Abstract
Dechlorination is the key step in biological degradation of dichloromethane (DCM), which is catalyzed by the enzyme DCM dehalogenase. In this study, the detailed structure and function of DCM dehalogenase in Methylobacterium rhodesianum H13 were investigated. Our results indicate that DCM dehalogenase (C1505H2255N403O430S7) is a stable hydrophilic protein localized in the cytoplasm, with an isoelectric point of 6.35. They also indicate that DCM dehalogenase is a frizzled protein with 52.43% of amino acid residues exposed on its surface, and that alpha-helices are a main structural component (53.47%). As one of the theta class glutathione S-transferases (GSTs), DCM dehalogenase presents as a dimeric structure and shows strong substrate selectivity. The catalytic tunnel radius and length of DCM dehalogenase are both 1.4 Å. Glu42 and Val43 are two of the essential amino acids in both the catalytic tunnel and catalytic pockets. The amino acid sequence of DCM dehalogenase was predicted to be homologous among various microorganisms, and its active site was conserved with eight potential sites. The recombinant DCM dehalogenase was expressed in E. coli BL21 strain. The optimum conditions for DCM dehalogenase are pH 8, temperature of 30 °C and presence of Mn2+. All the obtained information of DCM dehalogenase will lay the foundation for further study of its structural transformation and functional improvement.
Introduction
Dichloromethane (DCM) is one of the most common manufactured chlorinated organic compounds that has been commonly used as a solvent in chemical, film and pharmaceutical fields [Citation1]. DCM is a stable compound and can accumulate in surface water, posing a threat to ecosystems due to its toxic properties. These characteristics have led to its identification as a priority pollutant by the United States Environmental Protection Agency [Citation2]. The sustained increase of DCM in the atmosphere has the potential to delay the recovery of Earth’s ozone layer for up to thirty years [Citation3]. Also, due to bioaccumulation and carcinogenic effects, DCM poses a considerable human health risk [Citation4].
Since the toxicity of DCM is linked to the chlorine atom [Citation5], the dechlorination of DCM to produce methane is of great importance. Recently, a variety of reductive dechlorination methods have been developed [Citation6–12]. Among these approaches, biodegradation showed good promise due to its typically mild reaction conditions and low energy consumption [Citation13–15].
DCM dehalogenase, a glutathione-dependent dehalogenase of the glutathione S-transferase (GST; EC 2.5.1.18) family encoded by dcmA, plays an important role in DCM biodegradation [Citation16]. Genome-wide studies demonstrated that all isolated and purified DCM dehalogenases are glutathione (GSH) dependent, with similar subunit size and substrate specificity [Citation17]. The expression pattern of DCM dehalogenase consists of the structural gene dcmA and the regulatory gene dcmR [Citation18]. The genome sequence of Methylobacterium extorquens DM4 indicates that dcmA lies on a 126 kb dcm genomic island, which has not been observed in other DCM-dechlorinating strains so far [Citation19]. Furthermore, DCM dehalogenases are divided into two groups (group A and B) according to their reaction kinetics; group B has a greater vitality than group A [Citation20]. However, the crystal structure and functional domain information are still yet to be reported for DCM dehalogenase, which are key to revealing catalytic mechanisms behind enzymatic DCM dechlorination [Citation21].
The GST superfamily is composed of a wide group of multifunctional enzymes ubiquitous in bacteria, fungi, plants and animals [Citation22]. GSTs with known crystal structures can be used as templates for the prediction of DCM dehalogenase catalytic mechanism [Citation23–25]. In our previous work, the DCM dehalogenase gene sequence was obtained from M. rhodesianum H13, which is a highly efficient DCM-degrading bacterium [Citation26]. The specific activity of DCM dehalogenase from recombinant bacteria Escherichia coli BL21(DE3)-pET28b(+)-dcm was detected as 0.00686 ± 0.00005 U/mg, three times greater than from wild-type M. rhodesianum [Citation27]. In our study, we build on these previous efforts by reporting the physicochemical properties, structural and functional domains and catalytic tunnel of DCM dehalogenase that were predicted by bioinformatics analysis. A model of the mechanistic action between DCM dehalogenase and substrate is also reported from molecular docking simulations. We also analyzed the conservation and genetic relationships among DCM dehalogenases by the methods of homologous sequence alignment and phylogenetic tree analysis. These findings could aid to understand the structure–activity relationship and dechlorination mechanism of DCM dehalogenase.
Materials and methods
Sequences
The nucleotide and amino acid sequences of DCM dehalogenase were obtained from GenBank (http://www.ncbi.nlm.nih.gov). The accession numbers were KM243642 and AIN35010.1 for nucleotide and amino acid sequences, respectively.
Bioinformatics analysis
Molecular weight, number of amino acid residues, isoelectric point and instability index were predicted using the ProtParam tool (http://web.expasy.org/protparam/) [Citation28]. The subcellular localization, signal peptide and transmembrane domains were predicted by PSORT (http://psort.hgc.jp/), Signal P (http://www.cbs.dtu.dk/services/SignalP/) and TMpred (https://embnet.vital-it.ch/software/TMPRED_form.html), respectively. Secondary structural information was acquired by Predict Protein (https://www.predictprotein.org/). Functional domain and substrate binding site predictions were obtained using the Conserved Domains Database (CDD) (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Potential phosphorylation sites were determined using Net Phos2.0 (http://www.cbs.dtu.dk/services/Net Phos/). The sequence similarity search was performed using BLAST (www.ncbi.nlm.nih.gov/BLAST), and multiple sequence alignment was carried out using the ClustalW algorithm from DNAMAN software. The phylogenetic tree was created using the neighbor-joining method in MEGA software [Citation29]. Tertiary structure was established using SWISS-MODEL (http://swissmodel.expasy.org/) based on homology modeling [Citation30]. The putative catalytic tunnel, where DCM transits to the DCM dehalogenase active site was analyzed by HotSpot Wizard (https://loschmidt.chemi.muni.cz/hotspotwizard/) [Citation31], and the molecular docking prediction was achieved using Autodock 4.0 software [Citation32, Citation33].
Gene cloning and recombinant plasmid construction
The full-length sequence of DCM gene from M. rhodesianum H13 was cloned. A sequence of 864 bp with two restriction enzyme cutting sites of EcoRV and HindIII were inserted into a T vector. The construct was confirmed by polymerase chain reaction (PCR) and enzyme digestion. Then, the fine construction with DCM gene was transformed into Escherichia coli strain BL21 using an LB agar plate containing 100 mmol/L ampicillin. The T-DCM plasmids were isolated from E. coli cells using an extraction plasmid kit (Clontech) according to the manufacturer’s protocol. The DCM gene fragment was double digested and cloned into the recombinant expression vector pET21b. The obtained construct was checked using PCR with universal T7-promoter and T7-terminator primers.
Expression of recombinant protein in E. coli BL21 strain
The pET21b-DCM construct was transformed into the E. coli BL21 cells. To induce protein expression, 600 μL isopropyl β-D-1-thiogalactopyranoside (IPTG) was added and the culture was incubated at 20 °C for 12 h. After cultivation, E. coli cells were sonicated twice and pelleted by centrifugation at 14,000 g for 15 min at 4 °C. The supernatant was collected and added to a chromatography column that was loaded with packing materials. Then, five times of the column volume of working buffer containing 20 mmol/L Tris-H2SO4, 10 mmol/L imidazole, and 0.5 mol/L Na2SO4 was added to balance the column. After equilibration, the protein sample was added to the column about 10 times of the column volume per hour. The final sample was eluted using an appropriate amount of 500 nmol/L of imidazole.
To verify the purified DCM protein, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) experiment was performed. Proteins were separated by 12% SDS-PAGE in 250 mmol/LM Tris-HCl buffer (pH 6.8) and 0.5% (w/v) bromine blue; initial voltage of 80 V and voltage after 1 h of 150 V.
Protein concentration was assayed using a BCA protein assay kit (Thermo Scientific). For DCM protein purification, nickel affinity chromatography resin (Bio-Rad) was used. In brief, two resin-bed volumes of equilibration buffer was added and mixed until the resin is fully suspended. The sample protein extract was added to the resin tube and mixed on a rotator for 30 min. The tubes were centrifuged for 2 min at 700 g. The resin was washed with two resin-bed volumes of wash buffer, followed by centrifugation again. Bound His-tagged proteins were eluted using one resin-bed volume of dilution buffer. The tubes were centrifuged for 2 min at 700 g. The supernatant was carefully removed and stored.
Determination of DCM dehalogenase activity
Firstly, 1 mL of DCM dehalogenase sample solution was added to 5 mL of 50 mmol/L dipotassium phosphate solution buffer containing 12.5 mmol/L of DCM and 5 mmol/L of GSH and was incubated at 30 °C for 30 min. After filtration using a 0.45 μm filter, the sample was subjected to a C18 column. The level of formed Cl−1 was quantitatively determinated using a Dionex ICS-2100 ion chromatograph with a Chromeleon chromatography workstation DionexIonPac AS18 (250 × 4 mm) separation column. The chromatographic experiment was performed according to the published work [Citation34]. To evaluate the effect of pH on the activity of DCM dehalogenase, dipotassium hydrogen phosphate solution was adjusted to a pH ranging from 3.0 to 11.0, and the enzymatic reaction was carried out at 30 °C for 30 min. To evaluate the effect of temperature on the activity of DCM dehalogenase, temperatures ranging from 20 to 60 °C were selected. To evaluate the effect of metal ions on the activity of DCM dehalogenase, several metal ions, including Ca2+, Cu2+, Mg2+, Fe2+ and Mn2+, with a working concentration of 10 mmol/L were added to the reaction mixture, and the DCM dehalogenase activity was also determined at 30 °C for 30 min.
Data analysis
Data are representative images or mean values with standard deviation (±SD) (N = 3). Statistical analysis was done using the Wilcoxon texts with SPSS software 19.0 version (SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant at p < 0.05.
Results and discussion
Biochemical properties
The biochemical properties of DCM dehalogenases were obtained from the National Center for Biotechnology Information (NCBI) (). Most reported DCM dehalogenases are composed of 288 amino acids with average molecular weight of 33.1 kDa, except the DCM dehalogenase from M. extorquens. According to the formula, an instability index lower than 40 predicts a stable protein, whereas a value above 40 denotes a potentially unstable protein [Citation35]. The DCM dehalogenases were predicted as stable enzymes due to their instability index values ranging from 22.98 to 29.58. Stable enzymes are advantageous as they can be used for longer durations in biocatalysis.
Table 1. Predicted biochemical properties of DCM dehalogenase.
Hydrophobicity plays an important role in protein folding, which can provide evidence for protein secondary structure and functional domain predictions [Citation36]. For DCM dehalogenase from M. rhodesianum H13, most amino acids are strongly hydrophilic, and it is speculated to be a hydrophilic protein with the grand average value of −0.490.
Prediction of signal peptide, transmembrane domains and subcellular localization
In light of the significance of signal peptides for understanding the protein classification and localization, the signal peptide of DCM dehalogenase was predicted by Signal P software (). The analysis showed the following values: C-max = 0.144, Y-max = 0.138, S-max = 0.343 (< 0.5), S-mean = 0.165 and D = 0.151, with a low value of Y and C. According to the result, DCM dehalogenase does not have a signal peptide and may be a non-secreted protein. Meanwhile the signal peptide of DCM dehalogenase from Hyphomicrobium sp. GJ21 also was predicted (), and the obtained results were analogical.
Figure 1. Prediction of signal peptide and transmembrane domain of DCM dehalogenase. (a) Prediction of signal peptide of DCM dehalogenase from Methylobacterium rhodesianum H13. (b) Prediction of signal peptide of DCM dehalogenase from Hyphomicrobium sp. GJ21. (c) (i→o) Inside to outside helix; (o→i) outside to inside helix.
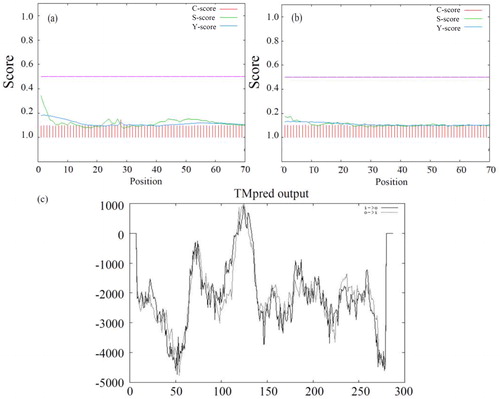
To gain insight into the structure and function of DCM dehalogenase, we predicted the transmembrane domains. The results suggest that there are two transmembrane helices of DCM dehalogenase (). One was from the inside to outside containing amino acids 116–135, and the other was from the outside to inside containing amino acids 110 to 135. A source states that DCM dehalogenase could also be transferred across the cell membrane [Citation37].
According to our subcellular localization prediction using Signal P, DCM dehalogenase is most likely located in the cytoplasm, which agrees with the previous findings [Citation38]. They found that DCM dehalogenases from Methylobacterium sp. are located in the cytoplasm, and occasionally in the interstitial space.
Prediction of secondary structure, phosphorylation sites and functional domains
The polypeptide chain is usually folded to form a relatively stable secondary structure in order to generate specific physiochemical properties and biological activity. Secondary structural information is the basis for understanding the tertiary structure of a protein [Citation39]. DCM dehalogenase was predicted as a coiled-coil protein, containing 53.47% α-helices, 9.72% β-sheets and 36.81% loops (). It was also found that 52.43% (>16%) of amino acid residues were exposed to the protein surface, which provided strong evidence for the hydrophilic properties of DCM dehalogenase.
Figure 2. Detail information of DCM dehalogenase. (a) Prediction of secondary structure of DCM dehalogenase. H: helix, E: extended (sheet), blank: other (loop). (b) Prediction of phosphorylation sites of DCM dehalogenase. (c) Prediction of functional domains of DCM dehalogenase.
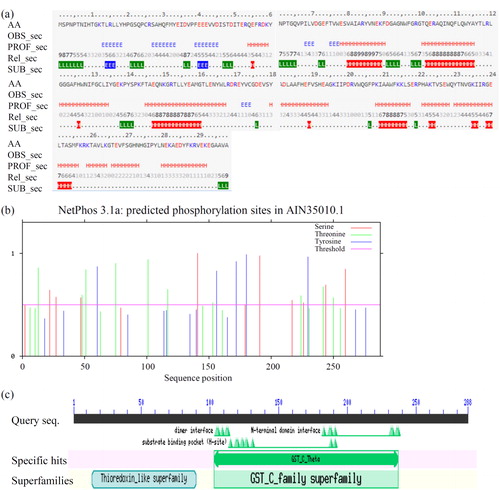
Protein kinase catalyzed phosphorylation is one of the most common protein post-translational modifications. The conformation and activity of a protein can be altered by the phosphorylation of key amino acid residues such as threonine (Thr, T), serine (Ser, S) and tyrosine (Tyr, Y) [Citation40, Citation41]. In total, 24 phosphorylation sites were found in DCM dehalogenase (). They are T13, T48, T51, T75, T101, T117, T153, T224, T242, T250, S22, S27, S47, S141, S191, S217, S226, S244, S260, Y60, Y156, Y172, Y180 and Y230. This indicates that DCM dehalogenase is very liable to phosphorylation. As an important post-translational modification, phosphorylation plays roles in the regulation of enzymatic activity. Our data identified several potential phosphorylation sites for artificial improvement of DCM dehalogenase.
The functional domains of DCM dehalogenase were analyzed by CDD (). The results indicated that DCM dehalogenase has two functional domains: one from the GST_C_family superfamily (130–237) and the other from the thioredoxin-like superfamily (14–90). The GST family is a multifunctional gene superfamily that contains a class of soluble proteases which are capable of nucleophilic attack on an electrophilic substrate. GST genes from bacteria can be divided into four categories (β, χ, ξ and θ) according to their basic nucleotide sequence information, and the DCM dehalogenase gene belongs to type θ, which reportedly encode more substrate-specific enzymes than other categories [Citation29, Citation42]. The dimer interface sites (105, 106, 109, 112 and 113), N-terminal domain interface sites (183, 187, 188, 191, 232, 233, 236 and 237) and substrate binding sites (115, 119, 120, 123, 124, 128, 188 and 191) were also obtained. Importantly, substrate binding sites are putative catalytic activity sites, which closely relate to enzyme catalytic activity.
Phylogenetic tree and multiple sequence alignment
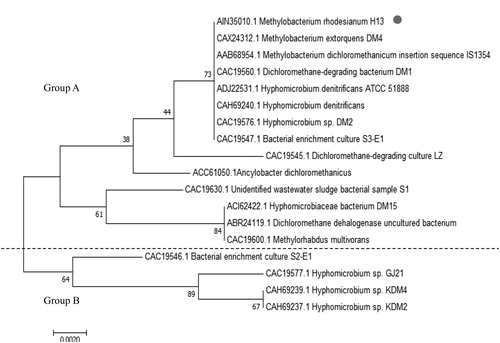
The multiple sequence alignment and phylogenetic tree analysis were performed to reveal the taxonomical variations in DCM dehalogenases from various microorganisms. Genetic evolutionary trees are an effective tool for studying biological evolution processes and relationships [Citation43]. Phylogenetic tree branch lengths relate to the degree of difference between the two groups (Group A and B). Thus, gene origin, evolution and possible structural/functional changes could be derived from related sequences. The phylogenetic tree of DCM dehalogenase was constructed by EMGA software using the neighbor-joining method (). Although similar phylogenetic trees have been constructed before, the strains included in the tree that was constructed here are more diverse [Citation27]. Our tree indicated that the DCM dehalogenases from M. rhodesianum H13 and eight other strains, such as M. extorquens DM4 and Hyphornicrobium sp. DM2 and Hyphornicrobium denitrificans ATCC 51888, share the same branch in Group A, suggesting they may be closely related to each other. This result is in accordance with the work of Muller et al. [Citation19]. Therefore, we speculate that the amino acid sequences of DCM dehalogenases are highly conserved among microorganisms.
In recent years, multiple bacterial strains have been found to be capable of dehalogenation [Citation16]. However, dehalogenases extracted from them differ in catalytic activity and substrate specificity, and their dehalogenation mechanisms remain poorly understood. In order to gain more comprehensive information on the mechanism of halogenated hydrocarbon degradation, in-depth bioinformatics analysis may be used. Sequence homology data can contribute to understanding the basic characteristics and conserved members of a gene family. We found that the amino acid sequence of DCM dehalogenase demonstrates high homology with those from other species selected using GenBank ().
Homologous modeling, catalytic tunnel analysis and molecular docking
The tertiary structure provides important information for the study and reconstruction of the protein function [Citation44]. Despite the great structural diversity of biologically important natural product scaffolds, a number of privileged structural motifs occur within them with high frequency [Citation45]. In this study, the tertiary structure of DCM dehalogenase was predicted by SWISS-MODEL (). The model was based on template 2c3t, a human GST T1-1, with a sequence identity of 30.33%, and the QMEAN score was −1.71. As reported, DCM dehalogenase usually shows distinct substrate specificity, and the substrate binding center as well as the catalytic sites were located between two α-helices, where a very conservative cysteine residue pocket formed as the receptor for the groups and electron during the reaction [Citation46].
Figure 5. Model structure of DCM dehalogenase. (a) Tertiary structure of DCM dehalogenase; (b) Catalytic tunnel and the key amino acids simulation of DCM dehalogenase; (c) Molecular docking of DCM dehalogenase.
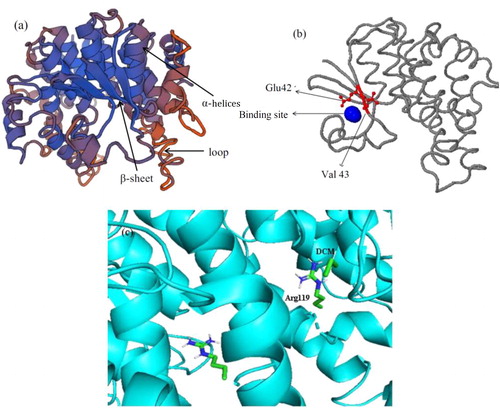
The tunnel analysis indicated that the tunnel radius and length of DCM dehalogenase were both 1.4 Å (). The larger tunnel radius contributes to speeding up the reaction rate by offering a convenient entrance for DCM to the active region of DCM dehalogenase. Meanwhile, the shorter length of the tunnel can shorten the contact time between DCM and the DCM dehalogenase, which is beneficial to the capture and catalysis of DCM [Citation47]. In addition, it is found that the amino acid residues Glu42 and Val43 in both the tunnel and catalytic pocket were likely to be closely related to the catalytic performance of DCM dehalogenase.
Molecular docking studies of DCM dehalogenase showed the interactions between substrate DCM and the active sites (). The haloalkane dehalogenases catalyze the hydrolases of ester and amid bonds via a two-step nucleophilic acyl substitution mechanism [Citation48]. In the first step, the aspartate of an Asp-His-Asp/Glu catalytic triad functions as a nucleophile that attacks the sp3-hybridized carbon atom of the scissile ester/amide bond, resulting in a covalently bound ester intermediate. Subsequently, a water molecule activated by the His-Asp or His-Glu pair hydrolyzes this ester intermediate in the second step.
Cloning and bacterial expression of recombinant DCM dehalogenase
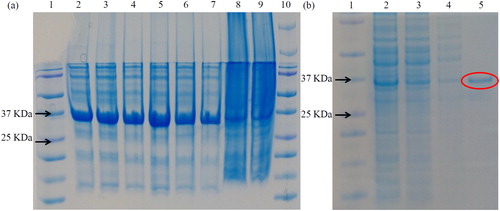
To confirm the biological function of DCM dehalogenase, full length CDS of its encoding gene was isolated and cloned from M. rhodesianum H13. After cloning DCM into a pET28b vector, the construct of pET28b-DCM was transformed into E. coli strain BL21 and the recombinant DCM dehalogenase protein was expressed in the host. Taking advantage of the His-tag in the pET28b vector, nickel affinity chromatography resin was used for DCM protein purification. Then, purified DCM dehalogenase protein was checked using SDS PAGE gel (). Our data showed that the purity of recombinant DCM dehalogenase meets the requirements of subsequent experiments ().
Figure 6. SDS PAGE of purified recombinant protein. (a) Lane 1: protein marker (Fermentas); lane 2: 10 mmol/L imidazole-repetition 1; lane 3: 10 mmol/L imidazole-repetition 2; lane 4: 10 mmol/L imidazole-repetition 3; lane 5: 15 mmol/L imidazole-repetition 1; lane 6: 15 mmol/L imidazole-repetition 2; lane 7: 15 mmol/L imidazole-repetition 3; lane 8: supernatant; lane 9: mixture; lane 10: marker. (b) lane 1: protein marker (…); 2: crude enzyme solution; 3: superior fluid penetration solution; 4: miscellaneous protein; 5: pure enzyme solution.
Enzyme activity analysis of DCM dehalogenase
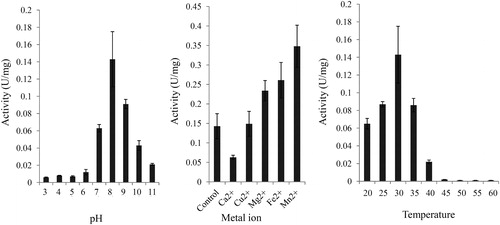
Several environmental factors, such as pH, temperature and metal ions, largely affected the activity of DCM dehalogenase [Citation34]. The effects of pH, temperature and metal ions on the activity of DCM dehalogenase were determined. Our results showed that DCM dehalogenase activity reached its peak at pH 8 and then sharply deceased with the increase of pH to 11 (). In addition, the activity of DCM dehalogenase was increased with the increase of temperature from 15 to 35 °C and quickly decreased over 40 °C, suggesting that the optimum temperature of DCM dehalogenase is 35 °C (). Moreover, several metal ions, including Ca2+, Cu2+, Mg2+, Fe2+ and Mn2+, were selected to test the effects of metal ions on the activity of DCM dehalogenase. Our data showed that Mg2+, Fe2+ and Mn2+ played positive roles in the activity of DCM dehalogenase and Ca2+ played a negative role in the activity of DCM dehalogenase. No significant role of Cu2+ on the activity of DCM dehalogenase was observed ().
Figure 7. Analysis of enzymatic properties of DCM dehalogenase. (a) pH dependence. (b) Influence of metal ions. (c) Temperature dependence. The measurement of enzyme activity was performed after purification of DCM dehalogenase from M. rhodesianum H13. Data are mean values with standard deviation (±SD) from three independent experiments. Asterisks indicate statistically significant differences (p < 0.05).
DCM dehalogenase is widespread in various microbes. However, the crystal structure and enzyme activity of DCM dehalogenase have not been analyzed yet. We first cloned the full length sequence of DCM dehalogenase from M. rhodesianum and predicted its potential active sites, providing an opportunity to understand the dechlorination mechanism of DCM dehalogenase. Taking advantage of the recombinant protein, the enzyme activity of DCM dehalogenase under different conditions was studied. Our results gave important information for a foundation of artificial modification and functional improvement of DCM dehalogenase.
Conclusions
The physicochemical properties, structural and functional characteristics of DCM dehalogenase from Methylobacterium rhodesianum H13 were analyzed by bioinformatics methods in this study. Our results support the hypothesis that DCM dehalogenase is a stable hydrophilic protein with frizzled structure found in the cytoplasm. In addition, DCM dehalogenase appears to belong to the θ class of glutathione transferases and has strong substrate selectivity. Multiple sequence alignment and phylogenetic tree analyses showed that the amino acid sequences of DCM dehalogenase are homologous among microorganisms, and all eight substrate binding sites are located in conserved areas. The radius and length of the catalytic tunnel were both predicted as 1.4 Å, which is relevant for the capture and catalysis of DCM. In addition, two amino acid residues in catalytic tunnels and pockets were predicted, which may play a key role in enzyme catalytic activity and stability. The recombinant DCM dehalogenase was expressed in E. coli BL21 strain. The optimum conditions for DCM dehalogenase are pH 8, temperature 30 °C and addition of Mn2+. The results of this study have important relevance for the structural transformation and functional improvement of DCM dehalogenase.
Disclosure statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Shestakova M, Sillanpaa M. Removal of dichloromethane from ground and wastewater: a review. Chemosphere. 2013;93(7):1258–1267.
- Yu J, Cai W, Cheng Z, et al. Degradation of dichloromethane by an isolated strain Pandoraea pnomenusa and its performance in a biotrickling filter. J Environ Sci (China). 2014;26(5):1108–1117.
- Hossaini R, Chipperfield MP, Montzka SA, et al. The increasing threat to stratospheric ozone from dichloromethane. Nat Commun. 2017;8:15962.
- Park AS, Ritz B, Ling C, et al. Exposure to ambient dichloromethane in pregnancy and infancy from industrial sources and childhood cancers in California. Int J Hyg Environ Health. 2017;220(7):1133–1140.
- Yu J, Song X, Wang J, et al. Bioelectrocatalytic dechlorination of trichloroacetic acid by hemoglobin modified graphite electrode in aqueous solutions of different pH and temperature. Int J Electrochem Sci. 2012;7:10519–10529.
- Chen X, Yao X, Yu C, et al. Hydrodechlorination of polychlorinated biphenyls in contaminated soil from an e-waste recycling area, using nanoscale zerovalent iron and Pd/Fe bimetallic nanoparticles. Environ Sci Pollut Res Int. 2014;21(7):5201–5210.
- Li YP, Cao HB, Zhang Y. Reductive dehalogenation of haloacetic acids by hemoglobin-loaded carbon nanotube electrode. Water Res. 2007;41(1):197–205.
- Liu Q, Yu J, Xu Y, et al. Bioelectrocatalytic dechlorination of trichloroacetic acid at gel-immobilized hemoglobin on multiwalled carbon nanotubes modified graphite electrode: kinetic modeling and reaction pathways. Electrochim Acta. 2013;92:153–160.
- Sun C, Baig SA, Lou Z, et al. Electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid using nanosized titanium nitride doped palladium/nickel foam electrodes in aqueous solutions. Appl Catal B Environ. 2014;158–159:38–47.
- Sun Z, Wei X, Han Y, et al. Complete dechlorination of 2,4-dichlorophenol in aqueous solution on palladium/polymeric pyrrole-cetyl trimethyl ammonium bromide/foam-nickel composite electrode. J Hazard Mater. 2013;244–245:287–294.
- Wang H, Hu J, Xu K, et al. Biodegradation and chemotaxis of polychlorinated biphenyls, biphenyls, and their metabolites by Rhodococcus spp. Biodegradation. 2018;29(1):1–10.
- Xu Y, Ding X, Ma H, et al. Selective hydrodechlorination of 3,5,6-trichloropicolinic acid at an activated silver cathode: synthesis of 3,5-dichloropicolinic acid. Electrochim Acta. 2015;151:284–288.
- Matturro B, Di Lenola M, Ubaldi C, et al. First evidence on the occurrence and dynamics of Dehalococcoides mccartyi PCB-dechlorinase genes in marine sediment during Aroclor1254 reductive dechlorination. Mar Pollut Bull. 2016;112(1–2):189–194.
- Wang S, Chng KR, Wilm A, et al. Genomic characterization of three unique Dehalococcoides that respire on persistent polychlorinated biphenyls. Proc Natl Acad Sci USA. 2014;111(33):12103–12108.
- Wu S, Zhang H, Yu X, et al. Identification and cloning of a gene encoding dichloromethane dehalogenase from a methylotrophic bacterium, Bacillus circulans WZ-12 CCTCC M 207006. Bioprocess Biosyst Eng. 2009;32(6):845–852.
- Muller EE, Hourcade E, Louhichi-Jelail Y, et al. Functional genomics of dichloromethane utilization in Methylobacterium extorquens DM4. Environ Microbiol. 2011;13(9):2518–2535.
- Vuilleumier S, Chistoserdova L, Lee MC, et al. Methylobacterium genome sequences: a reference blueprint to investigate microbial metabolism of C1 compounds from natural and industrial sources. PLoS One. 2009;4(5):e5584.
- Firsova JE, Doronina NV, Trotsenko YA. Analysis of the key functional genes in new aerobic degraders of dichloromethane. Microbiology. 2010;79(1):66–72.
- Muller EE, Bringel F, Vuilleumier S. Dichloromethane-degrading bacteria in the genomic age. Res Microbiol. 2011;162(9):869–876.
- Janssen DB, Oppentocht JE, Poelarends GJ. Microbial dehalogenation. Curr Opin Biotechnol. 2001;12(3):254–258.
- Xue Y-P, Cao C-H, Zheng Y-G, et al. Enzymatic asymmetric synthesis of chiral amino acids. Chem Soc Rev. 2018;47(4):1516–1561.
- Longkumer T, Parthasarathy S, Vemuri SG, et al. OxyR-dependent expression of a novel glutathione S-transferase (Abgst01) gene in Acinetobacter baumannii DS002 and its role in biotransformation of organophosphate insecticides. Microbiology (Reading). 2014;160(Pt 1):102–112.
- Allocati N, Federici L, Masulli M, et al. Cysteine 10 is critical for the activity of Ochrobactrum anthropi glutathione transferase and its mutation to alanine causes the preferential binding of glutathione to the H-site. Proteins. 2008;71(1):16–23.
- Stourman NV, Branch MC, Schaab MR, et al. Structure and function of YghU, a nu-class glutathione transferase related to YfcG from Escherichia coli. Biochemistry. 2011;50(7):1274–1281.
- Tars K, Larsson AK, Shokeer A, et al. Structural basis of the suppressed catalytic activity of wild-type human glutathione transferase T1-1 compared to its W234R mutant. J Mol Biol. 2006;355(1):96–105.
- Chen DZ, Ouyang DJ, Liu HX, et al. Effective utilization of dichloromethane by a newly isolated strain Methylobacterium rhodesianum H13. Environ Sci Pollut Res Int. 2014;21(2):1010–1019.
- Yu J, Liu Q, Liu L, et al. Cloning and characterization of dichloromethane dehalogenase from Methylobacterium rhodesianum for dichloromethane degradation. Biorem J. 2017;21(2):71–80.
- Wilkins MR, Gasteiger E, Bairoch A, et al. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–552.
- Mohsenzadeh S, Saffari B, Mohabatkar H. A new member of Tau-class glutathione S-transferase from barley leaves. EXCLI J. 2009;8:190–194.
- Arnold K, Bordoli L, Kopp J, et al. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22(2):195–201.
- Bendl J, Stourac J, Sebestova E, et al. HotSpot Wizard 2.0: automated design of site-specific mutations and smart libraries in protein engineering. Nucleic Acids Res. 2016;44(W1):W479–W487.
- Ballu S, Itteboina R, Sivan SK, et al. Rational design of methicillin resistance Staphylococcus aureus inhibitors through 3D-QSAR, molecular docking and molecular dynamics simulations. Comput Biol Chem. 2018;73:95–104.
- Khosla C, Tang Y, Chen AY, et al. Structure and mechanism of the 6-deoxyerythronolide B synthase. Annu Rev Biochem. 2007;76:195–221.
- Yu J, Shi J, Zhang Y, et al. Molecular docking and site-directed mutagenesis of dichloromethane dehalogenase to improve enzyme activity for dichloromethane degradation. Appl Biochem Biotechnol. 2020;190(2):487–505.
- Farmani J, Safari M, Roohvand F, et al. Conjugated linoleic acid‐producing enzymes: a bioinformatics study. Eur J Lipid Sci Technol. 2010;112(10):1088–1100.
- Bai Y, He S, Zhao G, et al. Toxoplasma gondii: bioinformatics analysis, cloning and expression of a novel protein TgIMP1. Exp Parasitol. 2012;132(4):458–464.
- Lygina AS, Meyenberg K, Jahn R, et al. Transmembrane domain peptide/peptide nucleic acid hybrid as a model of a SNARE protein in vesicle fusion. Angew Chem Int Ed Engl. 2011;50(37):8597–8601.
- Hohenberg H, Mannweiler K, Muller M. High-pressure freezing of cell suspensions in cellulose capillary tubes. J Microsc. 1994;175(Pt 1):34–43.
- Micsonai A, Wien F, Kernya L, et al. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc Natl Acad Sci USA. 2015;112(24):E3095–E3103.
- Madero-Perez J, Fdez E, Fernandez B, et al. Parkinson disease-associated mutations in LRRK2 cause centrosomal defects via Rab8a phosphorylation. Mol Neurodegener. 2018;13(1):3.
- Nitulescu II, Meyer SC, Wen QJ, et al. Mediator kinase phosphorylation of STAT1 S727 promotes growth of neoplasms with JAK-STAT activation. EBioMedicine. 2017;26:112–125.
- Wiktelius E, Stenberg G. Novel class of glutathione transferases from cyanobacteria exhibit high catalytic activities towards naturally occurring isothiocyanates. Biochem J. 2007;406(1):115–123.
- Li ZH, Tang ZX, Fang XJ, et al. Bioinformatics analysis of a non-specific nuclease from Yersinia enterocolitica subsp. palearctica. Comput Biol Chem. 2013;47:207–214.
- Hao X, Zhang G, Zhou X. Guiding exploration in conformational feature space with Lipschitz underestimation for ab-initio protein structure prediction. Comput Biol Chem. 2018;73:105–119.
- Scott TA, Heine D, Qin Z, et al. An L-threonine transaldolase is required for L-threo-β-hydroxy-α-amino acid assembly during obafluorin biosynthesis. Nat Commun. 2017;8:15935.
- Wu SJ, Zhang LL, Wang JD, et al. Bacillus circulans WZ-12 - a newly discovered aerobic dichloromethane-degrading methylotrophic bacterium. Appl Microbiol Biotechnol. 2007;76(6):1289–1296.
- Liu ZQ, Zhang XH, Xue YP, et al. Improvement of Alcaligenes faecalis nitrilase by gene site saturation mutagenesis and its application in stereospecific biosynthesis of (R)-(-)-mandelic acid. J Agric Food Chem. 2014;62(20):4685–4694.
- de Jong RM, Dijkstra BW. Structure and mechanism of bacterial dehalogenases: different ways to cleave a carbon-halogen bond. Curr Opin Struct Biol. 2003;13(6):722–730.