor-like kinase, initiates rice defense responses against a chewing herbivore. New Phytol. 2018;219(3):1097–1111.
- Guo JY, Dong L, Wan FH. Influence of Bt transgenic cotton on larval survival of common cutworm Spodoptera litura. Chin J Biol Control. 2003;19:145–148.
- Lü BB, Li XJ, Sun WW, et al. AtMYB44 regulates resistance to the green peach aphid and diamondback moth by activating EIN2-affected defences in Arabidopsis. Plant Biol (Stuttg). 2013;15(5):841–850.
- Shah AD, Ahmed M, Mukhtar Z, et al. Spider toxin (Hvt) gene cloned under phloem specific RSs1 and RolC promoters provides resistance against American bollworm (Heliothis armigera). Biotechnol Lett. 2011;33(7):1457–1463.
- Javaid S, Amin I, Jander G, et al. A transgenic approach to control hemipteran insects by expressing insecticidal genes under phloem-specific promoters. Sci Rep. 2016;6:34706–34734.
- Zhang JY, Wang QJ, Guo ZR. [Progresses on plant AP2/ERF transcription factors]. Yi Chuan. 2012;34(7):835–847.
- Amorim A, Lidiane LB, da Fonseca dos Santos R, et al. Transcription factors involved in plant resistance to pathogens. Curr Protein Pept Sci. 2017;18(4):335–351.
- Lu J, Ju H, Zhou G, et al. An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J. 2011;68(4):583–596.
- Wu C, Avila CA, Goggin FL. The ethylene response factor Pti5 contributes to potato aphid resistance in tomato independent of ethylene signalling. J Exp Bot. 2015;66(2):559–570.
- Nakayasu M, Shioya N, Shikata M, et al. JRE4 is a master transcriptional regulator of defense-related steroidal glycoalkaloids in tomato. Plant J. 2018;94(6):975–990.
- Song X, Li Y, Hou X. Genome-wide analysis of the AP2/ERF transcription factor superfamily in Chinese cabbage (Brassica rapa ssp. pekinensis). BMC Genomics. 2013;14:573.[cited 2020 07Jul 08]
- Lai Y, Dang F, Lin J, et al. Overexpression of a Chinese cabbage BrERF11 transcription factor enhances disease resistance to Ralstonia solanacearum in tobacco. Plant Physiol Biochem. 2013;62:70–78.
- Wang H, Ding Q, Wang HL. A new Na+/H+ antiporter gene KvNHX1 isolated from the halophyte Kosteletzkya virginica improves salt tolerance in transgenic tobacco. Biotechnol Biotechnol Equip. 2018;32(6):1378–1386.
- Tel-Zur N, Abbo S, Myslabodski D, et al. Modified CTAB procedure for DNA isolation from epiphytic cacti of the genera Hylocereus and Selenicereus (Cactaceae). Plant Mol Biol Rep. 1999;17(3):249–254.
- Tang QY, Zhang CX. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013;20(2):254–260.
- Sakuma Y, Liu Q, Dubouzet JG, et al. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun. 2002;290(3):998–1009.
- Kumari P, Kant S, Zaman S, et al. A novel insecticidal GroEL protein from Xenorhabdus nematophila confers insect resistance in tobacco. Transgenic Res. 2014;23(1):99–107.
- Chakraborty M, Reddy PS, Mustafa G, et al. Transgenic rice expressing the cry2AX1 gene confers resistance to multiple lepidopteran pests. Transgenic Res. 2016;25(5):665–678.
- Vos IA, Verhage A, Schuurink RC, et al. Onset of herbivore-induced resistance in systemic tissue primed for jasmonate-dependent defenses is activated by abscisic acid. Front Plant Sci. 2013;4:539. [cited year month day]
- Zhang H, Hedhili S, Montiel G, et al. The basic helix-loop-helix transcription factor CrMYC2 controls the jasmonate-responsive expression of the ORCA genes that regulate alkaloid biosynthesis in Catharanthus roseus. Plant J. 2011;67(1):61–71.
- Li S, Wang H, Li F, et al. The maize transcription factor EREB58 mediates the jasmonate-induced production of sesquiterpene volatiles. Plant J. 2015;84(2):296–308.
- Niu L, Lei P, Zeng W, et al. Dynamic transcriptomes of resistant and susceptible peach lines after infestation by green peach aphids (Myzus persicae Sülzer) reveal defence responses controlled by the Rm3 locus. BMC Genomics. 2018;19(1):846–861.
- Zhou X, Zhang ZL, Park J, et al. The ERF11 Transcription factor promotes internode elongation by activating gibberellin biosynthesis and signaling. Plant Physiol. 2016;171(4):2760–2770.
- Dubois M, Van den Broeck L, Claeys H, et al. The Ethylene response factors ERF6 and ERF11 antagonistically regulate mannitol-induced growth inhibition in Arabidopsis. Plant Physiol. 2015;169(1):166–179.
- Zheng X, Xing J, Zhang K, et al. Ethylene response factor ERF11 activates BT4 transcription to regulate immunity to Pseudomonas syringae. Plant Physiol. 2019;180(2):1132–1151.
