Abstract
Cathelicidin host defense peptides (cHDPs) widely exist in animals with the functions of innate immunity and acquired immunity. To investigate the tissue and sequential expression patterns of the dCATH gene, which encodes the only cHDPs found in waterfowls, we analyzed the internal organs of healthy ducks (Shaxoxing Sheldrake) from 1-day-old to 28-day-old raised in the natural environment. The dCATH mRNA expression level across tissues was determined via quantitative real-time polymerase chain reaction (qRT-PCR). Monoclonal antibody against the mature dCATH peptide used in enzyme-linked immunosorbent assay (ELISA) was prepared by the lymphocyte hybridoma technique for the first time. The concentration of the dCATH peptide in tissues was determined by ELISA. It showed that the dCATH mRNA had a clear tissue-specificity and age-dependent expression pattern, and it was highly expressed in the bone marrow and moderately expressed in the liver, kidney, spleen, pancreas and bursa of healthy duck. The peptide levels of dCATH in liver, kidney, spleen, pancreas and bursa changed with the age of duck, and the peptide concentration in these organs was between 88 and 128 ng/g tissue. For the first time, we characterized the concentration of the cHDP dCATH in vivo. Our study may represent new insights into the crosstalk of innate immunity, tissue development and age.
Introduction
Host defense peptides (HDPs) are a family of low-molecular-weight peptides produced by organisms. HDPs act in the protection of hosts from a broad range of pathogens including bacteria, viruses and fungi. HDPs are widely distributed among mammals, birds, insects and plants and possess many biological functions besides antimicrobial activity, such as immunological, anticancer and anti-mitogenic activity [Citation1]. As the critical components of animal innate immunity, HDPs play an important role in protecting animal organs from invading pathogens [Citation2]. HDPs play an indispensable role in vertebrates by killing invading microorganisms directly in the early stages. In addition, HDPs can cure infection by enhancing the adaptive immunity of vertebrates. Many studies have shown that HDPs not only kill microbes rapidly but also have subsequent immune modulatory activity [Citation3,Citation4].
Two types of HDPs (defensins and cathelicidins) have been identified in birds. All defensins found in birds belong to the β-defensins and are named avian β-defensins (AvBDs). Many AvBDs have been previously described in different birds including the chicken (Gallus gallus) and zebra finch (Taeniopygia guttata) [Citation5], mallard (Anas platyrhynchos) [Citation6], crested ibis (Nipponia nippon) [Citation7], goose (Anser cygnoides) [Citation8] and pigeon (Columba livia) [Citation9]. The name cathelicidin is derived from the similarity of the cathelicidin large middle domain to cathelin, a cathepsin L inhibitor originally isolated from porcine leukocytes [Citation10]. Cathelicidins are translated intracellularly as inactive prepropeptides, consisting of a short signal peptide, a conserved cathelin domain and a highly variable mature peptide domain. Upon release into the environment, the C-terminal mature peptide is activated by proteolytic cleavage and exerts its antimicrobial and immunomodulatory functions [Citation11].
Although cathelicidins have been identified from almost all vertebrate species, including humans, rabbits, guinea pigs, monkeys, horses, mice, cattle, sheep, goats, rats [Citation12,Citation13], there are very limited reports of cathelicidins in birds. At present, four cathelicidins have been described from the chicken (Gallus gallus), i.e. cathelicidin-1/fowlicidin-1 [Citation14], CMAP27/fowlicidin-2 [Citation15], fowlicidin-3 [Citation16] and cathelicidin-B1 [Citation17]. Three cathelicidins were characterized in ringnecked pheasant (Phasianus colchicus), i.e. Pc-CATH-1, −2 and −3, which share a high level of identity with chicken cathelicidins-1, −2 and −3, respectively [Citation18,Citation19]. A novel waterfowl cathelicidin ortholog named dCATH from duck was identified by Gao et al. [Citation20]. Besides having a direct inhibitory effect on pathogenic microorganisms, cathelicidins can also modulate the immune response. In addition, cathelicidins are involved in phagocytosis, neutralization of LPS-induced TLR4 activation and LTA-induced TLR2 activation [Citation21], and they can promote wound healing [Citation22], skew macrophage differentiation [Citation23] and regulate phagocytosis [Citation24]. In addition, because several functions have been described for a limited number of cathelicidins, it is unclear which properties are peptide-specific and which are related to general functions of cathelicidins [Citation21].
The cDNA sequence of the dCATH gene encodes a predicted 146-amino-acid polypeptide composed of a 17-residue signal peptide, a 109-residue conserved cathelin domain and a 20-residue mature peptide (the amino acid sequence: KRFWQLVPLAIKIYRAWKRR). The mature peptide of dCATH exerted strong antimicrobial activity against a broad range of bacteria in vitro by destroying the cell membrane, with most minimum inhibitory concentrations in the range of 2 to 4 μmol/L [Citation20]. Although the dCATH peptide may function as an important regulator of host defense against exogenous pathogens, the expression profile and biological functions of dCATH are still unclear.
This study investigated the expression of the dCATH gene with regard to tissue specificity and body development stages of duck at the mRNA and mature peptide level. The dCATH mRNA expression level in different tissues was determined via quantitative real-time polymerase chain reaction (qRT-PCR). Monoclonal antibody against the dCATH peptide was prepared by the lymphocyte hybridoma technique, and was used in enzyme-linked immunosorbent assay (ELISA) analysis of the level of the dCATH peptide in duck body. To our knowledge, it was the first time that avain cathelidin peptide concentration in vivo was quantified. Our study provided the basis for the functional expression of the dCATH gene and novel insights into the correlation between cathelicidins and functions of innate and acquired immunity.
Materials and methods
Ethics statement
The experimental protocol was conducted in accordance with the practices outlined in the Guide for the Care and Use of Agricultural Animals in Agriculture Research and Teaching of Northeast Agricultural University (Protocol number: NEAU-[2011]-9).
Animals and tissue sampling
Fifty 1-day-old male specific-pathogen-free (SPF) Shaoxing sheldrake were raised under SPF conditions and adjusted to diet after facility acclimation. Diet was weighed and recorded to ensure that a similar amount was fed to all ducks. Ducks were housed in five cages, and 10 replicates in one cage served as one treatment. All ducks were provided access to food and water ad libitum during the 28-d experimental period. Ducks were exposed to 12-h dark and light cycles. At the end of each trial cycle (1, 7, 14, 21 and 28 days old), three ducks randomly selected from each treatment group were euthanized with ether and decapitated, and their tissues were snap-frozen in liquid nitrogen and then stored at −80 °C until use.
Total RNA extraction and qRT-PCR
Total RNA of tissue samples was isolated using a reagent kit (TaKaRa, Japan) in accordance with the protocol recommended by manufacturers. Briefly, 2 μg of total RNA from each sample was converted into cDNA using oligo dT primers and SuperScript™ II reverse transcriptase followed by the manufacturer’s instructions (Tiangen Biotech Co. Ltd., Beijing, China). The expression patterns of the dCATH gene in different tissues were determined via qRT-PCR using the SYBR® Premix Ex Taq™ Kit (Takara Biotechnology Co., Ltd., Otsu, Shiga, Japan) in the ABI StepOne Plus™ Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The primers were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). For dCATH, 5′-GCAGCCTCAACTTCACCATCA-3′ and 5′-GAGTCCACGCAGGTGACAT-3′ were used. For the β-actin gene, 5′-ATGTCGCCCTGGATTTCG-3′ and 5′-CACAGGACTCCATACCCAAGAA-3′ were used. The PCR conditions were 94 °C for 5 min; 30 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 60 s followed by a final elongation step at 72 °C for 10 min. The resulting mean –ΔΔCt values from each group were used to calculate the relative expression ratio of the detected mRNA. All assays were repeated in triplicate.
Peptide synthesis and coupling
The mature dCATH peptide was synthesized by solid-phase methods using N-(9-fluorenyl) methoxycarbonyl (Fmoc) chemistry [Citation25]. To couple with keyhole limpet hemocyanin, a cysteine was added at the N-end of the peptide. The carboxyl end of the mature dCATH peptide was amidated to avoid the generation of an antibody against the carboxyl group due to the immunogenicity of the carboxyl group. The purity of the peptide was analyzed by reversed phase high performance liquid chromatography (RP-HPLC). Molecular weight was measured by electrospray ionization mass spectrometry (ESI-MS). 3-Maleimidobenzoic acid N-hydroxysuccinimide ester (MBS) was used as the coupling agent, and 0.1 mL dimethyl sulfoxide solution (DMSO) containing MBS (10 mg/mL) was mixed with 0.5 mL aqueous solution of hemocyanin. After a 30-min reaction at room temperature, the mixture was purified by a G10 chromatographic column and eluted with phosphate buffered saline (PBS) (1 mmol/L ethylenediaminetetraacetic acid (EDTA), pH 7.2), and the target elution peak was collected. Two milliliters of hemocyanin elution was mixed with 1 mL PBS (1 mmol/L EDTA, 5 mg/mL peptide) and reacted at room temperature for 2 h and stored at 4 °C until use.
Preparation of monoclonal antibody (mAb)
The hemocyanin-coupled dCATH peptide was used as an antigen to produce mAbs according to the method of Gao et al. [Citation26]. Three female Balb/c mice (6 to 8 weeks old) were injected at multiple sites subcutaneously and intraperitoneally with the coupled peptide solution (containing 20 μg of dCATH peptide) or hemocyanin thoroughly emulsified with an equal volume of complete Freund’s adjuvant (Sigma-Aldrich) as first immunization. Two boosts were given at days 14 and 28 with 20 μg of the peptide thoroughly emulsified with incomplete Freund’s adjuvant (Sigma-Aldrich). The antibody titer of the antisera was examined by indirect ELISA using the mature dCATH peptide. Three days later, splenocytes from the immunized mouse were mixed with murine SP2/0 myeloma cells at a ratio of 10:1 in the presence of 1 mL polyethylene glycol (PEG) 1500 (Roche) to generate hybridoma cells. The hybridomas were cultured in RPMI 1640 medium and the titer of the supernatant of culture medium was determined by indirect ELISA for the antibody activity of the dCATH peptide. After three subclonings, about 1 × 106 positive hybridoma cells were injected into the paraffin primed mouse intraperitoneally. The ascites fluid was collected after at least one week, and the mAb was purified with Protein G (GE Healthcare, Chicago, IL, USA). The purified mAb was analyzed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and iELISA as described above. The mAb isotype was analyzed using the mouse monoclonal antibody isotyping Kit (the Clonotyping System-HRP Kit, Southern Biotech). Firstly, IgA, IgG1, IgG2a, IgG2b, IgM, Lambda and Kappa reagents were diluted by coating buffer (1:1000) and coated overnight at 4 °C. The purified mAb was added onto plates and incubated at 37 °C for 1 h. The following procedure was the same as that of the indirect ELISA.
Enzyme-linked immunosorbent assay (ELISA)
The concentration of the mature dCATH peptide in different tissues was measured via ELISA. Protein extract was prepared from tissues using tissue protein extraction reagent (Thermo Scientific, USA). The protein extract was coated on an ELISA plate at 4 °C for 10-12 h and then washed three times with PBS with 0.1% Tween-20 (PBST). Blocking was conducted with 5.0% nonfat dry milk at 37 °C for 2 h. The mAb (1:400) against the dCATH peptide was added to 96-well plates and incubated at 37 °C for 1 h. After washing three times with PBST, goat anti-mouse IgG (H + L) antibody (horseradish peroxidase conjugated) (Beyotime Biotechnology®, Shanghai, China) was added to the plates, TMB (Solarbio Life Science®, Shanghai, China) was added as a substrate, and the plates were incubated at 37 °C in a dark environment. Fifteen minutes later, the reaction was terminated with 1.0 mol/L H2SO4. The OD of 96-well plates was determined at 450 nm. All assays were repeated in triplicate.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software version 6.0 (GraphPad Software Inc., San Diego, CA). Data were expressed as the mean values with standard deviation (±SD) values. Statistical significance was determined by Student's t-test, with p < 0.05 indicating statistical significance.
Results and discussion
mRNA level of the dCATH gene expressed in tissues
More comprehensive study work on AvBDs has been reported on their gene expression, tissue distribution, expression modulation and bioactivities in vivo and in vitro, and so on [Citation5, Citation7, Citation12, Citation27]. But very limited research work has been carried out on the avian cathelicidins. As the sole cHDP in duck, knowledge pertaining dCATH expression and tissue distribution patterns in the host is unknown. In this study, the expression of the dCATH gene in 18 tissues of duck at different ages was analyzed by qRT-PCR. As shown in , the dCATH gene was differentially expressed in the internal tissues of 1-, 7-, 14-, 21- and 28-day-old ducks, and the dCATH mRNA level had clear specificity and correlation with the different development phases.
Figure 1. dCATH mRNA level in different tissues at post-hatch day 1 (a), day 7 (b), day 14 (c), day 21 (d) and day 28 (e).
Note: β-actin was used to normalize the target gene expression profiles. Data relate to 3 healthy ducks/group and are presented as means ± SD
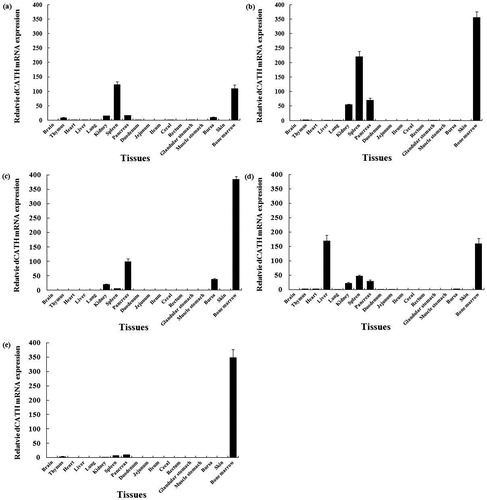
Many studies showed that avian HDPs (AvBDs and cathelicidins) expression was characterized comprehensively and differentially in different tissues [Citation28–30]. However, different avian HDPs genes have various tissue specificities in their expression characteristics in vivo. The expression levels of chCATH-1 and −2 (fowlicidin 1 and 2) were notably high in the bursa and bone marrow and much higher than in other tissues [Citation17]. The expression of HDPPc-CATH1 in the bursa and bone marrow was higher than that in other tissues [Citation19]. AvBD9 (Gal-6) mRNA was highly expressed in the esophagus and crop, moderately expressed in the glandular stomach and most weakly expressed in the intestinal tract of chicken [Citation27]. The mRNA of AvBD10 (Gal-8) was highly expressed in the small intestine and liver and moderately expressed in the chick tongue and lung, and the mRNA of host peptide AvBD5 (Gal-9) was highly expressed in the tongue, small intestine, lung, heart, proventriculus, spleen, liver and thymus of chicken [Citation29]. The dCATH mRNA was highly expressed in bone marrow of ducks of different age. Besides bone marrow, several tissues including kidney, spleen, pancreas and bursa, also contained high dCATH mRNA level at specific development stages of duck. The dCATH mRNA was not detected or was present in very low level in other tissues of duck from 1 to 28 days old. These results demonstrated that the expression of the dCATH gene was highly expressed at the mRNA level in duck immune organs and exhibited tissue specificity.
The expression of chicken cathelicidins is developmentally regulated. The expression levels of all four cathelicidin mRNAs were generally increased in the course of embryo development [Citation31]. During the first 28 days after hatching, CATH1-3 showed an age-dependent increase both in the cecal tonsil and lung, whereas all four cathelicidins were peaked in the bursa on day 4 after hatching, with a gradual decline by day 28. On the other hand, CATH1-3 showed a peak expression in the cecum on day 28, whereas the highest expression of CATH-B1 was seen in both the lung and cecal tonsil on day 14 [Citation28]. The mRNA expression of the dCATH gene in tissues also showed developmental stage-dependence except that the gene was constitutively expressed in bone marrow (). For instance, the mRNA level of the dCATH gene in kidney tissue was higher only on the first day of age, but lower on other age-days. High-level dCATH mRNA expression in spleen occurred at the ages of 1 and 7 days (). The liver only expressed high dCATH mRNA level at 21 days of age, but no expression or very low level was detected on other age-days (). Interestingly, no high expression was found for the dCATH mRNA in all tissues except for bone marrow.
The development of the innate immune system in neonatal avian is less well described. Bar-Shira et al. [Citation32] found that neonatal and even embryonic chicks could produce humoral immune responses, but these were generally very low. They also found that the antibody titer against BSA was higher in chicks that were orally or intramuscularly vaccinated for more than 10 days compared with animals vaccinated in the first week after hatching, which demonstrated that acquired immune function development of chicks occurred over the first week of life. Heterophils of 4- and 7-day-old chicks showed higher phagocytic and bactericidal capacities compared with cells of 1-day-old chicks [Citation33].
The expression of the dCATH gene has been confirmed in the bone marrow of Anas platyrhynchos [Citation20]. Except for muscle stomach, the expression levels of the dCATH gene in some tissues (such as the thymus, heart, liver, lung, bursa, kidney, spleen, pancreas and bone marrow) were much higher than those in other tissues. The Pc-CATH1 gene was more highly expressed in the bone marrow and bursa but to a lesser extent in the lung, heart, brain, testis and spleen, and almost no Pc-CATH1 expression was detected in the thymus and liver [Citation19]. In this study, the dCATH gene expression was higher compared to chicken Pc-CATH1 expression in the thymus, heart, liver, lung, bursa, kidney, spleen, pancreas and bone marrow, which demonstrated that in the process of innate immunity, dCATH might play a more important role in these internal organs. High-level expression of the dCATH gene in the bone marrow and other tissues of the healthy physiological state ducks suggested that the dCATH peptide might possess other important functions apart from its antimicrobial activities. The findings of this study support our research results that dCATH could be expressed in the thymus, liver, lung, bursa and spleen, and different HDPs might be expressed in different tissues in different species. The highest expression for the CATH-1 gene was reported in the spleen, oviduct and large intestine; that of the CATH-2 gene, in the stomach, spleen, oviduct and large intestine, and that of the CATH-3 gene, in the large intestine and spleen [Citation34]. A study reported that the AvBD9 gene was expressed at low levels in the cecum of 6-week-old Ross broilers [Citation27], which was supported by our results. The expression distribution of the dCATH gene in this study was almost similar to the HDP gene expression pattern in different tissues and species.
The dCATH peptide synthesis
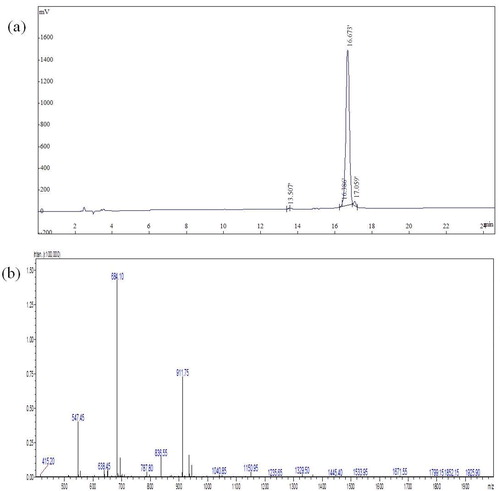
Since dCATH is a small peptide, it is difficult to analyze the product. An immunological assay based on monoclonal antibody for the peptide was unavailable prior to our study. To obtain the antigen for antibody preparation, the dCATH peptide with C-terminal amidation was synthesized by solid-state chemical synthesis. After purification with a G10 column, the peptide purity reached 98.28%, as determined by RP-HPLC (). The peptide molecular mass was 2732.40 Da as measured with mass spectrometry () consistent with its theoretical molecular mass (2731.37 Da), suggesting that the peptide was successfully synthesized.
Immunization and screening of hybridoma clones against dCATH
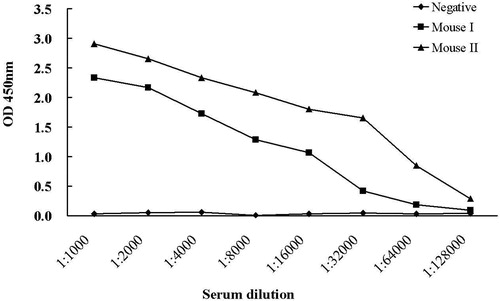
Due to the low antigenicity of the small peptides of dCATH, it is difficult to prepare antibodies by using dCATH as the antigen directly. In order to increase the antigenicity of dCATH, keyhole limpet hemocyanin was used to couple with the dCATH peptide. The coupled CATH peptide as antigen was injected to immune mice, and the dCATH peptide was adopted to coat ELISA plates. After immunization three times, the titer of antiserum from mouse was determined by iELISA. As shown in , the titers from two immunized mice were significantly higher than that from the negative control injected hemocyanin. The spleen cells of mouse I and II were harvested aseptically and used to fuse with myeloma cells. The fusion result that yielded mAbs was presented in . Hybridoma cell clones of mouse I from 673 wells out of 682 wells showed positive activity of anti-dCATH mAb determined by iELISA with the positive clone rate of 2.35%, while 670 wells out of 674 wells showed positive activity with the positive clone rate of 3.97% for mouse II. Among these hybridoma cell clones, one stable hybridoma cell that showed highly significant specificity against dCATH was successfully screened out from mouse II, and selected for further experiments.
Table 1. Cell fusion and screening of hybridoma clones against dCATH.
Production and characterization of mAb
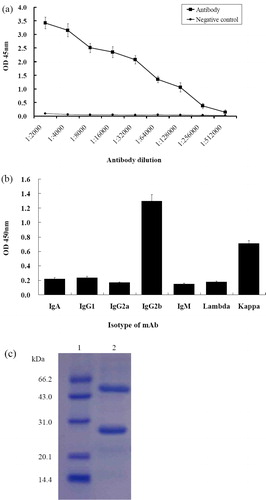
By injecting the hybridoma cells into paraffin primed Balb/c mice, ascetic fluid was produced and collected. Antibodies prepared by ascites are often contaminated with other proteins, such as albumin, transferrin, lipoprotein, macroglobulin and serum, and do not meet the requirements of further functional studies [Citation35]. Therefore, further purification of antibodies is required. After purification by affinity chromatography using Protein G columns, the concentration of the mAb was detected to be 0.310 mg/mL. The titer of the mAb was determined by iELISA, and the results showed that the titer of anti-dCATH mAb reached 1.28 × 105 (). To identify the antibody isotype of the mAb produced from the cell line, a capture ELISA method was performed by using isotyping mAb kit, and it showed that the mAb belonged to the IgG2b subclass (). SDS-PAGE analysis of the purified mAb showed that the molecular weight of the light and heavy chains of the anti-dCATH mAb were about 27 kDa and 55 kDa, respectively (). These results demonstrated that the active mAb was successfully purified and could be used for further characterization.
Figure 4. Analysis of the purified dCATH mAb. (a) Titer of the mAb determined by iELISA. The minimum OD value greater than 1 is at a titer of 1.28 × 105, indicating that the antibody remained active after purification. Negative control was ascites from negative mouse. Three replicates were performed for each test. (b) The isotype of dCATH mAb determined by isotyping kit (IgA, IgG1, IgG2a, IgG2b, IgM, Lambda, Kappa). (c) SDS-PAGE analysis of the dCATH mAb. Lane M: Marker (RP1400, Solarbio Life Science®, Shanghai, China), lane 1: the purified mAb.
There are little commercial mAbs available for the research of HDPs to date, which greatly hampers the study of the biological properties and activity of HDPs in vivo. We here reported the generation of a new mAb to the mature dCATH peptide. MAbs against dCATH were developed to recognize native and synthetic dCATH and are useful for the analysis of dCATH in tissue extracts or biological fluids. This well-characterized specific mAb to dCATH will have the potential for development of detection kits, and to allow for multiplex measurement of dCATH isoforms in duck bodily fluids. The present study provides insights into establishing an easy, feasible and reliable method for rapid preparation of such novel mAbs against cHDPs.
Content of dCATH peptide in tissues
After biosynthesis as precursors, cHDPs lay in the granules of host cells in an inactive form. Upon microbial invasion and inflammation, mature active cHDPs are released quickly by proteolytic processing from precursor peptides [Citation36]. The concentration of mature cHDPs is crucial for their biofunction since the activity of cHDPs showed a concentration gradient dependent pattern in vitro [Citation16, Citation20]. A lot of researches were carried out to determine the cHDPs solely based on mRNA level, but there are few reports on the concentration of cHDPs in vivo to date. In this study, the mature peptide concentration of dCATH in duck tissues was determined for the first time by ELISA method using C-terminal peptide-specific mAb. As shown in , the mature dCATH peptide had a broad tissue distribution (liver, bursa, kidney, spleen and pancreas), and the concentration of the peptide was between 88 and 128 ng/g tissue in these tissues. In the liver, the dCATH peptide had the highest expression at days 1 and 21 and decreased from 1 to 14 and 21 to 28 days (p < 0.05) (). In the kidney, the dCATH peptide expression changed slightly from 1 to 7 days but was significantly downregulated in 14-day-old animals (p < 0.05) (). After that step, the dCATH peptide expression increased but was not significant (p < 0.05). In the spleen, the dCATH peptide expression was not significantly different from day 1 to day 7 but was significantly decreased between 14 and 21 days (p < 0.05) (). After that point, the dCATH peptide expression significantly increased at day 28 (p < 0.05). In the pancreas, the peak of the dCATH peptide expression was at day 7 and then its expression was dramatically downregulated in 14- and 21-day-old duck (). At 28 days of age, the dCATH peptide increased but this was not significant (p > 0.05). In bursa, the dCATH peptide expression gradually decreased from 1 to 14 days and increased significantly from 14 to 28 days of age (p < 0.05) (). However, no tissue specificity for the peptide concentration was observed, which was different from the trend observed in the dCATH mRNA level. Further research is required to determine the cell-specific transcriptional regulation and posttranscriptional regulation of dCATH.
Figure 5. Tissue concentration of the dCATH peptide in healthy ducks. Liver (a), kidney (b), spleen (c), pancreas (d) and bursa (e) sampled from healthy ducks at day 1, day 7, day 14, day 21 and day 28 after hatching. Data relate to 3 healthy ducks/group and are presented as means ± SD.
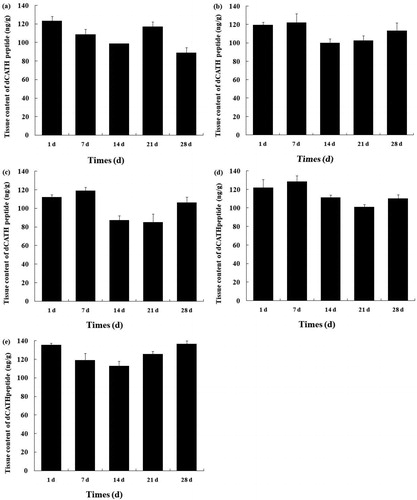
The concentration of the mature dCATH peptide in tissues showed significant difference between different ages in statistics, but there was no age-dependent manner as shown in the dCATH mRNA expression in these tissues. For instance, the dCATH mRNA in liver, bursa, kidney, spleen and pancreas was not detectable or was present in a very low level at 28 day-old, but the concentrations of the mature dCATH peptide were in high level in these tissues. These results indicated that the dCATH mature peptide could be transported from other tissues such as bone marrow before or after peptide cleavage from the preproprotein. Goitsuka et al. [Citation17] found that chicken cathelicidin-B1 was synthesized by the interfollicular secretory epithelial cells, but the mature peptide must be transported to deposit onto a fibrillar network lining the M cell basolateral surfaces through a likely route of transport via M cell transcytosis. Another research found that the CATH-2 expression was not induced in intestinal tissues by Salmonella enteritidis challenge, but the bacterial infection did result in recruitment of CATH-2 containing heterophils to the site of infection [Citation37]. However, cell localization of the dCATH mature peptide and transport mechanism in tissues need to be further investigated.
Conclusions
The dCATH mRNA expression showed distinct tissue specificity and age dependence. The bone marrow is the most important tissue expressing the dCATH gene, and has consistently high level of dCATH mRNA. Five tissues, the liver, kidney, spleen, pancreas and bursa, also showed high level of the dCATH mRNA in specific periods after hatch. There was almost no mRNA expression other tissues. The content of the dCATH mature peptide in tissues was quantified for the first time and the concentration was between 88 and 128 ng/g tissue. The present study has successfully demonstrated a rapid and effective method to prepare mAb for the HDPs.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Xingjun Feng, upon reasonable request.
Additional information
Funding
References
- Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals (Basel). 2014;7(5):545–594.
- Feng X, Jin S, Wang M, et al. The critical role of tryptophan in the antimicrobial activity and cell toxicity of the duck antimicrobial peptide dCATH. Front Microbiol. 2020;11:1146
- Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30(3):131–141.
- Yount NY, Yeaman MR. Emerging themes and therapeutic prospects for anti-infective peptides. Annu Rev Pharmacol Toxicol. 2012;52:337–360.
- Hellgren O, Ekblom R. Evolution of a cluster of innate immune genes (beta-defensins) along the ancestral lines of chicken and zebra finch. Immunome Res. 2010;6:3.
- Huang Y, Li Y, Burt DW, et al. The duck genome and transcriptome provide insight into an avian influenza virus reservoir species. Nat Genet. 2013;45(7):776–783.
- Lan H, Chen H, Chen LC, et al. The first report of a pelecaniformes defensin cluster: characterization of β-defensin genes in the crested ibis based on BAC libraries. Sci Rep. 2014;4:6923
- Ma DY, Zhou CY, Zhang MY, et al. Functional analysis and induction of four novel goose (Anser cygnoides) avian β-defensins in response to salmonella enteritidis infection. Compar Immunol Microbiol Infectious Dis. 2012;35(2):197–207.
- Li Y, Xu Q, Zhang T, et al. Host avian beta-defensin and toll-Like receptor responses of pigeons following infection with pigeon paramyxovirus type 1. Appl Environ Microbiol. 2015;81(18):6415–6424.
- Ritonja A, Kopitar M, Jerala R, et al. Primary structure of a new cysteine proteinase inhibitor from pig leucocytes. FEBS Lett. 1989;255(2):211–214.
- Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. 2005;7(2):179–196.
- Cuperus T, Coorens M, Dijk AV, et al. Avian host defense peptides. Dev Comp Immunol. 2013;41(3):352–369.
- Wassing GM, Bergman P, Lindbom L, et al. Complexity of antimicrobial peptide regulation during pathogen-host interactions. Int J Antimicrob Agents. 2015;45(5):447–454.
- Lynn DJ, Higgs R, Gaines S, et al. Bioinformatic discovery and initial characterisation of nine novel antimicrobial peptide genes in the chicken. Immunogenetics. 2004;56(3):170–177.
- van Dijk A, Veldhuizen EJA, van Asten AJAM, et al. CMAP27, a novel chicken cathelicidin-like antimicrobial protein. Vet Immunol Immunopathol. 2005;106(3-4):321–327.
- Xiao Y, Cai Y, Bommineni YR, et al. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J Biol Chem. 2006;281(5):2858–2867.
- Goitsuka R, Chen CL, Benyon L, et al. Chicken cathelicidin-B1, an antimicrobial guardian at the mucosal M cell gateway. Proc Natl Acad Sci USA. 2007;104(38):15063–15068.
- Feng F, Chen C, Zhu W, et al. Gene cloning, expression and characterization of avian cathelicidin orthologs, Cc-CATHs, from Coturnix coturnix. FEBS J. 2011;278(9):1573–1584.
- Wang Y, Lu Z, Feng F, et al. Molecular cloning and characterization of novel cathelicidin-derived myeloid antimicrobial peptide from Phasianus colchicus. Dev Comp Immunol. 2011;35(3):314–322.
- Gao W, Xing L, Qu P, et al. Identification of a novel cathelicidin antimicrobial peptide from ducks and determination of its functional activity and antibacterial mechanism. Sci Rep. 2015;5:17260
- Coorens M, Scheenstra MR, Veldhuizen EJ, et al. Interspecies cathelicidin comparison reveals divergence in antimicrobial activity, TLR modulation, chemokine induction and regulation of phagocytosis. Sci Rep. 2017;7:40874
- Carretero M, Escámez MJ, García M, et al. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol. 2008;128(1):223–236.
- van der Does AM, Beekhuizen H, Ravensbergen B, et al. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J Immunol. 2010;185(3):1442–1449.
- Wan M, van der Does AM, Tang X, et al. Antimicrobial peptide LL-37 promotes bacterial phagocytosis by human macrophages. J Leukoc Biol. 2014;95(6):971–981.
- Cantor S, Vargas L, Rojas A O, et al. Evaluation of the antimicrobial activity of cationic peptides loaded in surface-modified nanoliposomes against foodborne bacteria. IJMS. 2019;20(3):680. pii:
- Gao Y, Sang FF, Meng L, et al. Preparation of a novel monoclonal antibody against caprine interleukin-17A and its applications in immunofluorescence and immunohistochemistry assays. BMC Biotechnol. 2019;19(1):47.
- van Dijk A, Veldhuizen EJA, Kalkhove SIC, et al. The beta-defensin gallinacin-6 is expressed in the chicken digestive tract and has antimicrobial activity against food-borne pathogens. Antimicrob Agents Chemother. 2007;51(3):912–922.
- Achanta M, Sunkara LT, Dai G, et al. Tissue expression and developmental regulation of chicken cathelicidin antimicrobial peptides. J Anim Sci Biotechnol. 2012;3(1):15
- Ma DY, Liu SW, Han ZX, et al. Expression and characterization of recombinant gallinacin-9 and gallinacin-8 in Escherichia coli. Protein Expr Purif. 2008;58(2):284–291.
- Sugiarto H, Yu P. Identification of three novel ostricacins: an update on the phylogenetic perspective of beta-defensins. Int J Antimicrob Agents. 2006;27(3):229–235.
- Meade KG, Higgs R, Lloyd AT, et al. Differential antimicrobial peptide gene expression patterns during early chicken embryological development. Dev Compar Immunol. 2009;33(4):516–524.
- Bar-Shira E, Sklan D, Friedman A. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev Comp Immunol. 2003;27(2):147–157.
- Wells LL, Lowry VK, Deloach JR, et al. Age-dependent phagocytosis and bactericidal activities of the chicken heterophil. Dev Comp Immunol. 1998;22(1):103–109.
- Yacoub HA, Elazzazy AM, Mahmoud MM, et al. Chicken cathelicidins as potent intrinsically disordered biocides with antimicrobial activity against infectious pathogens. Dev Comp Immunol. 2016;65:8–24.
- Zha M, Yang J, Zhou L, et al. Preparation of mouse anti-human rotavirus VP7 monoclonal antibody and its protective effect on rotavirus infection. Exp Ther Med. 2019;18(2):1384–1390.
- Salzman NH, Hung K, Haribhai D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11(1):76–83.
- van Dijk A, Tersteeg-Zijderveld MHG, Tjeerdsma-van Bokhoven JLM, et al. Chicken heterophils are recruited to the site of Salmonella infection and release antibacterial mature Cathelicidin-2 upon stimulation with LPS. Mol Immunol. 2009;46(7):1517–1526.